Abstract
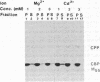
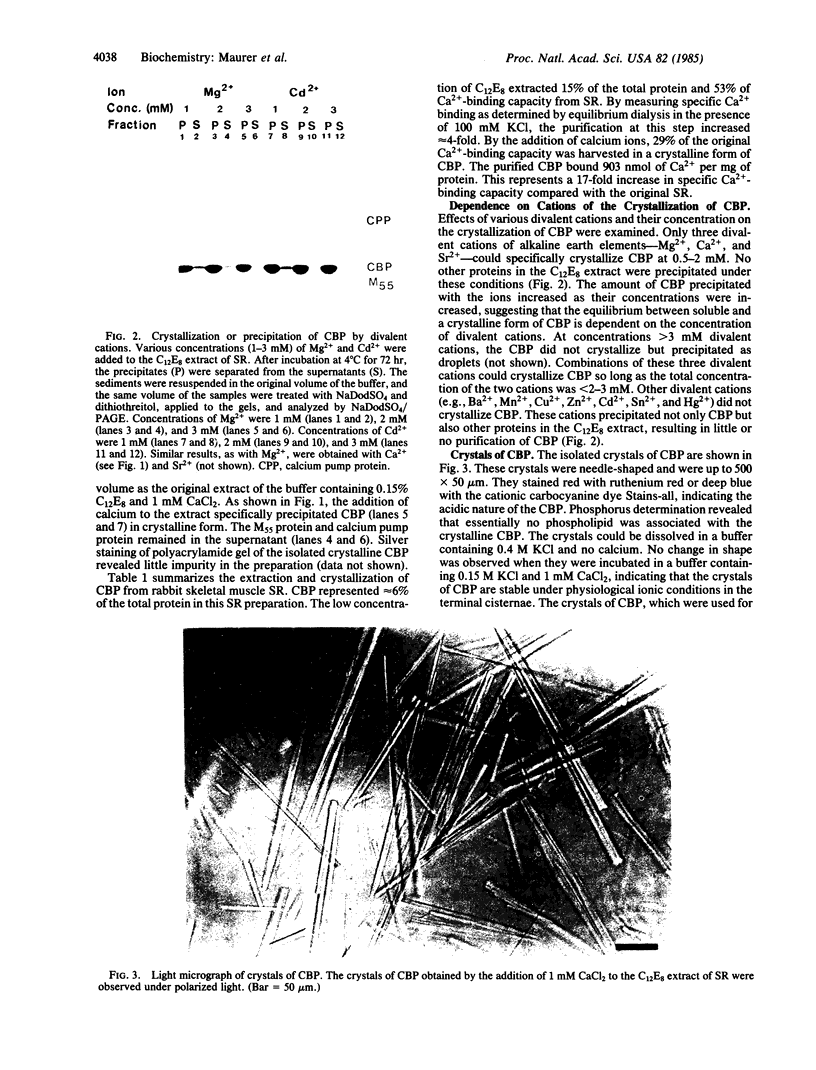
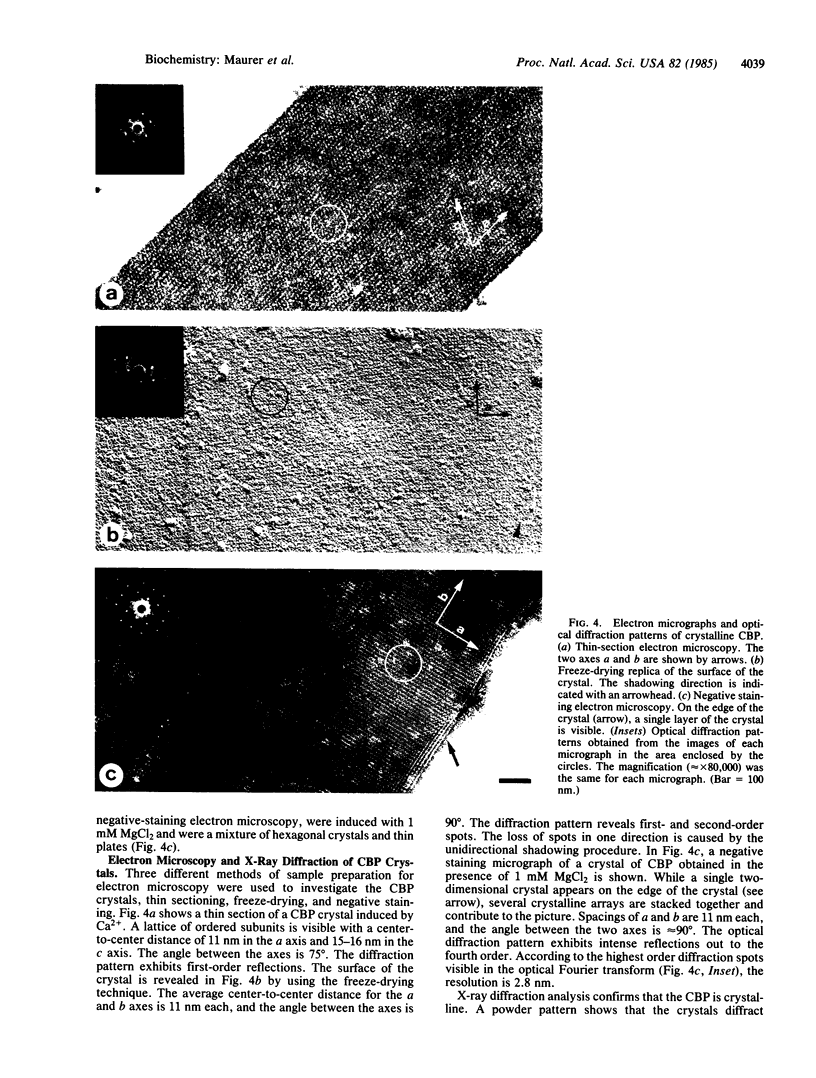
The calcium binding protein of skeletal muscle sarcoplasmic reticulum (also referred to as calsequestrin) was purified by release from the compartment of the vesicles with the detergent octaethyleneglycol mono-n-dodecyl ether (C12E8) and by subsequent precipitation of the calcium binding protein with specific divalent cations. The isolated protein exhibited a single band on NaDodSO4/PAGE and bound 903 nmol of Ca2+ per mg of protein. The calcium binding protein could be crystallized in the presence of Ca2+, Mg2+, Sr2+, or combinations of these three cations used in a narrow concentration range. Needle-shaped crystals of up to 500 X 50 micron were obtained. The removal of the divalent cations resulted in solubilization of the crystals. The spacings and angles of the crystals were obtained by electron microscopy using three different methods of sample preparation. By freeze-drying and negative staining electron microscopy, the spacings along axes a and b were determined to be 10-11 nm each, and the angle between the two axes was 90 degrees. By thin section electron microscopy, the spacing along axis a was 11 nm, along axis c was 15-16 nm, and the angle between the two axes was 75 degrees. This study reports (i) a simple and rapid method for purification of the calcium binding protein; (ii) conditions to crystallize the protein using Ca2+, Mg2+, Sr2+, or combinations of the three; and (iii) some preliminary characteristics of the crystals. The crystalline nature was characterized by electron microscopy and x-ray diffraction. The larger crystals diffracted beyond 3-A Bragg spacing.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cala S. E., Jones L. R. Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J Biol Chem. 1983 Oct 10;258(19):11932–11936. [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H., Jorgensen A. O. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye "Stains-all". J Biol Chem. 1983 Sep 25;258(18):11267–11273. [PubMed] [Google Scholar]
- Cozens B., Reithmeier R. A. Size and shape of rabbit skeletal muscle calsequestrin. J Biol Chem. 1984 May 25;259(10):6248–6252. [PubMed] [Google Scholar]
- Green D. E. Membrane proteins: a perspective. Ann N Y Acad Sci. 1972 Jun 20;195:150–172. [PubMed] [Google Scholar]
