Abstract
Background
Little is known about the patterns of utilization of surveillance imaging after treatment of hepatocellular carcinoma (HCC). We sought to define population-based patterns of surveillance and investigate if intensity of surveillance impacted outcome following HCC treatment.
Methods
The Surveillance, Epidemiology, and End Results-Medicare database was used to identify patients with HCC diagnosed between 1998 and 2007 who underwent resection, ablation, or intra-arterial therapy (IAT). The association between imaging frequency and long-term survival was analyzed.
Results
Of the 1,467 patients, most underwent ablation only (41.5 %), while fewer underwent liver resection only (29.6 %) or IAT only (18.3 %). Most patients had at least one CT scan (92.7 %) during follow-up, while fewer had an MRI (34.1 %). A temporal trend was noted with more frequent surveillance imaging obtained in post-treatment year 1 (2.5 scans/year) vs. year 5 (0.9 scans/year; P=0.01); 34.5 % of alive patients had no imaging after 2 years. Frequency of surveillance imaging correlated with procedure type (total number of scans/5 years, resection, 4.7; ablation, 4.9; IAT, 3.7; P<0.001). Frequency of surveillance imaging was not associated with a survival benefit (three to four scans/year, 49.5 months vs. two scans/year, 71.7 months vs. one scan/year, 67.6 months; P=0.01)
Conclusion
Marked heterogeneity exists in how often surveillance imaging is obtained following treatment of HCC. Higher intensity imaging does not confer a survival benefit.
Keywords: Hepatocellular carcinoma, Imaging, Surveillance, Recurrence, Surgery
Introduction
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver in both men and women. It is one of the fastest growing causes of cancer-related death in the USA with many patients being over 65 years of age.1 The incidence of HCC in the USA has risen at a rate of 4.5 % per year in the last three decades and is currently 4.9 per 100,000 persons.2 Surgery is the mainstay of treatment for HCC and offers the best hope of long-term disease control. Other therapeutic options for patients include ablation and intra-arterial therapies (IAT) such as trans-arterial chemoembolization (TACE) and trans-arterial embolization (TAE). While short- and long-term post-treatment survival rates have significantly improved over the last two decades, up to 80 % of patients with HCC will recur within 5 years of treatment.2–4
Given the relatively high incidence of recurrence, surveillance with post-surgical cross-sectional imaging to detect asymptomatic recurrent disease is a widespread practice. There is no clear consensus on the intensity and usefulness of follow-up imaging. Recent guidelines from the National Comprehensive Cancer Network (NCCN) advocate imaging at 3–6-month intervals for the first 2 years and then every 6–12 months.5 There is a paucity of information on patterns of imaging surveillance among patients with HCC, and evidence on whether intensity of imaging correlates with outcomes is lacking. It is also not known if physician consultation patterns influence the likelihood and intensity of surveillance for HCC, as has been noted for other cancer sites.6,7 Most recurrences of HCC are intrahepatic, and therefore, early detection may facilitate secondary interventions.4 While intense, close surveillance with cross-sectional imaging may intuitively seem appealing, there has been concern about unnecessary exposure to radiation,8 as well as associated avoidable, high health care costs.9
In the present report, we sought to define the pattern of utilization, as well as intensity of post-operative abdominal imaging following HCC treatment using the population based Surveillance, Epidemiology and End Results (SEER)-linked Medicare database. In addition, we investigated whether the intensity/frequency of surveillance impacted long-term survival.
Methods
Source of Data
We performed a retrospective cohort study using the SEER-linked Medicare database. The details of this database have been noted elsewhere.10 Briefly, the SEER database is sourced from cancer registries from specific geographical areas that represent 28 % of the US population. For the present study, we considered patients age 66 years or older, with a pathologically confirmed diagnosis of HCC (International Classification of Diseases for Oncology, third edition,11 histology codes 8170 to 8175 for HCC and site code C22.0 for liver) between January 1, 1998 and December 31, 2007, who had continuous enrollment in Medicare part A and B for a minimum of 12 months preceding and 30 months following the diagnosis, and were not enrolled in an HMO plan at any point in this time period. We excluded patients with a diagnosis of another primary cancer within 5 years of the diagnosis of HCC; patients diagnosed on autopsy or by death certificate were also excluded. We restricted the cohort to patients with loco-regional disease as classified by the SEER historic staging criteria and excluded those who had distant disease. Patients with early-stage disease were defined by an established algorithm.12
Medicare data were used to ascertain type of treatment received and date of delivery. Data on liver resection (hemihepatectomy or lobectomy), ablation (radiofrequency, alcohol, cryoablation, or NOS), and TACE/TAE were abstracted from the Medicare inpatient, outpatient, and provider files; data on transplantation patients were not included. Repeat procedures were defined as either of these procedures types performed after 90 days of the index procedure. Demographic covariates such as age at diagnosis, gender, and race/ethnicity were extracted from the SEER database. Cancer-related covariates including: tumor stage, size, and focality were also determined using SEER data. Elixhauser's comorbidities were extracted as a measure of preoperative comorbidity burden.13
Surveillance Testing
The surveillance period extended from 90 days following surgery to 5 years or until the date of last follow-up available in the claims data. The 90-day lag was used to exclude patients undergoing imaging and physician consultations related to post-operative or post-procedural complications.7 For each monthly window of surveillance, we included only those patients who survived at least until the end of that month. We censored patients who were referred to, or were billed for, by hospice. For all analyses, we included a patient in a particular time period if he or she was alive and uncensored by the end of that period.
