Abstract
Mutations in genes encoding ribosomal proteins cause the Minute phenotype in Drosophila and mice, and Diamond-Blackfan syndrome in humans. Here we report two mouse dark skin (Dsk) loci caused by mutations in Rps19 (ribosomal protein S19) and Rps20 (ribosomal protein S20). We identify a common pathophysiologic program in which p53 stabilization stimulates Kit ligand expression, and, consequently, epidermal melanocytosis via a paracrine mechanism. Accumulation of p53 also causes reduced body size and erythrocyte count. These results provide a mechanistic explanation for the diverse collection of phenotypes that accompany reduced dosage of genes encoding ribosomal proteins, and have implications for understanding normal human variation and human disease.
Mutants with the dominantly inherited Minute phenotype, first described in flies more than 85 years ago, share common features, including delayed larval development, abnormal bristles and recessive lethality1. Because of these phenotypic similarities, it is not surprising that Minute loci encode genes in a common pathway: thus far, all known mutations affect ribosomal proteins, in both the 40S (encoded by Rps genes) and 60S (encoded by the Rpl genes) subunits2. Rps and Rpl mutations have also been described in other species, including mice and humans3,4. RPS19 and, more recently, RPS17 and RPS24 sequence alterations have been identified in individuals with Diamond-Blackfan syndrome, in which congenital red blood cell aplasia is associated with growth retardation and limb and/or craniofacial malformations4–7. In most affected individuals, RPS mutations are heterozygous and sporadic; inherited forms of familial Diamond-Blackfan syndrome also exist in which RPS mutations segregate in an autosomal dominant manner7–9. Akin to Minute flies, the genotype–phenotype association between RPS mutations and the human disorder is strong, yet the molecular mechanisms underlying the specific phenotypes observed in humans remain enigmatic.
Studies in unicellular organisms and cultured mammalian cells have shown that Rps and Rpl mutations compromise ribosome biogenesis and protein synthesis3,10–12. In addition, mutant cells show decreased rates of proliferation and a survival disadvantage in mosaic and chimeric animals3,11,13. Although some phenotypes may be the direct result of abnormal cell cycle kinetics, it is not known how mutations that impair protein synthesis and cell cycle dynamics yield tissue-specific phenotypes such as anemia.
During the course of a forward genetic screen in mice for pigmentary abnormalities, we identified missense alterations of Rps19 and Rps20 in two mutants with dominantly inherited dark skin, Dark skin 3 (Dsk3) and Dark skin 4 (Dsk4), respectively. Using a combination of cell biological and genetic approaches, we show that abnormalities caused by mutations in genes encoding ribosomal proteins are mediated by a common pathophysiologic mechanism that involves activation and/or stabilization of p53. Often referred to as the guardian of the genome because its activation in response to genomic stress helps to prevent cancer, p53 also contributes to the pathogenesis of several human diseases when activated14. Here we show that the ‘dark side’ of p53 is pleiotropic, with distinct phenotypic outcomes that depend on the underlying cell type in which activation occurs, providing new insight into pigmentary biology, erythrocyte development and human disease.
RESULTS
Epidermal melanocytosis in Dsk mutants
Dsk3 and Dsk4 were identified in a large-scale chemical mutagenesis screen for dominant traits affecting morphology, blood chemistry or behavior15. From approximately 32,000 animals, 15 Dsk mutants were recovered that represent three phenotypic categories: adult-onset dark skin associated with epidermal thickening, accumulation of dermal melanocytes during development and accumulation of epidermal melanocytes during development16. We have previously described molecular defects responsible for the first two categories16,17; Dsk3 and Dsk4 represent the third category.
