Abstract
BACKGROUND
Hospitals are increasingly adopting 24-hour intensivist physician staffing as a strategy to improve intensive care unit (ICU) outcomes. However, the degree to which nighttime intensivists are associated with improvements in the quality of ICU care is unknown.
METHODS
We conducted a retrospective cohort study involving ICUs that participated in the Acute Physiology and Chronic Health Evaluation (APACHE) clinical information system from 2009 through 2010, linking a survey of ICU staffing practices with patient-level outcomes data from adult ICU admissions. Multivariate models were used to assess the relationship between nighttime intensivist staffing and in-hospital mortality among ICU patients, with adjustment for daytime intensivist staffing, severity of illness, and case mix. We conducted a confirmatory analysis in a second, population-based cohort of hospitals in Pennsylvania from which less detailed data were available.
RESULTS
The analysis with the use of the APACHE database included 65,752 patients admitted to 49 ICUs in 25 hospitals. In ICUs with low-intensity daytime staffing, nighttime intensivist staffing was associated with a reduction in risk-adjusted in-hospital mortality (adjusted odds ratio for death, 0.62; P = 0.04). Among ICUs with high-intensity daytime staffing, nighttime intensivist staffing conferred no benefit with respect to risk-adjusted in-hospital mortality (odds ratio, 1.08; P = 0.78). In the verification cohort, there was a similar relationship among daytime staffing, nighttime staffing, and in-hospital mortality. The interaction between nighttime staffing and daytime staffing was not significant (P = 0.18), yet the direction of the findings were similar to those in the APACHE cohort.
CONCLUSIONS
The addition of nighttime intensivist staffing to a low-intensity daytime staffing model was associated with reduced mortality. However, a reduction in mortality was not seen in ICUs with high-intensity daytime staffing. (Funded by the National Heart, Lung, and Blood Institute.)
Daytime intensivist physician staffing has been consistently associated with improved outcomes among patients admitted to an intensive care unit (ICU).1 This observation has led to policy initiatives calling for expansion of the intensivist staffing model to encompass all critically ill patients.1,2 More recently, some experts have proposed further extending the intensivist staffing model to provide care around the clock, with intensivist physicians remaining in the ICU overnight.3-6
Proponents of 24-hour intensivist staffing suggest that nighttime intensivist staffing may result in earlier establishment of treatment plans, more timely resuscitation of patients in unstable condition, uninterrupted provision of complex care, and more consistent bedside medical decision making at all hours of the day. However, others have questioned the potential benefit of nighttime coverage, citing the cost of this investment and the untested assumption that nighttime intensivist staffing improves outcomes.7,8 To date, data are lacking from multicenter studies, and single-center studies have had mixed results.9,10
In this article, we retrospectively examine the relationship between nighttime intensivist physician staffing and mortality among ICU patients. The data were obtained from the Acute Physiology and Chronic Health Evaluation (APACHE) clinical outcomes database, and replication was attempted in a second database from acute care hospitals in Pennsylvania.
METHODS
STUDY DESIGN
We performed a retrospective study involving patients admitted to ICUs in 34 hospitals that used the APACHE clinical information system (Cerner, Kansas City, MO) from 2009 through 2010. APACHE collects detailed clinical, physiological, and outcome data on adult ICU patients at participating hospitals for benchmarking and quality improvement. APACHE data are collected at each site by trained local coordinators. Specific data elements include the primary diagnosis at admission, the patient’s age, location of the patient before admission to the ICU, length of hospital stay before admission, and clinical and physiological variables during the first 24 hours after admission to the ICU. The APACHE database has been used for numerous observational studies involving critically ill patients.11,12
We linked patient-level outcome data from APACHE to data from a 2010 organizational survey about ICU-level structures and care processes conducted in APACHE ICUs13 (see the Supplementary Appendix, available with the full text of this article at NEJM.org, for the complete survey). We developed this survey on the basis of a literature review and qualitative content analysis of 64 interviews with multidisciplinary staff members at seven nonstudy hospitals, and we pretested the survey with 12 ICU nurse managers at three nonstudy hospitals (one academic hospital and two community hospitals). We invited APACHE clinical coordinators from APACHE hospitals to complete the online survey in exchange for a $50 gift card, with up to four e-mail messages and telephone calls to coordinators who did not respond.