For the present study, we abstracted data on all CT (CPT code 74150, 74160, 74170; ICD-9 codes 88.01, 88.02) and MRI (CPT codes 74818, 74182, 74183; ICD-9 code 88.97) scans of the abdomen performed for any indication during each month of follow-up during surveillance.
Physician Consultation
We extracted all records for physician consultations around the date of initial diagnosis and HCC treatment for each patient. Physician specialty was determined by the Healthcare Financing Administration (HCFA) specialty codes. Bills from physicians who noted specialty designation as general practice, general surgery, family practice, gastroenterology, internal medicine, geriatric medicine, hematology, medical oncology, surgical oncology, radiation oncology, and interventional radiology (HCFA Specialty codes 01, 02, 08, 10, 11, 38, 83, 90, 91, 92, 94) were retained. One specialty code was allocated to each physician identification number. Physicians who billed both as a specialist and a generalist (i.e., gastroenterologist and internist) were given their specialist designation. Physicians were classified as a primary care practitioner (general practice, family practice, internal medicine, geriatric medicine), medical oncologist (hematology, medical oncology), surgeon (general surgery, surgical oncology), radiation oncologist, and interventional radiologist. We dichotomized specialty designation between primary care practitioner, and cancer specialist (surgeon, medical oncologist, radiation oncologist, and interventional radiologist).6,7
Statistical Analysis
Mean and median values were used to describe continuous data, with discrete variables displayed as totals and frequencies. Cells with <11 cases per variable cell were relabeled as “<11 (<%)” in compliance with the National Cancer Institute regulation for reporting of SEER–Medicare data. Univariate comparisons were assessed using the two-sample Student's t test for continuous variables and the Chi-square test for dichotomous and categorical variables. For purposes of analyses, the distribution of the total number of comorbid conditions per patient was divided into tertiles: 0, 1–2, or 3+ comorbidities. Cumulative event rates were calculated using the method of Kaplan and Meier, and survival curves were compared using the log-rank test. Overall survival time was calculated from the date of the first HCC directed treatment to the date of last follow-up. In order to assess the impact of imaging on long-term survival, we performed a more restricted analysis on patients who had had survived for at least 2 years following the HCC procedure. This mitigated against the effect of differential scanning frequency due to varying time of follow-up due to early death. In the case of differences in distribution of covariates among comparison groups, propensity score matching was used to create comparable cohorts. Univariate comparisons of survival were performed using the log-rank test. Multivariate modeling of survival was performed using Cox proportional hazards models. Covariates were included in the multivariate Cox model based on statistical significance in the univariate models (P≤.20). The overall fit of the multivariate models was assessed using the likelihood ratio test. Relative risks were expressed as hazard ratios (HR) with a 95 % confidence interval (CI). Adherence to the proportional hazards assumption was assessed using Schoenfeld residuals and log–log plots. All reported P values are two-tailed. All statistical analyses were performed using SAS 9.3 (SAS Corp., Cary, NC).
Results
Patient, Disease, and Treatment Characteristics
Utilizing the SEER database, 1,710 patients were identified who underwent liver resection, ablation, or IAT of HCC and met inclusion criteria; of these 1,467 patients survived for at least 90 days, defined as the start of surveillance period. Most patients were diagnosed between years 2003 and 2007 (n=912, 62.2 %), while the remaining cohort of 555 patients was diagnosed in 1998–2002. The demographic and clinical characteristics of the patients are outlined in Table 1. The median patient age was 74 years (IQR, 70–78). The majority of patients were white (n=985, 67.1 %) and male (n=965, 65.8 %). Most patients lived in an urban location (n=1,329, 90.6 %), were from the Western United States (n=809, 55.2 %), and belonged to the lower three quintiles of socioeconomic status (n=842, 57.0 %). At the time of the procedure, most patients had non-hepatitis-related liver cirrhosis (n=888, 60.5 %), while fewer had chronic HCV infection (n=524, 35.7 %) or HBV infection (n=206, 14.0 %). The majority of patients had non-liver-related comorbidities (n=791, 53.6 %); 485 (33.1 %) patients had one to two comorbidities, and 297 (20.3 %) had three or more comorbidities. The most commonly noted comorbidities were hypertension (n=476, 32.5 %), diabetes mellitus (n=337; 23.0 %), anemia (n=173, 21.1 %), and chronic pulmonary disease (n=144, 9.8 %).
Table 1.
Demographic and clinical characteristics of the cohort
| Demographic characteristics | Total (n=1,476) |
|---|---|
| Age, years | |
| 66–69 | 378 (25.8) |
| 70–74 | 480 (32.7) |
| 75–79 | 372 (25.4) |
| 80 + | 237 (16.2) |
| Male gender | 965 (65.8) |
| Race | |
| White | 985 (67.1) |
| Other | 482 (32.9) |
| Urban location | 1,329 (90.6) |
| Top two quintiles of SES | 634 (43.2) |
| US Geographic region | |
| Northeast | 338 (23.0) |
| West | 809 (55.2) |
| Midwest | 159 (10.8) |
| South | 161 (11.0) |
| Clinical characteristics | |
| Non-liver-related comorbidities | |
| 0 | 685 (46.7) |
| 1–2 | 485 (33.1) |
| 3+ | 297 (20.3) |
| Liver-related comorbidities | |
| HCV infection | 524 (35.7) |
| HBV infection | 206 (14.0) |
| Non-hepatitis-related cirrhosis | 888 (60.5) |
| Cancer characteristics | |
| Localized | 1,023 (69.7) |
| Regional | 295 (20.1) |
| No stage recorded | 149 (10.2) |
| Multiple tumor focia | 398 (31.8) |
| Bilobar diseaseb | 199 (17.9) |
| Size≥5 cmb | 600 (47.9) |
| Limited/potentially curable disease | 571 (38.9) |
Data were missing for 217 patients
Data were missing for 353 patients
Data were missing for 215 patients
The majority of patients had tumors that were staged as localized disease (n=1,023; 69.7 %), while fewer had regional disease (n=295; 20.1 %). Median tumor size was 4.6 cm (IQR, 3.0–7.0 cm), and multi-focal disease was present in 398 (31.8 %) patients; 199 (17.9 %) patients had disease involving both lobes of the liver. Only a subset of patients (n=571; 38.9 %) had early-stage disease. Around the time of HCC diagnosis, the majority of patients visited a primary care physician (n=1,332; 90.8 %). A smaller proportion saw a cancer specialist: surgeon (n=976; 66.5 %), medical oncologist (n=976; 66.5 %), or interventional radiologist (n=857; 58.4 %).