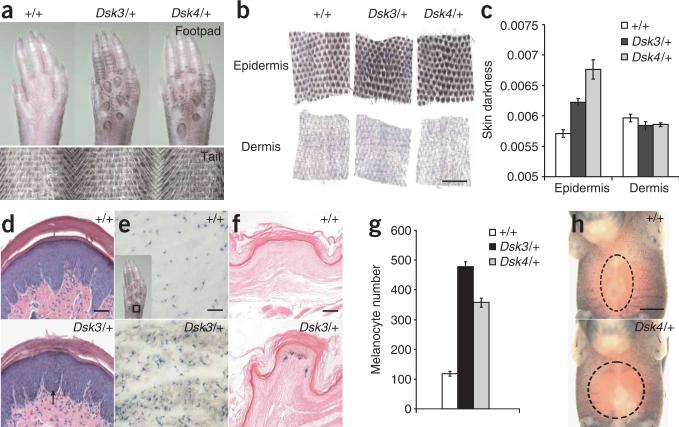
Increased pigmentation in Dsk3 and Dsk4 becomes apparent by 3 weeks of age in the footpad, tail and ears of mutant animals and persists throughout adult life (Fig. 1a and data not shown). Using a quantitative assay for the degree of pigmentation in separate layers of tail skin, we found that pigment accumulates in the mutant epidermis compared to the nonmutant epidermis, whereas the dermis is unaffected (Fig. 1b,c). This conclusion was confirmed by histological analysis showing excess pigment in the epidermis of Dsk3 and Dsk4 footpads, tail and ear (Fig. 1d and data not shown).
Figure 1.
Dsk3 and Dsk4 pigmentary phenotype. (a,b) Whole footpads and tails (a) or separated tail epidermis and dermis (b) from adult animals. (c) Skin darkness (mean ± s.e.m.) of epidermis and dermis for each genotype. (d) Histological sections of footpad epidermis show pigment accumulation in the epidermis (arrow). (e–h) Xgal-stained adult footpad epidermis (e, inset), whole footpad (f) and E15.5 embryos (h) from +/+ and Dsk4/+ animals that also carry the Dct-lacZ transgene. A dashed circle denotes the area without Xgal-positive cells. (g) Number of Xgal-positive cells (mean ± s.e.m.) from the location shown in e (inset). For c and g, P values for mutant versus control (based on a two-tail t-test) are 1.53 × 10–5 and 0.0036 (c), and 0.0017 and 0.0036 (g), for Dsk3/+ and Dsk4/+, respectively. Scale bars: b, 500 μm; d,f, 50 μm; e,h, 300 μm.
We used a histochemical marker for pigment cells, a lacZ transgene driven by regulatory elements from the Dct (dopachrome tautomerase) gene18, to determine whether excess epidermal pigmentation is caused by an increased number of melanocytes or by increased melanin production. We found that Dsk3 and Dsk4 footpad epidermis is characterized by an increased number of Xgal-positive cells located in the basal layer (Fig. 1e–g). Excess cells are first apparent in the mutant footpad at postnatal day 3 (P3), and persist in the adult footpad (Supplementary Fig. 1a online).
We studied the ontogeny of this process by examining midgestation mutant and nonmutant embryos. At embryonic day 10 (E10), the Dct-lacZ transgene marks melanoblasts as they leave the neural crest; by E15, pigment cell precursors have moved to the developing skin in a dorsal to ventral expansion (Fig. 1h)18–20. Of note, we found that Dsk3 and Dsk4 mutant embryos have fewer Xgal-positive cells than nonmutant animals at all embryonic time points examined (Fig. 1h, Supplementary Fig. 1b and data not shown). In fact, a white belly spot, a cutaneous location in which melanocytes fail to populate during development21,22, was observed in approximately 10% of Dsk3 adults, and was enhanced when placed on a genetic background sensitized by the presence of the KitWv mutation (Supplementary Fig. 1c). Thus, epidermal melanocytosis in Dsk3 and Dsk4 occurs postnatally. Furthermore, these mutations have opposite effects at different times: during embryogenesis, the Dsk3 and Dsk4 mutations impair pigment cell development, but after birth, they cause pigment cells to accumulate progressively in the adult epidermis.