Apart from contributing data, Cerner made no financial or material contribution to this study. All aspects of the study were reviewed and approved by the institutional review board of the University of Pittsburgh. Because this research was deemed to pose minimal risk to participants, the need for individual informed consent was waived.
PATIENT SELECTION
Patients who were older than 17 years of age and who were admitted to an ICU with completed survey data were eligible for inclusion in this analysis. For patients with multiple ICU admissions, subsequent admissions were included in the analysis.
VARIABLES
The primary outcome variable was in-hospital mortality. Patients who were discharged to hospice care were classified as dead at discharge. The primary exposure variable was nighttime intensivist staffing, defined as an intensivist attending physician who was physically present in the ICU or elsewhere in the hospital and immediately available to manage ICU emergencies during nighttime hours.
Covariates included variables specified a priori as potential confounders between nighttime staffing and mortality on the basis of previous studies.11,12 Patient-level covariates included age, race or ethnic group, sex, acute physiology score (a measure of the severity of illness ranging from 0 to 252, with higher scores indicating more severe illness and a higher risk of death), the presence or absence of selected coexisting conditions (the acquired immunodeficiency syndrome, lymphoma, myeloma, cirrhosis, liver failure, immunosuppression, and metastatic cancer), the location of the patient before admission to the ICU (emergency department, operating room, hospital floor, other hospital, or other location), the length of the hospital stay before ICU admission, the annualized ICU volume of admissions, the admission diagnosis,14 and the patient’s status with respect to the need for invasive mechanical ventilation at the time of admission. We also included the teaching status of the hospital, based on the ratio of residents to beds (with a ratio of 0 indicating a non-teaching hospital, 0 to <0.25 a minor teaching hospital, and ≥0.25 a major teaching hospital); the daytime intensivist staffing model, based on the role of the daytime intensivist in the ICU (with optional consultation with the intensivist categorized as low intensity and mandatory consultation with the intensivist or primary transfer of care to the intensivist categorized as high intensity)1; geographic region; and type of ICU.15
SENSITIVITY ANALYSIS
We assessed the sensitivity of our findings by repeating the primary analysis with a less restrictive definition of nighttime intensivist staffing that included nighttime ICU resident coverage. We also assessed the association between nighttime staffing and in-hospital mortality for five prespecified patient subpopulations: patients who received active treatment on ICU admission rather than being admitted simply for observation,14 patients who received mechanical ventilation on ICU admission, patients admitted to the ICU at night (between 7 p.m. and 7 a.m.), patients with the highest acute physiology scores (highest third), and patients with an admission diagnosis of sepsis. We hypothesized a priori that these populations would be most likely to benefit from the presence of an in-house intensivist at night.
VERIFICATION ANALYSIS
To verify the results of our initial analysis, we conducted a second retrospective cohort study using discharge data from the Pennsylvania Health Care Cost Containment Council (PHC4). This independent state agency collects detailed clinical and administrative data on all patients admitted to Pennsylvania hospitals, for the purposes of benchmarking and research. Like the APACHE database, PHC4 data have been used for numerous observational studies involving critically ill patients.16-18 Data on adult medical discharges between July 1, 2004, and June 30, 2006, were used in this analysis. We linked patient-level outcome data from PHC4 to a 2005 survey of Pennsylvania acute care hospital practices, which included questions on daytime and nighttime staffing in ICUs (see the Supplementary Appendix for the complete survey).18-20 We used logistic regression to evaluate the relationship between nighttime intensivist staffing and in-hospital mortality, controlling for patient age, predicted probability of death, location of patient before ICU admission, race or ethnic group, type of ICU, teaching status of the hospital, annualized volume of discharges, and selected coexisting conditions. This analysis was stratified according to daytime staffing intensity, as defined in our primary analysis.
STATISTICAL ANALYSIS
We performed bivariate analyses comparing the characteristics of hospitals between clinical coordinators who responded to the survey and those who did not respond and comparing the demographic and clinical characteristics of patients between ICUs with nighttime intensivist staffing and those without such staffing, using the Mann–Whitney test for continuous variables and chi-square tests or Fisher’s exact test for categorical variables. Multivariable modeling of the association between the nighttime staffing model and in-hospital mortality was performed with the use of logistic regression, with adjustment for covariates specified as potential confounders of the relationship between ICUs with nighttime intensivist staffing and outcome as described above. The patients’ age and acute physiology score were modeled with restricted linear splines.21 Categorical variables were modeled with the use of indicator covariates. Generalized estimating equations with robust variance estimators were used to account for ICU-level patient clustering.22 Given that prior studies1 have shown an association between daytime intensivist staffing and improved outcomes, we tested for an interaction between daytime intensivist staffing and nighttime intensivist staffing. All analyses were performed with Stata software, version 11.0.