At the time of liver-directed therapy, most patients underwent ablation only (n=608, 41.5 %), while 434 (29.6 %) patients underwent liver resection only. Other therapies included IAT only (n=269; 18.3 %), concurrent hepatectomy and tumor ablation (n=20; 1.4 %), some combination of hepatectomy and IAT (n=37; 2.5 %) or ablation and IAT (n=91; 6.2 %). Surgical resection consisted of at least a hemi-hepatectomy in 250 (50.1 %) patients, while 249 (49.9 %) patients had a partial/wedge resection.
Repeat procedures were carried out in 285 (19.4 %) patients. Most often the second procedure involved ablation (n=170, 11.6 %), IAT (n=123, 8.4 %), or hepatectomy (n=31, 2.1 %). Median interval to any second procedure was 11.8 months. Median time interval to individual procedures was: ablation 12.9 months, IAT 8.8 month, and hepatectomy 15.7 months.
Intensity and Factors Associated with Follow-up Imaging
Among the 1,476 patients with HCC who underwent a liver-directed procedure, a total of 6,735 imaging procedures were performed from the time of treatment until being censored at 5 years following surgery or death. Overall yearly utilization of surveillance abdominal imaging following therapy for HCC increased over time (Fig. 1). Of note, a majority (n=1,327, 90.5 %) of patients had some type of abdominal imaging following treatment, a small subset of patients (n=140, 9.5 %) did not have any record of abdominal imaging after treatment. Among the 1,327 patients who had an imaging scan, most patients had a CT scan (n=1,230, 92.7 %), while fewer had an MRI (n=453, 34.1 %). The overall utilization of CT increased slightly over time, but the greatest increase in imaging was largely attributable to increased utilization of MRI scanning (Fig. 2).
Fig. 1.
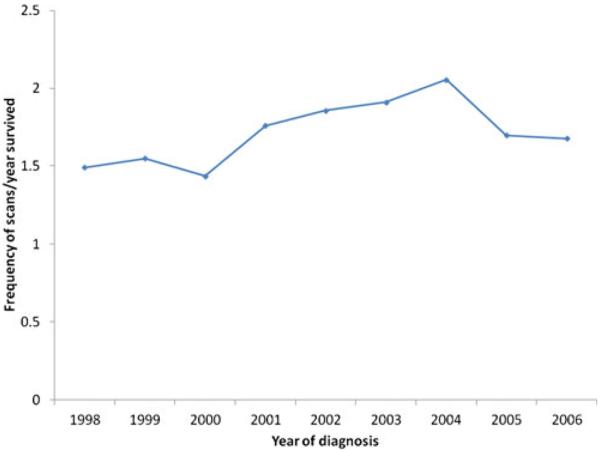
Mean number of imaging scans per patient within 5 years of surgery, ablation, or hepatic IAT for HCC
Fig. 2.
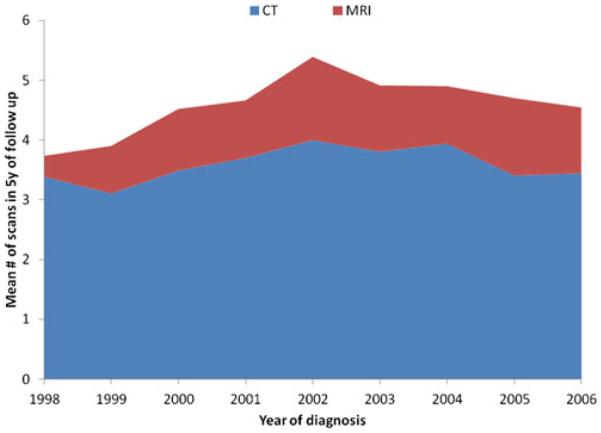
Annual rate of imaging utilization per patient, adjusted for postoperative or procedural survival
In the first year following treatment, 1,270 (86.6 %) patients had at least one abdominal scan; with each subsequent year of follow-up, the proportion of patients having at least one imaging scan decreased (year 2, n=745, 73.9 %; year 3, n=418, 58.5 %; year 4, n=262, 48.1 %; year 5, n=149, 46.4 %). After adjusting for post-procedure survival time, among the subset of patients who had at least one scan per year, the intensity of scanning similarly decreased (year 1, 2.5 scans/year vs. year 2, 1.8 scans/year vs. year 3, 1.3 scan/year vs. year 4, 1.0 scan/year vs. year 5, 0.9 scan/year; P value for trend 0.01). Overall, 34.5 % (n=246) of patients who were alive 2 years post-procedure did not receive any abdominal imaging scans during subsequent follow-up. A number of factors were associated with receipt of any post-procedure surveillance imaging (Table 2). Specifically, there were differences in both patient and non-patient factors between individuals who had at least one imaging procedure recorded and those who did not. Patients who had post-surgical surveillance imaging were more likely to have higher socioeconomic status (OR, 1.62; 95 % CI, 1.12–2.36), live in the Midwest or South region (Midwest/South vs. West, OR, 2.00; 95 % CI, 1.18–3.35), have one or more comorbidities at diagnosis (OR, 1.58; 95 % CI, 1.07–2.18), have tumors>5 cm (OR, 1.21; 95 % CI, 1.10–1.68), multiple tumors (OR, 1.15; 95 % CI, 1.01–1.34), and have undergone a hepatectomy or tumor ablation as the index procedure (hepatectomy vs. IAT, OR, 3.02; 95 % CI, 1.70–5.34; ablation vs. IAT, OR, 2.36; 95 % CI, 1.47–3.79; all P<0.05).