Positional cloning of Dsk3 and Dsk4
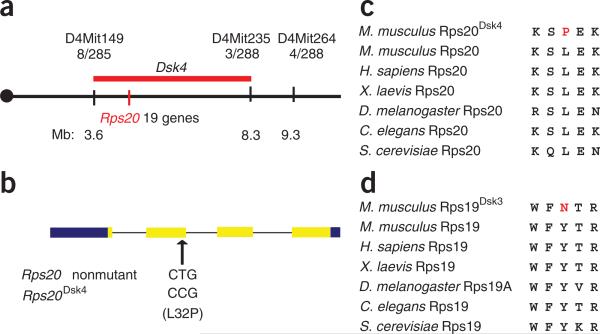
Dsk3 and Dsk4 were initially localized to 4.49- and 3.85-cM intervals on chromosomes 7 and 4, respectively16. By generating and evaluating ~1,500 additional backcross progeny, we narrowed these intervals to 0.6 and 4.7 Mb, respectively (Fig. 2a and Supplementary Fig. 2a online), within which no known pigmentation genes were apparent. Despite its smaller size, the Dsk3 critical interval is very gene-dense compared to that of Dsk4; therefore, we focused initially on the latter. We sequenced the exons and proximal 5′ flanking regions of all 19 candidate genes in the critical interval (chr4: 3.6–9.3 Mb, genome assembly mm8) and identified two nucleotide substitutions in Dsk4-mutant mice compared to the strain of origin: a point mutation (T2201A) in the 3′ UTR of Impad1 (inositol monophosphatase domain containing 1; NM_177730), and a point mutation (T209C) that predicts a L32P amino acid substitution in Rps20 (ribosomal protein S20; NM_026147) (Fig. 2b).
Figure 2.
Positional cloning of Dsk mutations. (a) Genetic and physical maps of the Dsk4 critical interval on mouse chromosome 4. Recombination frequencies (stated as the number of recombinant chromosomes between the marker and Dsk3, over the number of informative chromosomes evaluated) are given immediately below each marker. Approximate physical coordinates in megabases (Mb) are given below. (b) The position and sequence of the Dsk4 point mutation is shown relative to the exon–intron structure of Rps20 where untranslated and protein-coding regions are represented by blue and yellow, respectively. (c,d) Predicted protein sequences for Rps20Dsk4 (c) and Rps19Dsk3 (d), aligned with homologous sequence in other species.
We then realized that the Dsk3 critical interval contained a related gene, Rps19, which, like Rps20, encodes a protein component of the 40S small ribosomal subunit23. Within the protein-coding region of Rps19, we identified a point mutation (T316A) that predicts a Y54N amino acid substitution (Supplementary Fig. 2b).
The aforementioned three mutations were the only sequence alterations identified in a total of approximately 125 kb of sequence, and the predicted amino acid substitutions in Rps19 and Rps20 occur in residues that are evolutionarily conserved among their respective orthologs in unicellular and multicellular organisms (Fig. 2c,d). Besides the pigmentary phenotype observed in heterozygous animals, homozygosity for Dsk3 or Dsk4 causes early (before E7.5) embryonic lethality (data not shown), a phenotype shared by other ribosomal protein mutants2,3. Taken together with the data described below for a third ribosomal protein gene, we conclude that Dsk3 and Dsk4 are caused by loss-of-function alterations in Rps19 and Rps20, respectively.