RESULTS
CHARACTERISTICS OF HOSPITALS AND PATIENTS
We received a completed survey from APACHE coordinators at 25 of 34 hospitals (74%). Collectively, these hospitals included 49 ICUs, and we analyzed data from 65,752 of 99,727 ICU admissions (66%) (Fig. 1). Twelve ICUs with nighttime intensivist staffing contributed data on 14,424 admissions (22%), and 37 ICUs without nighttime intensivist staffing contributed data on 51,328 admissions (78%).
Figure 1.
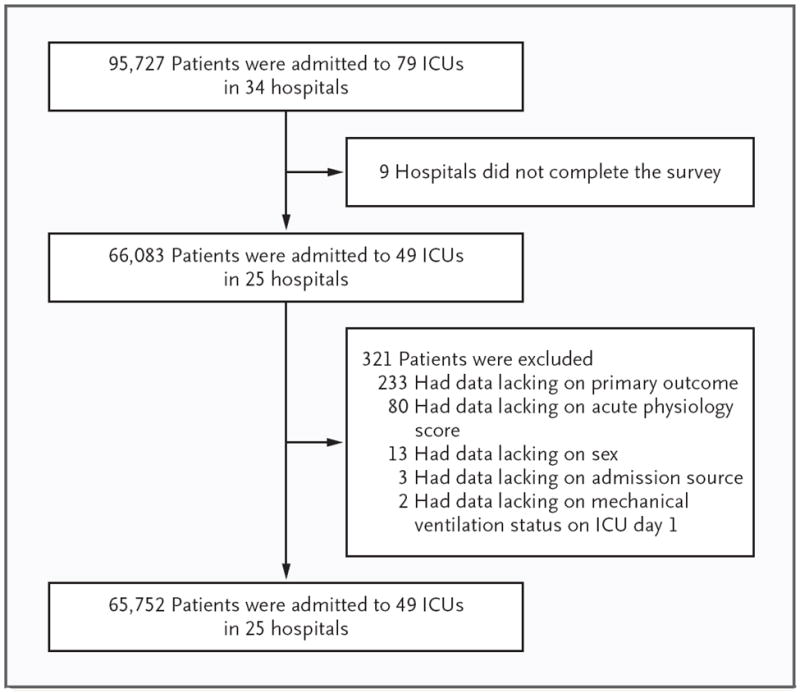
Numbers of Patients, Intensive Care Units (ICUs), and Hospitals in the Study.
The hospitals were diverse with respect to the number of beds, number of ICUs, academic status, and geographic region. There were no significant differences between hospitals in which the APACHE coordinator responded to the survey and hospitals in which the APACHE coordinator did not respond (Table 1). Characteristics of the 49 ICUs in the study are shown in Table 2. Among ICUs without nighttime intensivist staffing, the most common nighttime staffing model was resident coverage (in 25 ICUs [51% of the total]), followed by no in-house coverage (in 6 ICUs [12%]), coverage by physicians who were not intensivists (in 5 ICUs [10%]), and coverage by a nurse practitioner or physician assistant (in 1 ICU [2%]).
Table 1.
Characteristics of the Hospitals According to Survey Response Status.*
| Characteristic | Respondents (N = 25) | Nonrespondents (N = 9) | P Value |
|---|---|---|---|
| Annualized ICU admissions — no. | 0.19 | ||
| Median | 1694 | 1200 | |
| Interquartile range | 820–2172 | 686–1396 | |
| Hospital beds — no. (%) | 0.58 | ||
| <250 | 4 (16) | 1 (11) | |
| 250–500 | 8 (32) | 5 (56) | |
| >500 | 13 (52) | 3 (33) | |
| ICUs — no. (%) | 0.95 | ||
| 1 | 11 (44) | 3 (33) | |
| 2 | 5 (20) | 2 (22) | |
| 3 | 5 (20) | 2 (22) | |
| ≥4 | 4 (16) | 2 (22) | |
| Academic status — no. (%) | 0.89 | ||
| Large teaching hospital | 11 (44) | 4 (44) | |
| Small teaching hospital | 6 (24) | 3 (33) | |
| Nonteaching hospital | 8 (32) | 2 (22) | |
| Region — no. (%) | 0.78 | ||
| Midwest | 11 (44) | 6 (67) | |
| Northeast | 3 (12) | 0 | |
| Southeast | 6 (24) | 1 (11) | |
| West | 5 (20) | 2 (22) |
ICU denotes intensive care unit.