Table 2.
Characteristics of 828 patients by scanning intensity Characteristics of 828 patients by scanning intensity among patients who survived for more than 2 years and did not have a second procedure during these years
| Zero to one scan in 2 years (n=230) | Regular, periodic scanning over 2 years |
P value | ||
|---|---|---|---|---|
| One to two scans/year (n=455) | Three to four scans/year (n=143) | |||
| Age, n (%) | ||||
| 66–69 | 51 (22.2) | 135 (29.7) | 48 (33.6) | 0.10 |
| 70–74 | 76 (33.0) | 145 (31.9) | 47 (32.9) | |
| 75+ | 103 (44.8) | 175 (38.5) | 48 (33.6) | |
| Male gender; n (%) | 142 (61.7) | 298 (65.5) | 94 (65.7) | 0.59 |
| White race; n (%) | 152 (66.1) | 292 (64.2) | 85 (59.4) | 0.42 |
| Urban location | 202 (87.8) | 413 (90.8) | 132 (92.3) | 0.16 |
| Top two quintiles of SES | 79 (34.4) | 219 (48.1) | 67 (46.9) | <0.001 |
| Geographic location | ||||
| Northeast | 40 (17.4) | 98 (21.5) | 37 (25.9) | 0.18 |
| West | 140 (60.9) | 263 (57.8) | 86 (60.1) | |
| Midwest/South | 50 (21.7) | 94 (20.7) | 20 (14.0) | |
| SEER historic stage of HCC | ||||
| Localized/in situ | 180 (79.1) | 353 (77.6) | 98 (68.5) | 0.15 |
| Regional | 33 (14.4) | 69 (15.2) | 33 (23.1) | |
| No stage recorded | 15 (6.5) | 33 (7.3) | 12 (8.4) | |
| Any comorbidity | 133 (57.8) | 263 (57.8) | 83 (58.0) | 0.99 |
| First procedure following diagnosis | ||||
| Hepatectomy | 125 (54.4) | 198 (43.5) | 31 (21.7) | <0.001 |
| Ablation | 76 (33.0) | 210 (46.2) | 86 (60.1) | |
| IAT | 29 (12.6) | 47 (10.3) | 26 (18.2) | |
| Period of treatment | ||||
| 1998–2002 | 131 (57.0) | 292 (64.2) | 91 (63.6) | 0.17 |
| 2003–2007 | 99 (43.0) | 163 (35.8) | 52 (36.4) | |
| Physician consultation | ||||
| Surgeon | 156 (67.8) | 303 (66.6) | 92 (64.3) | 0.79 |
| Medical oncologist | 129 (56.1) | 274 (60.2) | 87 (60.8) | 0.53 |
| IR | 105 (45.7) | 236 (51.9) | 94 (65.7) | <0.001 |
Factors associated with intensity of surveillance imaging were examined (≤2 scans/year vs. ≥3 scans/year) among patients who had at least 2 years of follow-up and at least one imaging event after treatment for HCC (n=792; Table 3). On univariate analysis factors associated with higher intensity of scanning were: undergoing ablation or IAT (ablation vs. hepatectomy, OR, 3.16; 95 % CI, 2.03–4.90; IAT vs. hepatectomy, OR, 3.61; 95 % CI, 2.06–6.44), consultation with an interventional radiologist following diagnosis (OR, 1.95; 95 % CI, 1.34–2.85), and geographical region (Northeast vs. Midwest/South, OR, 1.93; 95 % CI, 1.07–3.50; all P<0.05; Table 3). On multivariate analysis, the only factor that remained associated with increased intensity of scanning was the index procedure type: undergoing ablation or IAT as initial treatment (ablation vs. hepatectomy, OR, 2.77; 95 % CI, 1.76–4.38; P=0.01; IAT vs. hepatectomy, OR, 2.66; 95 % CI, 1.40–5.06; P=0.04). The mean number of scans performed within 5 years of surgery was higher among patients undergoing ablation (4.9) compared with resection (4.7) or IAT (3.7; P<0.001).
Table 3.