Dark skin is keratinocyte autonomous
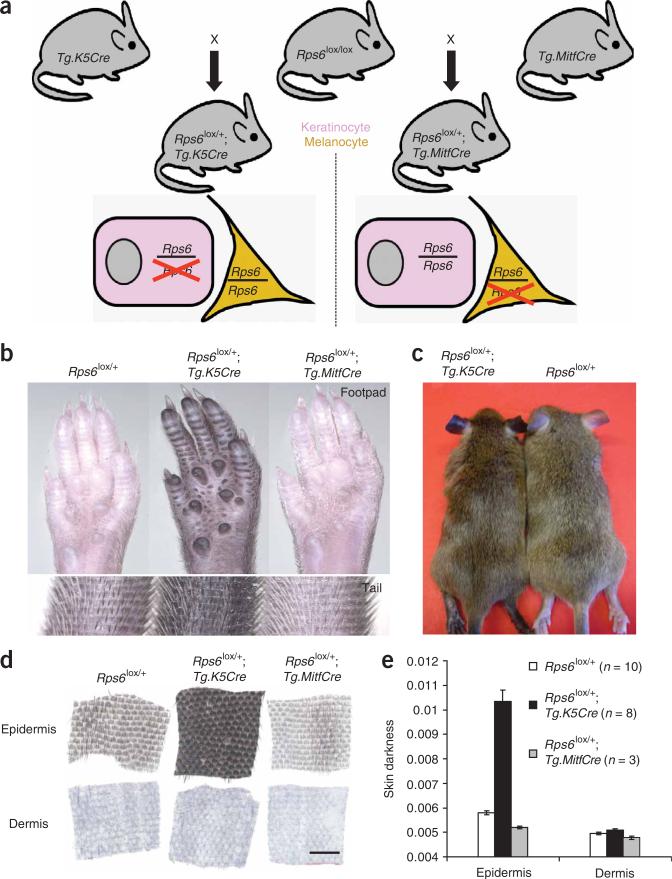
To further investigate the effect of ribosomal 40S alterations on pigmentation, and to determine in which cell type those alterations act to cause dark skin, we made use of a conditional knockout allele11 for a third ribosomal protein subunit gene, Rps6lox. Animals homozygous for Rps6lox were crossed to those carrying either a Cre driver expressed in keratinocytes (Tg.K5Cre)24 or in melanocytes (Tg.MitfCre)25 (Fig. 3a).
Figure 3.
Tissue-specific modulation of Rps6 gene dosage. (a) One copy of Rps6 was removed either from keratinocytes (pink, using Tg.K5Cre) or from melanocytes (yellow, using Tg.MitfCre). (b–d) Whole footpads and tails (b), ears (c) and separated tail epidermis and dermis (d) from animals of the indicated genotype. (e) Skin darkness (mean ± s.e.m.); P values for mutant versus control (based on a two-tail t-test) are 1.7 × 10–5 and 6.7 × 10–5 for keratinocyte (Tg.K5Cre)- and melanocyte (Tg.MitfCre)-specific Rps6, respectively. Scale bar in d, 500 μm.
We found that animals with keratinocyte-specific hemizygosity for Rps6 (Rps6lox/+; Tg.K5Cre/+) show markedly darkened footpads, ears, tail and hair compared to control animals (Fig. 3b,c). Examination of tail skin showed that pigment accumulates in the epidermis, whereas the dermis is unaffected (Fig. 3d,e), a phenocopy of Rps19Dsk3 and Rps20Dsk4. Thus, mutations affecting three different components of the 40S ribosome give rise to similar pigmentary phenotypes, and for at least one of those components, action in keratinocytes is sufficient to cause the mutant phenotype.
By contrast, melanocyte-specific hemizygosity for Rps6 (in Rps6lox/+;Tg.MitfCre/+ animals) causes lighter skin (Fig. 3b,d,e) and affects both the epidermis and, to a lesser degree, the dermis (Fig. 3d,e). This phenotype is consistent with a reduction in the number and/or proliferative capacity of melanoblasts, as occurs in animals with impaired Kit signaling, and as we observed in Rps19Dsk3 and Rps20Dsk4 mutant embryos (Supplementary Fig. 1b)21,22. Taken together, these results suggest that the pigmentary phenotypes of the original mutants represent the combination of two opposing processes: mutations of Rps19 or Rps20 in melanocytes decrease skin pigmentation, whereas the same mutations in keratinocytes increase skin pigmentation. Consistent with this idea, keratinocyte-specific hemizygosity for Rps6 causes much darker skin than observed in Rps19Dsk3 or Rps20Dsk4 mutant animals (Figs. 1 and 3).