Table 2.
Characteristics of the ICUs According to the Presence or Absence of Nighttime Intensivists.
| Characteristic | Nighttime Intensivists | No Nighttime Intensivists | P Value |
|---|---|---|---|
| High-intensity daytime staffing | |||
| Annualized ICU admissions — no. | 0.24 | ||
| Median | 517 | 770 | |
| Interquartile range | 418–603 | 514–979 | |
| ICU type— no./total no. (%) | >0.99 | ||
| Mixed | 3/6 (50) | 11/21 (52) | |
| Specialty | 3/6 (50) | 10/21 (48) | |
| Full-time physician ICU director — no./total no. (%) | 2/6 (33) | 20/21 (95) | <0.01 |
| Routine participation of medical students, residents, or other physician trainees — no./total no. (%) | 5/6 (83) | 21/21 (100) | 0.22 |
| Daily multidisciplinary rounds — no./total no. (%) | 6/6 (100) | 17/21 (81) | 0.55 |
| Low-intensity daytime staffing | |||
| Annualized ICU admissions — no. | 0.10 | ||
| Median | 1377 | 815 | |
| Interquartile range | 741–1735 | 368–1198 | |
| ICU type — no./total no. (%) | >0.99 | ||
| Mixed | 4/6 (67) | 9/16 (56) | |
| Specialty | 2/6 (33) | 7/16 (44) | |
| Full-time physician ICU director — no./total no. (%) | 6/6 (100) | 15/16 (94) | >0.99 |
| Routine participation of medical students, residents, or other physician trainees — no./total no. (%) | 3/6 (50) | 10/16 (62) | 0.66 |
| Daily multidisciplinary rounds — no./total no. (%) | 6/6 (100) | 15/16 (94) | >0.99 |
There were no significant differences between patients admitted to ICUs with nighttime intensivist coverage and those treated in ICUs without such coverage with respect to demographic characteristics, admitting diagnosis, severity of illness according to the acute physiology score, active treatment on admission, use or nonuse of mechanical ventilation on admission, and length of hospital stay before admission to the ICU (Table 3). Unadjusted in-hospital mortality was similar for ICUs with and those without nighttime intensivist staffing (12.8% and 13.4%, respectively; P = 0.053).
Table 3.
Characteristics of the Patients.
| Characteristic | ICUs with Nighttime Intensivists (N = 14,424) | ICUs without Nighttime Intensivists (N = 51,328) | P Value |
|---|---|---|---|
| Age — yr | <0.001 | ||
| Median | 66 | 62 | |
| Interquartile range | 54–76 | 51–74 | |
| Female sex — % | 42.4 | 44.6 | <0.001 |
| Race — no. (%)* | <0.001 | ||
| White | 11,888 (82.4) | 40,481 (78.9) | |
| Black | 538 (3.7) | 5,376 (10.5) | |
| Other | 1,265 (8.8) | 1,750 (3.4) | |
| Data missing | 733 (5.1) | 3,721 (7.2) | |
| Admission source — no. (%) | <0.001 | ||
| Emergency department | 4,625 (32.1) | 22,495 (43.8) | |
| Operating room | 4,760 (33.0) | 13,371 (26.1) | |
| Medical or surgical ward | 2,807 (19.5) | 10,725 (20.9) | |
| Transfer | 1,263 (8.8) | 3,832 (7.5) | |
| Other | 969 (6.7) | 905 (1.8) | |
| Reason for ICU admission — no. (%) | <0.001 | ||
| Surgery | 3,878 (26.9) | 10,290 (20.0) | |
| Cardiac disorder | 2,253 (15.6) | 7,865 (15.3) | |
| Respiratory disorder | 1,867 (12.9) | 7,074 (13.8) | |
| General medical disorder | 1,233 (8.5) | 6,152 (12.0) | |
| Sepsis | 1,047 (7.3) | 4,769 (9.3) | |
| Trauma | 718 (5.0) | 3,862 (7.5) | |
| Neurosurgery | 615 (4.3) | 1,681 (3.3) | |
| Cardiac arrest | 496 (3.4) | 1,534 (3.0) | |
| Other | 2,317 (16.1) | 8,101 (15.8) | |
| Acute physiology score† | <0.001 | ||
| Median | 36 | 38 | |
| Interquartile range | 24–53 | 25–56 | |
| Active treatment on day of ICU admission — no. (%)‡ | 9,509 (65.9) | 34,911 (68.0) | <0.001 |
| Mechanical ventilation on day of ICU admission — no. (%) | 6,687 (46.4) | 22,811 (44.4) | <0.001 |
| Emergency surgery — no. (%) | 534 (3.7) | 2,630 (5.1) | <0.001 |
| Length of hospital stay before ICU admission — days | <0.001 | ||
| Median | 0.50 | 0.46 | |
| Interquartile range | 0.20–1.10 | 0.12–1.06 | |
| In-hospital death — no. (%) | 1,842 (12.8) | 6,872 (13.4) | 0.053 |
Race was determined on admission by the admitting clinical team.