Factors associated with higher intensity of imaging on univariate and multivariate logistic regression
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| OR (95 % CI) | P value | OR (95 % CI) | P value | |
| Age | ||||
| 66–69 | 1.51 (0.97–2.36) | 0.12 | 1.43 (0.91–2.25) | 0.22 |
| 70–74 | 1.24 (0.78–1.92) | 0.97 | 1.24 (0.79–1.95) | 0.86 |
| 75+ | Reference | |||
| Male gender | 1.01 (0.66–1.57) | 0.95 | – | |
| White race | 0.80 (0.55–1.15) | 0.22 | – | |
| Urban location | 1.70 (0.83–3.49) | 0.14 | 1.34 (0.63–2.84) | 0.44 |
| Top two quintiles of SES | 1.14 (0.79–1.64) | 0.47 | – | |
| Geographic location | ||||
| Northeast | 1.93 (1.07–3.50) | 0.04 | 1.53 (0.82–2.85) | 0.22 |
| West | 1.55 (0.92–2.61) | 0.57 | 1.30 (0.75–2.23) | 0.81 |
| Midwest/South | Reference | Reference | ||
| SEER historic stage of HCC primary | ||||
| Localized/in situ | Reference | Reference | ||
| Regional | 1.76 (1.12–2.75) | 0.12 | 1.55 (0.98–2.47) | 0.21 |
| No stage recorded | 1.76 (0.70–2.65) | 0.94 | 1.22 (0.61–2.44) | 0.95 |
| Any comorbidity | 1.00 (0.69–1.44) | 0.99 | – | |
| Index procedure | ||||
| Hepatectomy | Reference | Reference | ||
| Ablation | 3.16 (2.03–4.90) | <0.001 | 2.77 (1.76–4.38) | 0.01 |
| IAT | 3.61 (2.06–6.44) | <0.001 | 2.66 (1.40–5.06) | 0.04 |
| HCC diagnosis after 2002 | 1.09 (0.75–1.58) | 0.67 | – | |
| Specialist consultation | ||||
| Surgeon | 0.88 (0.60–1.28) | 0.49 | – | |
| Medical oncologist | 1.08 (0.75–1.57) | 0.67 | – | |
| Interventional radiologist | 1.95 (1.33–2.85) | <0.001 | 1.33 (0.87–2.04) | 0.19 |
The proportion of patients undergoing a secondary procedure for recurrence differed significantly by intensity of surveillance imaging (three to four scans/year, 36.2 % vs. two scans/year, 14.0 % vs. one scan/year, 15.2 %; P<0.001).
Impact of Imaging Surveillance on Survival
The median survival of patients who underwent surgical management of HCC was 29.0 months (95 % CI, 26.3–30.8 months) with 1-, 3-, and 5-year survival of 81.2, 42.0, and 26.6 %, respectively. A number of well-described factors were associated with worse survival such as older age (HR 1.35, 95 % CI 1.12–1.62), index treatment with ablation (HR 1.72, 95 % CI 1.44–2.06), or IAT (HR 1.98, 95 % CI 1.54–2.54), consultation with a medical oncologist (HR 1.31, 95 % CI 1.12–1.54), and multiple tumors at diagnosis (HR 1.57, 95 % CI 1.31–1.88); receipt of post-operative imaging was also associated with survival (HR 1.47, 05 % CI 1.02–2.13; all P<0.05).
To further evaluate the potential relationship between survival and imaging surveillance, and to avoid patients who had fewer scans due to early death, we compared survival among the 475 patients who had survived for at least 2 years following the index procedure and did not have a second procedure. Paradoxically, overall median survival was lowest for patients with the highest frequency of post-surgical imaging (three to four scans/year, 49.5 months vs. two scans/year, 71.7 months vs. one scan/year, 67.6 months; P=0.01; Fig. 3). The paradoxical effect of increased scanning frequency and worse survival was not as pronounced among patients undergoing ablation (0.60) or IAT (0.72). Of note, among the 260 patients who survived to 5 years, only 77 (29.6 %) had received imaging at intervals of 1 year or greater.
Fig. 3.
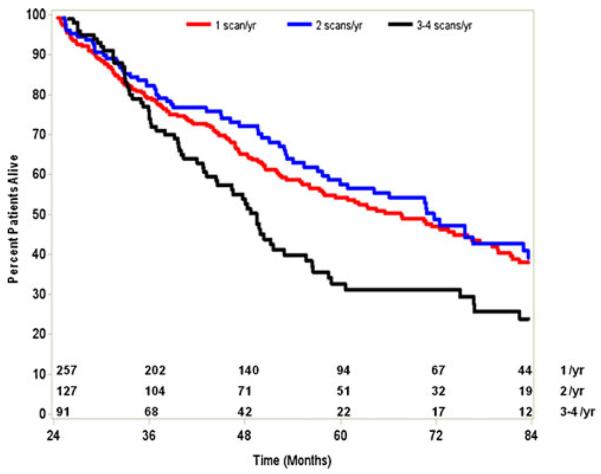
Overall survival stratified by intensity of post-procedural imaging during surveillance period (3 to 60 months) following surgery, ablation, or IAT for HCC
Discussion
Post-treatment surveillance is of growing importance as the number of cancer survivors is dramatically increasing due to improved early detection and more effective therapies.14 Although there are no uniform standards of coordinated care for survivors, surveillance is a long-standing element of oncologic practice. Current surveillance practices include interval clinic visits, serum tumor markers, and cross-sectional imaging. Routine post-operative surveillance imaging is employed in the majority of patients with gastrointestinal malignancy. Both providers and patients are concerned about detecting recurrences early, which may drive higher use of surveillance imaging.15,16 The current NCCN guidelines for surveillance among patients with HCC after resection or local regional therapy recommend routine AFP levels, if initially elevated, and imaging every 3 to 6 months for 2 years and then every 6 to 12 months.5 Although several studies have looked at the impact of surveillance on outcomes, as well as cost associated with imaging patients with cancers such as colorectal, breast, prostate, and lymphomas, there are limited data on outcomes associated with surveillance after HCC therapy.17–21 The current study is important because we used national, population-based data to evaluate the utilization of post-operative surveillance imaging among patients undergoing management of HCC. Specifically, we demonstrated a significant rise in the use of surveillance imaging over time following surgery, ablation, and IAT among patients with HCC. We also noted dramatic variations in the use of surveillance imaging that were associated with procedure type, type of specialist seen by the patient, and geographic location of the hospital.