Requirement for Kit signaling
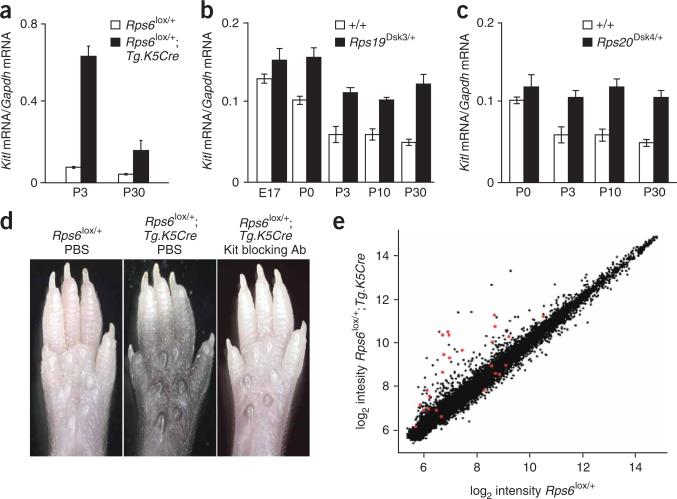
Keratinocytes produce a number of paracrine factors that affect pigment cell development, proliferation and behavior, and whose altered expression might cause epidermal melanocytosis26. We used quantitative RT-PCR to measure levels of Kitl (Kit ligand), Pomc (proopiomelanocortin) and Edn1 (endothelin-1) in mRNA from footpad epidermis of mutant (Rps6lox/+;Tg.K5Cre/+) and nonmutant (Rps6lox/+) littermates. Pomc and Edn1 mRNA levels were unchanged (Supplementary Fig. 3a,b online); however, Kitl mRNA levels were elevated 8.2- and 3.8-fold at P3 and P30, respectively, in mutant compared to nonmutant animals (Fig. 4a). We also found Kitl to be expressed at higher levels in the footpad epidermis of Rps19Dsk3/+ and Rps20Dsk4/+ compared to nonmutant animals (Fig. 4b,c).
Figure 4.
Gene expression and Kit signaling in Rps mutants. (a–c) Expression of Kitl mRNA in footpad epidermis relative to Gapdh mRNA (mean ± s.e.m.) in animals of the indicated genotype at different times. Mutant versus control P values (two-tail) are Rps6 P3 P = 0.002, P30 P = 0.003; Rps19Dsk3/+ P0 P = 0.039, P3 P = 0.007, P10 P = 0.005, P30 P = 0.0004; Rps20Dsk4/+ P3 P = 0.017, P10 P = 0.036, P30 P = 0.038 (n = 3–4 for each assay). (d) Appearance of P8 whole footpads from animals of the indicated genotype 6 days after intraperitoneal injection with PBS or Kit blocking antisera. Representative results are shown for each genotype and treatment condition (n = 4–6 for each class). (e) Microarray results for keratinocyte-specific Rps6 versus control footpad epidermis for each of 24,611 transcripts represented on the array. Each dot represents the mean for three mutant (Rps6lox/+;Tg.K5Cre/+) and three control (Rps6lox/+) samples; transcripts associated with P values ≤0.05 (after multiple testing correction) are indicated in red and identified in Supplementary Table 1.