The acute physiology score is a measure of severity of illness ranging from 0 to 252, with higher scores indicating more severe illness and a higher risk of death.
Active treatment was defined as any of 33 active life-supporting intensive care treatments.14
NIGHTTIME INTENSIVIST STAFFING AND MORTALITY
In a multivariable model that did not include our prespecified interaction term, nighttime intensivist staffing was not associated with reduced mortality (odds ratio for death, 1.02; 95% confidence interval [CI], 0.73 to 1.41; P = 0.92), nor was high-intensity daytime staffing associated with reduced mortality (odds ratio, 0.78; 95% CI, 0.57 to 1.07; P = 0.13). In our final model, the term for interaction between daytime staffing intensity and nighttime intensivist staffing was significant (P = 0.02); consequently, we report the effect of nighttime staffing separately for ICUs on the basis of daytime staffing intensity (Table 4). In the 22 ICUs with low-intensity daytime staffing (45% of the total), nighttime intensivist staffing was associated with a reduction in risk-adjusted in-hospital mortality (odds ratio, 0.62; P = 0.04). However, in ICUs with high-intensity daytime staffing, nighttime intensivist staffing conferred no benefit with respect to risk-adjusted in-hospital mortality (odds ratio, 1.08; P = 0.78). In all prespecified subpopulations, the association between nighttime staffing and in-hospital mortality mirrored the findings with the base model (Table 4).
Table 4.
Odds Ratio for Death in ICUs with Nighttime Intensivist Staffing in the APACHE and PHC4 Cohorts.*
| Cohort | No. of Patients | Low-Intensity Daytime Staffing | High-Intensity Daytime Staffing | Interaction Term | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | P Value | ||
| APACHE† | 65,752 | 0.62 (0.39–0.97) | 0.04 | 1.08 (0.63–1.84) | 0.78 | 0.02 |
|
| ||||||
| Patients with active treatment on admission | 44,420 | 0.59 (0.36–0.97) | 0.04 | 1.15 (0.71–1.88) | 0.57 | <0.01 |
|
| ||||||
| Patients who underwent mechanical ventilation‡ | 29,498 | 0.60 (0.37–0.96) | 0.03 | 1.36 (0.86–2.15) | 0.19 | <0.01 |
|
| ||||||
| Patients admitted at night§ | 29,088 | 0.51 (0.32–0.82) | 0.01 | 1.01 (0.71–1.44) | 0.95 | <0.01 |
|
| ||||||
| Patients in highest third of acute physiology scores | 21,532 | 0.56 (0.38–0.82) | <0.01 | 1.16 (0.75–1.79) | 0.51 | <0.01 |
|
| ||||||
| Patients with sepsis¶ | 5,816 | 0.46 (0.29–0.74) | <0.01 | 0.88 (0.58–1.33) | 0.54 | <0.01 |
|
| ||||||
| Alternative definition of nighttime staffing: nighttime intensivist or resident physician | 65,752 | 0.42 (0.29–0.59) | <0.01 | 0.47 (0.34–0.65) | <0.01 | <0.01 |
|
| ||||||
| PHC4 cohort∥ | 107,319 | 0.83 (0.69–0.99) | 0.049 | 0.97 (0.67–1.39) | 0.86 | 0.18 |
APACHE denotes Acute Physiology and Chronic Evaluation, and PHC4 Pennsylvania Health Care Cost Containment Council.