The utilization of non-invasive diagnostic radiologic imaging has increased over time.22,23 Dinan et al. reported an increase in the use of radiological studies with CT accounting for the most pronounced increase compared with more modest increases in the use of PET and MRI.9 Bhargavan et al. similarly reported a roughly 10 % higher use of imaging modalities over the last decade.23 Similarly, our own group has described an increase in the overall utilization of cross-sectional surveillance imaging following curative-intent surgery among patients with colorectal liver metastasis.24 In the current study, we expand on our previous work and examine the use of surveillance imaging following treatment of HCC. We found that utilization of surveillance imaging for patients with HCC undergoing hepatic resection, ablation, or IAT increased over time. In fact, the median number of scans performed per year per patient following HCC treatment increased from two/year in 1998 compared with four/year in 2007. In looking at the increase in surveillance imaging for HCC, there was a relative increase in MRI over time, but the increased use of CT accounted for the greatest absolute increase in imaging. The dramatic overall increase in CT use in the USA has been questioned not only due to the associated increased radiation exposure but also due to the associated costs and questionable benefits.8,25 The benefit of high-frequency “aggressive” surveillance imaging following cancer surgery is controversial. Edelman et al. reported that the aggressive routine surveillance testing with radiologic and biochemical studies did not detect the vast majority of cancer relapses or prolonged survival among patients with breast cancer, prostate cancer, lung cancer, or lymphomas.18 In contrast, two more recent studies reported that frequent measurement of serum tumor markers and surveillance imaging led to earlier and increased detection of isolated recurrences, as well as an overall survival benefit among patients with primary colorectal cancer.26,27 With regard to HCC, strategies for surveillance have primarily focused on strategies to detect primary HCC in high-risk populations.28–31 In these studies, data have suggested that early detection of primary HCC may lead to a higher chance at curative intent therapy and better outcomes. Whether primary surveillance data can be extrapolated to inform secondary surveillance strategies to detect recurrent disease remains unclear. Only limited data are available for surveillance after transplantation for HCC. In one study, Roberts reported on the role of routine surveillance following transplantation of HCC.32 In this study, the author noted that recurrence ranged from 11 to 18 % in the post-transplant population and that screening all patients would not be cost-effective, while screening for high-risk patients may be warrented.32 In the current study, we specifically excluded patients undergoing transplantation. Instead, we focused on HCC patients treated with resection, ablation, and IAT because of the lack of data on the trends and patterns of post-treatment surveillance in this population.
Significant variation was noted in the intensity of surveillance imaging obtained after resection, ablation, and IAT for HCC. In fact, we found that a subset of patients—approximately 10 %—did not have any recorded instance of abdominal imaging after management of HCC. Interestingly, among patients who did undergo post-treatment surveillance both non-clinical and clinical factors were associated with the frequency of imaging. Non-clinical factors associated with imaging after therapy included higher socioeconomic status and certain geographic locations, such as living in the Midwest/South region. Clinical and tumor factors associated with frequency of post-treatment imaging included patient age, as well as tumor size and number. Previous studies had demonstrated variation based on socioeconomic status, geographic and age variation with regard to receipt of treatment for some cancers.33,34 The current work expands on these previous studies and demonstrates that non-tumor-related factors not only impact receipt of therapy but also how patients are followed in the post-treatment period. For example, as patient age increased, provider utilization of surveillance imaging declined, perhaps suggesting the belief that detection of early recurrence was less important among the very elderly. We also noted that provider and type of index procedure was associated with variation in surveillance imaging intensity. Specifically, patients who were treated by an interventional radiologist and who had ablation were more like to have a higher intensity of imaging. These data are consistent with the known increased utilization of cross-sectional imaging to assess tumor response following local regional therapies.35–37
In addition to defining overall trends in post-treatment surveillance imaging of HCC, we sought to examine the impact that imaging frequency had on long-term survival. We failed to detect a beneficial effect of increased surveillance imaging frequency on prognosis. Among long-term survivors (e.g., those patients who survived to 5 years), only a small subset (29.6 %) had received high-intensity surveillance imaging at intervals of 1 year or greater. In fact, overall median survival was lowest for patients with the highest frequency of post-surgical imaging. The paradoxical effect of increased scanning frequency and worse survival was not seen among patients undergoing ablation or IAT. While the reason for this is undoubtedly multi-factorial, it likely relates to patient selection, as well as possibly the indication for obtaining additional imaging in the different groups. In the ablation and IAT groups, higher frequency imaging may be used to assess treatment response. In contrast, in the surgical group, providers may be more likely to obtain imaging for patients with advanced disease, especially those who have a worsening clinical course. As such, this may account, in part, for why patients who had the highest frequency of scans appeared to have the worse prognosis on Kaplan–Meier analysis (Fig. 3). Notwithstanding this, the data do seem to suggest that patients do not necessarily derive a survival benefit from more intense surveillance strategies. For example, there was no difference in survival among patients who had one scan/year versus two scans/year surveillance strategies. These findings are consistent with data reported on surveillance strategies for other primary cancers such as pancreatic adenocarcinoma. Witkowski et al. noted that routine surveillance imaging following pancreatic cancer resection had no survival benefit.38 While patients with recurrent HCC may have more therapeutic options, it was interesting to note that in the current study only a very small subset of patients had any type of repeat liver-directed procedure.