- Hymel L., Maurer A., Berenski C., Jung C. Y., Fleischer S. Target size of calcium pump protein from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1984 Apr 25;259(8):4890–4895. [PubMed] [Google Scholar]
- Ikemoto N., Bhatnagar G. M., Nagy B., Gergely J. Interaction of divalent cations with the 55,000-dalton protein component of the sarcoplasmic reticulum. Studies of fluorescence and circular dichroism. J Biol Chem. 1972 Dec 10;247(23):7835–7837. [PubMed] [Google Scholar]
- Ikemoto N., Bhatnager G. M., Gergely J. Fractionation of solubilized sarcoplasmic reticulum. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1510–1517. doi: 10.1016/s0006-291x(71)80257-7. [DOI] [PubMed] [Google Scholar]
- Ikemoto N., Nagy B., Bhatnagar G. M., Gergely J. Studies on a metal-binding protein of the sarcoplasmic reticulum. J Biol Chem. 1974 Apr 25;249(8):2357–2365. [PubMed] [Google Scholar]
- KLUG A., BERGER J. E. AN OPTICAL METHOD FOR THE ANALYSIS OF PERIODICITIES IN ELECTRON MICROGRAPHS, AND SOME OBSERVATIONS ON THE MECHANISM OF NEGATIVE STAINING. J Mol Biol. 1964 Dec;10:565–569. doi: 10.1016/s0022-2836(64)80081-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Isolation of a second form of calsequestrin. J Biol Chem. 1974 Feb 10;249(3):980–984. [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- MacLennan D. H., Wong P. T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer A., Mühlethaler K. Isolation and characterization of paracrystalline arrays of the plasma membrane of baker's yeast Saccharomyces cerevisiae. Eur J Cell Biol. 1981 Jun;24(2):216–225. [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Ostwald T. J., MacLennan D. H., Dorrington K. J. Effects of cation binding on the conformation of calsequestrin and the high affinity calcium-binding protein of sarcoplasmic reticulum. J Biol Chem. 1974 Sep 25;249(18):5867–5871. [PubMed] [Google Scholar]
- Ozawa T., Suzuki H., Tanaka M. Crystallization of part of the mitochondrial electron transfer chain: cytochrome c oxidase--cytochrome c complex. Proc Natl Acad Sci U S A. 1980 Feb;77(2):928–930. doi: 10.1073/pnas.77.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Wang C. T., Fleischer S. Membrane asymmetry and enhanced ultrastructural detail of sarcoplasmic reticulum revealed with use of tannic acid. J Cell Biol. 1978 Dec;79(3):601–616. doi: 10.1083/jcb.79.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]