Odds ratios and 95% confidence intervals compare nighttime intensivist staffing in the ICU with any other nighttime staffing. Models were adjusted for age, sex, race or ethnic group, acute physiology score, presence or absence of chronic coexisting conditions, preadmission location of the patient, diagnosis, type of ICU, daytime intensivist staffing model, annualized ICU volume of admissions, and use or nonuse of mechanical ventilation on the day of admission. Confidence intervals take into account clustering at the ICU level.
Active treatment was defined as any of 33 active life-supporting intensive care treatments.14
Nighttime admission was defined as admission between 7 p.m. and 7 a.m.
The primary admission diagnosis of sepsis was determined by the admitting clinicians.
Odds ratios and 95% confidence intervals are for nighttime intensivist staffing in the ICU with any other nighttime staffing. Models were adjusted for age, sex, race, predicted probability of death, presence or absence of chronic coexisting conditions, teaching status of the hospital, type of ICU, daytime intensivist staffing model, and annualized ICU volume of admissions. Confidence intervals take into account clustering at the ICU level.
A sensitivity analysis that included coverage by residents in the definition of nighttime intensivist staffing also showed a significant interaction between daytime and nighttime intensivist staffing. With the use of this definition, nighttime staffing was associated with reduced in-hospital mortality in both low-intensity ICUs (odds ratio, 0.42; 95% CI, 0.29 to 0.59; P<0.01) and high-intensity ICUs (odds ratio, 0.47; 95% CI, 0.34 to 0.65; P<0.01).
Our verification analysis of PHC4 data showed a similar relationship among daytime staffing, nighttime staffing, and in-hospital mortality. The interaction between nighttime staffing and daytime staffing was not significant (P = 0.18), yet the direction of the findings was similar to that in the APACHE cohort. In ICUs with high-intensity daytime staffing, nighttime staffing conferred no additional benefit with respect to mortality reduction (odds ratio, 0.97; 95% CI, 0.67 to 1.39; P = 0.86). In ICUs that had low-intensity daytime staffing, in-house nighttime intensivists were associated with reduced in-hospital mortality (odds ratio, 0.83; 95% CI, 0.69 to 0.99; P = 0.05).
Unadjusted analyses in both cohorts showed no association between nighttime staffing and inhospital mortality. (See Tables 1, 2 and 3 in the Supplementary Appendix for a more detailed description of the PCH4 cohort, the unadjusted results, and a comparison of the interaction terms in the APACHE and PHC4 cohorts.)
DISCUSSION
These data show an association between nighttime intensivist staffing and reduced in-hospital mortality among ICU patients, which is contingent on the daytime intensivist role in the ICU. Nighttime intensivist staffing was associated with decreased risk-adjusted in-hospital mortality in ICUs that used a low-intensity physician staffing model (i.e., optional consultation with the intensivist). However, no additional mortality reduction was observed when nighttime intensivist staffing was present in ICUs that used a high-intensity staffing model (i.e., those in which consultation with an intensivist was mandatory for all admissions or those in which the intensivist had primary responsibility for patient care). This association was not observed in the unadjusted analysis, indicating that the finding was due to differences in risk-adjusted mortality rather than differences in crude mortality.
Our primary findings were largely replicated in a secondary population-based cohort, with an attenuated effect of nighttime staffing in high-intensity ICUs in this cohort. We speculate that the different effect sizes are due to different risk-adjustment strategies between the two cohorts; risk adjustment in the PHC4 cohort incompletely captured the increased severity of illness in ICUs with nighttime staffing. Although the interaction term between nighttime staffing and daytime staffing was not significant, the point estimate was contained in the confidence interval for the interaction term in the APACHE cohort (Table 4, and see the Supplementary Appendix).
There are several possible explanations for the relationship between nighttime staffing and outcome in low-intensity ICUs. As compared with providers who are not intensivists, nighttime intensivists may direct more timely resuscitation of patients in unstable condition, initiate appropriate medical therapies sooner, and adjust complex therapies more efficiently. Nighttime intensivists are more accessible to nursing staff and other providers for clarification on the plan of care; this could reduce medical errors. Indeed, the benefit of nighttime intensivist staffing appeared to be greatest for the subgroup of patients admitted with sepsis, a condition for which early administration of antibiotics and rapid resuscitation are known to improve outcomes.23-25 These factors may be most likely to affect outcomes in ICUs with less intensive daytime staffing.