The current study has several limitations. Due to the administrative nature of the SEER-Medicare database, we had to rely on billing codes to ascertain the utilization of CT and MRI. Medicare data have previously been shown, however, to be accurate in coding for relative high reimbursement procedures such as CT and MRI.39 While the number of scans performed was therefore most likely to have been captured accurately, there were no data available on the quality of the scans. Specifically, we do not know if the scans that were obtained were liver protocol, contrast enhanced, or non-contrast. However, any limitation in reporting of CT and MRI or differences in scan quality among patients would most likely be random in nature and be unlikely to influence the overall trends in procedure utilization herein reported. While we also did not have information on the indication for postoperative imaging, we specifically excluded any imaging within 90 days of the procedure to avoid including imaging that was not likely to be indicative of surveillance imaging. Finally, as with all data derived from SEER-linked Medicare data, the study cohort was limited to patients who were ≥65 years of age. As such, patterns and impact of imaging surveillance on younger patient populations may be different, and further study is warranted in this population.
In conclusion, utilization of surveillance imaging following surgery, ablation, and IAT for HCC has increased. The predominant imaging used for surveillance was CT scanning, although overall MRI utilization did increase over time. Despite the dramatic increase in surveillance imaging, approximately 10 % of Medicare beneficiaries had no imaging event recorded in the post-operative period following HCC therapy. For those patients who received post-procedural surveillance imaging, there was significant variation in the intensity of annual scans related to both patient and non-patient factors. Intensity of surveillance image was not associated with improved survival. These data may spur future investigation and discussion regarding post-therapeutic surveillance imaging guidelines for HCC patients.
Footnotes
Presented at the AHPBA Annual Meeting, February 20–24, 2013, Miami, Florida
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–76. doi: 10.1053/j.gastro.2007.04.061. Epub 2007/06/16. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2009;27(9):1485–91. doi: 10.1200/JCO.2008.20.7753. Epub 2009/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. Journal of Hepatology. 2003;38(2):200–7. doi: 10.1016/s0168-8278(02)00360-4. Epub 2003/01/28. [DOI] [PubMed] [Google Scholar]
- 4.Shindoh J, Hasegawa K, Inoue Y, Ishizawa T, Nagata R, Aoki T, et al. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2013;15(1):31–9. doi: 10.1111/j.1477-2574.2012.00552.x. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson AB, 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. Journal of the National Comprehensive Cancer Network: JNCCN. 2009;7(4):350–91. doi: 10.6004/jnccn.2009.0027. Epub 2009/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Surveillance testing among survivors of early-stage breast cancer. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2007;25(9):1074–81. doi: 10.1200/JCO.2006.08.6876. Epub 2007/03/21. [DOI] [PubMed] [Google Scholar]
- 7.Sheffield KM, Crowell KT, Lin YL, Djukom C, Goodwin JS, Riall TS. Surveillance of pancreatic cancer patients after surgical resection. Annals of Surgical Oncology. 2012;19(5):1670–7. doi: 10.1245/s10434-011-2152-y. Epub 2011/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. The New England journal of medicine. 2007;357(22):2277–84. doi: 10.1056/NEJMra072149. Epub 2007/11/30. [DOI] [PubMed] [Google Scholar]
- 9.Dinan MA, Curtis LH, Hammill BG, Patz EF, Jr., Abernethy AP, Shea AM, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006. JAMA: the journal of the American Medical Association. 2010;303(16):1625–31. doi: 10.1001/jama.2010.460. Epub 2010/04/29. [DOI] [PubMed] [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical care. 2002;40(8 Suppl) doi: 10.1097/01.MLR.0000020942.47004.03. IV-3-18. Epub 2002/08/21. [DOI] [PubMed] [Google Scholar]
- 11.Fritz JM, George S. The use of a classification approach to identify subgroups of patients with acute low back pain. Interrater reliability and short-term treatment outcomes. Spine. 2000;25(1):106–14. doi: 10.1097/00007632-200001010-00018. Epub 2000/01/27. [DOI] [PubMed] [Google Scholar]
- 12.Nathan H, Hyder O, Mayo SC, Hirose K, Wolfgang CL, Choti MA, et al. Surgical Therapy for Early Hepatocellular Carcinoma in the Modern Era: A 10-Year SEER-Medicare Analysis. Annals of Surgery. 2013 doi: 10.1097/SLA.0b013e31827da749. Epub 2013/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. Epub 1998/02/07. [DOI] [PubMed] [Google Scholar]
- 14.McCabe MS, Bhatia S, Oeffinger KC, Reaman GH, Tyne C, Wollins DS, et al. American Society of Clinical Oncology Statement: Achieving High-Quality Cancer Survivorship Care. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2013 doi: 10.1200/JCO.2012.46.6854. Epub 2013/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kievit J. Colorectal cancer follow-up: a reassessment of empirical evidence on effectiveness. European Journal of Surgical Oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2000;26(4):322–8. doi: 10.1053/ejso.1999.0893. Epub 2000/06/30. [DOI] [PubMed] [Google Scholar]