Although we found no mortality reduction in high-intensity ICUs, nighttime staffing may have other benefits. A recent single-center study showed that a model in which intensivists worked in around-the-clock shifts was associated with less evidence of intensivist burnout.26 At the same time, however, this model was associated with greater nursing conflict (i.e., more situations in which daytime and nighttime physicians provided conflicting plans or incompatible instructions for patient care) and less autonomy of house staff. Data are lacking to more clearly determine the effect of various staffing models on clinician satisfaction and trainee education.
Our study has several limitations. The hospitals participating in this study were not a random sample from the United States; rather, they were a self-selected group of hospitals that were highly motivated to improve the quality of ICU care. APACHE hospitals are generally larger and more likely to have an academic affiliation than the average U.S. hospital. However, the hospitals in our study were still diverse with respect to size, region, and academic status; this bolsters the generalizability of our findings. Furthermore, our more generalizable validation cohort replicated the findings of the primary analysis.
In addition, our definition of nighttime staffing was based on the title assigned to nighttime providers; we did not explicitly measure their clinical behavior. The definition thereby identifies a structural dimension of intensivists rather than process elements directly associated with improved care. Nonetheless, the link between structure and outcome by itself can be an important component of quality improvement in acute care.1,11,27,28
When residents were included in the definition of nighttime intensivist, we observed lower overall mortality in all ICUs with nighttime staffing. This result indicates that the presence of any physician in the ICU at night, whether a physician in training or a trained intensivist, was associated with improved outcomes. However, adding a nighttime intensivist to an ICU already staffed with physicians in training at night appeared to offer no marginal improvements in outcomes. When taken together with the results of the primary analysis, this finding suggests that the presence of nighttime attending intensivists may provide little incremental benefit with respect to mortality reduction in ICUs with in-house physician trainees operating under their supervision. We did not collect data on the level of experience of trainees at each institution, and it is possible that ICUs with less experienced residents would benefit from the presence of a nighttime intensivist. There may be other benefits, such as a reduced ICU stay and fewer procedural complications, but our analysis did not take these factors into consideration. We were also unable to examine the role of ICU tele-medicine, another method of improving access to intensivist expertise at night.29 Only three ICUs in our cohort used telemedicine; this small number precluded a detailed analysis.
Our results reconcile the findings of two previous studies of nighttime intensivist staffing. The first investigation, which showed a benefit of nighttime staffing, involved an ICU with low-intensity daytime staffing.9 Conversely, the second investigation, performed in a large academic center, involved an ICU with high-intensity daytime staffing in which intensivists were added to baseline nighttime resident staffing.10 In that study, there was no reduction in in-hospital mortality after the addition of nighttime intensivists. In the context of their daytime ICU staffing models, the results of the two studies are consistent with our findings.
Following two single-center investigations that had seemingly conflicting results, our multicenter evaluation showed that nighttime intensivist staffing was associated with reduced in-hospital mortality among patients admitted to ICUs with low-intensity daytime staffing, but with no incremental benefit among patients admitted to ICUs with high-intensity daytime staffing. These findings suggest that blanket endorsement of 24-hour intensivist coverage is premature, although such coverage appears to be useful in some clinical settings. In any case, intensivists are a scarce resource, and the feasibility of broad-based expansion of ICU staffing is questionable. Individual hospitals and ICUs will need to weigh the anticipated benefits of expanding intensivist nighttime coverage against those of other quality-improvement efforts in order to best serve their patients, staff, and community.
Supplementary Material
Acknowledgments
Supported by grants (K23-HL096651 and T32-HL07820) from the National Heart, Lung, and Blood Institute.