- 16.Audisio RA, Robertson C. Colorectal cancer follow-up: perspectives for future studies. European journal of Surgical Oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2000;26(4):329–37. doi: 10.1053/ejso.1999.0894. Epub 2000/06/30. [DOI] [PubMed] [Google Scholar]
- 17.Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow-up after curative surgery for colorectal carcinoma. Randomized comparison with no follow-up. Diseases of the Colon and Rectum. 1995;38(6):619–26. doi: 10.1007/BF02054122. Epub 1995/06/01. [DOI] [PubMed] [Google Scholar]
- 18.Edelman MJ, Meyers FJ, Siegel D. The utility of follow-up testing after curative cancer therapy. A critical review and economic analysis. Journal of General Internal Medicine. 1997;12(5):318–31. doi: 10.1046/j.1525-1497.1997.012005318.x. Epub 1997/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjeldsen BJ, Kronborg O, Fenger C, Jorgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. The British Journal of Surgery. 1997;84(5):666–9. Epub 1997/05/01. [PubMed] [Google Scholar]
- 20.Mortazavi A, Shaukat A, Othman E, Kepner JL, Fakih MG, Kuvshinoff BW, et al. Postoperative computed tomography scan surveillance for patients with stage II and III colorectal cancer: worthy of further study? American Journal of Clinical Oncology. 2005;28(1):30–5. doi: 10.1097/01.coc.0000139188.46296.d0. Epub 2005/02/03. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Moranta F, Salo J, Arcusa A, Boadas J, Pinol V, Bessa X, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2006;24(3):386–93. doi: 10.1200/JCO.2005.02.0826. Epub 2005/12/21. [DOI] [PubMed] [Google Scholar]
- 22.Maitino AJ, Levin DC, Parker L, Rao VM, Sunshine JH. Nationwide trends in rates of utilization of noninvasive diagnostic imaging among the Medicare population between 1993 and 1999. Radiology. 2003;227(1):113–7. doi: 10.1148/radiol.2272020617. Epub 2003/04/02. [DOI] [PubMed] [Google Scholar]
- 23.Bhargavan M, Sunshine JH. Utilization of radiology services in the United States: levels and trends in modalities, regions, and populations. Radiology. 2005;234(3):824–32. doi: 10.1148/radiol.2343031536. Epub 2005/02/01. [DOI] [PubMed] [Google Scholar]
- 24.Hyder O, Dodson RM, Mayo SC, Schneider E, Weiss M, Herman J, et al. Post-Treatment Surveillance of Patients with Colorectal Liver Metastases: Does Intensity of Follow-up Imaging Impact Outcomes manusript submitted for publication to HPB. 2013. publication pending. [Google Scholar]
- 25.Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA: the journal of the American Medical Association. 2012;307(22):2400–9. doi: 10.1001/jama.2012.5960. Epub 2012/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324(7341):813. doi: 10.1136/bmj.324.7341.813. Epub 2002/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007;(1):CD002200. doi: 10.1002/14651858.CD002200.pub2. Epub 2007/01/27. [DOI] [PubMed] [Google Scholar]
- 28.Peterson MS, Baron RL. Radiologic diagnosis of hepatocellular carcinoma. Clinics in Liver Disease. 2001;5(1):123–44. doi: 10.1016/s1089-3261(05)70157-4. Epub 2001/02/24. [DOI] [PubMed] [Google Scholar]
- 29.Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. Journal of Medical Screening. 2003;10(4):204–9. doi: 10.1258/096914103771773320. Epub 2004/01/24. [DOI] [PubMed] [Google Scholar]
- 30.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology. 2004;130(7):417–22. doi: 10.1007/s00432-004-0552-0. Epub 2004/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36. doi: 10.1002/hep.20933. Epub 2005/10/27. [DOI] [PubMed] [Google Scholar]
- 32.Roberts JP. Tumor surveillance-what can and should be done? Screening for recurrence of hepatocellular carcinoma after liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;(11 Suppl 2):S45–6. doi: 10.1002/lt.20605. Epub 2005/10/21. [DOI] [PubMed] [Google Scholar]
- 33.Krupski TL, Kwan L, Afifi AA, Litwin MS. Geographic and socioeconomic variation in the treatment of prostate cancer. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2005;23(31):7881–8. doi: 10.1200/JCO.2005.08.755. Epub 2005/10/06. [DOI] [PubMed] [Google Scholar]
- 34.Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 1995;13(1):93–100. doi: 10.1200/JCO.1995.13.1.93. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 35.Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115(3):616–23. doi: 10.1002/cncr.24050. Epub 2009/01/01. [DOI] [PubMed] [Google Scholar]
- 36.Halappa VG, Bonekamp S, Corona-Villalobos CP, Li Z, Mensa M, Reyes D, et al. Intrahepatic cholangiocarcinoma treated with local-regional therapy: quantitative volumetric apparent diffusion coefficient maps for assessment of tumor response. Radiology. 2012;264(1):285–94. doi: 10.1148/radiol.12112142. Epub 2012/05/26. [DOI] [PubMed] [Google Scholar]
- 37.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2011;29(30):3960–7. doi: 10.1200/JCO.2011.37.1021. Epub 2011/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witkowski ER, Smith JK, Ragulin-Coyne E, Ng SC, Shah SA, Tseng JF. Is it worth looking? Abdominal imaging after pancreatic cancer resection: a national study. Journal of Gastrointestinal Surgery: official journal of the Society for Surgery of the Alimentary Tract. 2012;16(1):121–8. doi: 10.1007/s11605-011-1699-z. Epub 2011/10/06. [DOI] [PubMed] [Google Scholar]
- 39.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Annals of Surgical Oncology. 2008;15(2):415–23. doi: 10.1245/s10434-007-9658-3. Epub 2007/11/08. [DOI] [PubMed] [Google Scholar]