We thank Nichole Benson for her valuable assistance in conducting the survey of APACHE ICUs.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–62. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 2.Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization of three levels of care. Crit Care Med. 2003;31:2677–83. doi: 10.1097/01.CCM.0000094227.89800.93. [DOI] [PubMed] [Google Scholar]
- 3.Burnham EL, Moss M, Geraci MW. The case for 24/7 in-house intensivist coverage. Am J Respir Crit Care Med. 2010;181:1159–60. doi: 10.1164/rccm.201004-0651ED. [DOI] [PubMed] [Google Scholar]
- 4.Cartin-Ceba R, Bajwa EK. 24-Hour on-site intensivist in the intensive care unit: yes. Am J Respir Crit Care Med. 2010;181:1279–80. doi: 10.1164/rccm.201004-0676ED. [DOI] [PubMed] [Google Scholar]
- 5.Jones SF, Gaggar A. Is there a doctor in the house? The downside of 24/7 attending coverage in academic intensive care units. Am J Respir Crit Care Med. 2010;181:1280–1. doi: 10.1164/rccm.201005-0681ED. [DOI] [PubMed] [Google Scholar]
- 6.Lindell KO, Chlan LL, Hoffman LA. Nursing perspectives on 24/7 intensivist coverage. Am J Respir Crit Care Med. 2010;182:1338–40. doi: 10.1164/rccm.201007-1129ED. [DOI] [PubMed] [Google Scholar]
- 7.Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016–24. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 8.Kahn JM, Hall JB. More doctors to the rescue in the intensive care unit: a cautionary note. Am J Respir Crit Care Med. 2010;181:1160–1. doi: 10.1164/rccm.201004-0557ED. [DOI] [PubMed] [Google Scholar]
- 9.Blunt MC, Burchett KR. Out-of-hours consultant cover and case-mix-adjusted mortality in intensive care. Lancet. 2000;356:735–6. doi: 10.1016/S0140-6736(00)02634-9. [DOI] [PubMed] [Google Scholar]
- 10.Gajic O, Afessa B, Hanson AC, et al. Effect of 24-hour mandatory versus ondemand critical care specialist presence on quality of care and family and provider satisfaction in the intensive care unit of a teaching hospital. Crit Care Med. 2008;36:36–44. doi: 10.1097/01.CCM.0000297887.84347.85. [DOI] [PubMed] [Google Scholar]
- 11.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 13.Wallace DJ, Barnato AE, Kramer AA, Angus D, Kahn JM. Structures and processes of care in intensivist-staffed critical care units. Am J Respir Crit Care Med. 2011;183 A102abstract. [Google Scholar]
- 14.Zimmerman JE, Kramer AA. A model for identifying patients who may not need intensive care unit admission. J Crit Care. 2010;25:205–13. doi: 10.1016/j.jcrc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Lott JP, Iwashyna TJ, Christie JD, Asch DA, Kramer AA, Kahn JM. Critical illness outcomes in specialty versus general intensive care units. Am J Respir Crit Care Med. 2009;179:676–83. doi: 10.1164/rccm.200808-1281OC. [DOI] [PubMed] [Google Scholar]
- 16.Boucher KM, Slattery ML, Berry TD, Quesenberry C, Anderson K. Statistical methods in epidemiology: a comparison of statistical methods to analyze doseresponse and trend analysis in epidemiologic studies. J Clin Epidemiol. 1998;51:1223–33. doi: 10.1016/s0895-4356(98)00129-2. [DOI] [PubMed] [Google Scholar]
- 17.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 18.Barnato AE, Chang C-CH, Farrell MH, Lave JR, Roberts MS, Angus DC. Is survival better at hospitals with higher “end-of-life” treatment intensity? Med Care. 2010;48:125–32. doi: 10.1097/MLR.0b013e3181c161e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn JM, Ten Have TR, Iwashyna TJ. The relationship between hospital volume and mortality in mechanical ventilation: an instrumental variable analysis. Health Serv Res. 2009;44:862–79. doi: 10.1111/j.1475-6773.2009.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MM, Barnato AE, Angus DC, Fleisher LA, Kahn JM. The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170:369–76. doi: 10.1001/archinternmed.2009.521. Erratum, Arch Intern Med 2010; 170:867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CY, Arnold RM, Lave JR, Angus DC, Barnato AE. Acute care practices relevant to quality end-of-life care: a survey of Pennsylvania hospitals. Qual Saf Health Care. 2010;19(6):e12. doi: 10.1136/qshc.2008.030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CY, Farrell MH, Lave JR, Angus DC, Barnato AE. Organizational determinants of hospital end-of-life treatment intensity. Med Care. 2009;47:524–30. doi: 10.1097/MLR.0b013e31819261bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellanos-Ortega A, Suberviola B, García-Astudillo LA, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010;38:1036–43. doi: 10.1097/CCM.0b013e3181d455b6. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 25.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 26.Garland A, Roberts D, Graff L. Twenty-four-hour intensivist presence: a pilot study of effects on ICU patients, families, doctors and nurses. Am J Respir Crit Care Med. 2012;185:738–43. doi: 10.1164/rccm.201109-1734OC. [DOI] [PubMed] [Google Scholar]
- 27.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 28.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305:2175–83. doi: 10.1001/jama.2011.697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