A previous study27 using a Kitl transgene driven by a keratinocyte promoter indicated that high expression of Kitl gives rise to epidermal melanocytosis, similar to, albeit more severe than, the dark skin in our Rps mutants. To investigate a potential requirement for Kit signaling in the Rps dark skin mutants, we made use of a neutralizing antibody for Kit, Ack2 (ref. 28). Homozygotes for the Rps6lox allele were crossed to animals carrying Tg.K5Cre, and mutant (Rps6lox/+;Tg.K5Cre/+) and nonmutant (Rps6lox/+) littermates were injected at P2 with either Ack2 antisera or phosphate-buffered saline (PBS). In footpads examined at P8, we observed that PBS injection had no effect on pigmentation in either mutant or nonmutant animals, but that Ack2 injection prevented dark skin in the mutant animals (Fig. 4d). These results demonstrate that Kit signaling is required for dark skin caused by Rps mutations, and suggest that the elevated levels of Kitl mRNA have a critical role in that process.
Gene expression signatures for melanocytes and p53
To explore whether increased expression of Kitl was part of a larger transcriptional program caused by reduced Rps gene dosage, we used whole-genome microarrays to measure gene expression in mRNA from P3 footpad epidermis. We prepared RNA for each hybridization from the epidermis of four rear footpads (two animals) and carried out three biological replicates for mutant (Rps6lox/+;Tg.K5Cre/+) and nonmutant (Rps6lox/+) epidermis.
We analyzed the microarray data by directly comparing the expression for each gene in mutant compared to nonmutant, and identifying genes whose expression between the two groups seemed significantly different. A global view of these results (Fig. 4e) revealed that the effects of reduced Rps6 gene dosage are subtle: only 25 of the 24,611 transcripts (~0.1%) represented on the array were associated with P values <0.05 (Benjamini-Hochman correction for false-discovery rate)29. In addition, most of the changes in the mutant sample represent induction rather than repression (Fig. 4e); only 5 of the 25 genes associated with a P value <0.5 were repressed (χ2 test P = 0.0027), and the average change as a multiple of control of induced genes was 4.12 compared to 0.82 for repressed genes (Supplementary Table 1 online). These observations suggest that reduced gene dosage of Rps6 elicits a specific biological response rather than a global effect on the transcriptome.
Two transcriptional signatures emerge from the microarray data that provide insight into the pathogenesis of dark skin. First, four melanocyte-selective genes, including Slc45a2 (membrane associated transporter), Sox10 (SRY-box containing gene 10), Si (silver) and Ednrb (endothelin receptor type b), seem induced in mutant epidermis; as such, these are likely to reflect an increased number of melanocytes rather than altered transcription per se (Supplementary Table 1). Second, and more importantly, several genes known to be transcriptional targets of p53 were induced in the mutant tissue, including Mdm2 (mouse double minute 2), Ddit4l (DNA-damage inducible transcript 4-like) (Supplementary Table 1)30,31, and others (L. Attardi, unpublished data).
Dark skin and erythrocyte hypoplasia are mediated by Trp53
On the basis of the microarray data, we hypothesized that p53 stabilization and/or activation might be a critical event linking reduced Rps gene dosage in keratinocytes to increased expression of Kitl and consequent epidermal melanocytosis. This idea is supported by previous studies demonstrating that reduced dosage of Rps6 in the early mouse embryo32 or in T lymphocytes33 triggers apoptosis and cell cycle arrest in a p53-dependent manner.
We first looked for evidence of altered p53 expression by immunohistochemistry of footpad epidermis. From tissues processed in parallel, we observed a 73-fold increase in p53 staining that was strongest in the basal layer of the epidermis in Rps6lox/+;Tg.K5Cre animals compared to nonmutant controls (Rps6lox/+) (Fig. 5a and Supplementary Fig. 4b,c online). Similar results were observed in Rps19Dsk3 and Rps20Dsk4 animals (Supplementary Fig. 5a online). A modest elevation of p53 staining (sevenfold) was also observed in the footpad epidermis of Tg.K5Cre heterozygous animals compared to nonmutant animals (an observation that is consistent with the findings of others34,35), but it did not affect footpad pigmentation (Supplementary Fig. 5a–c).
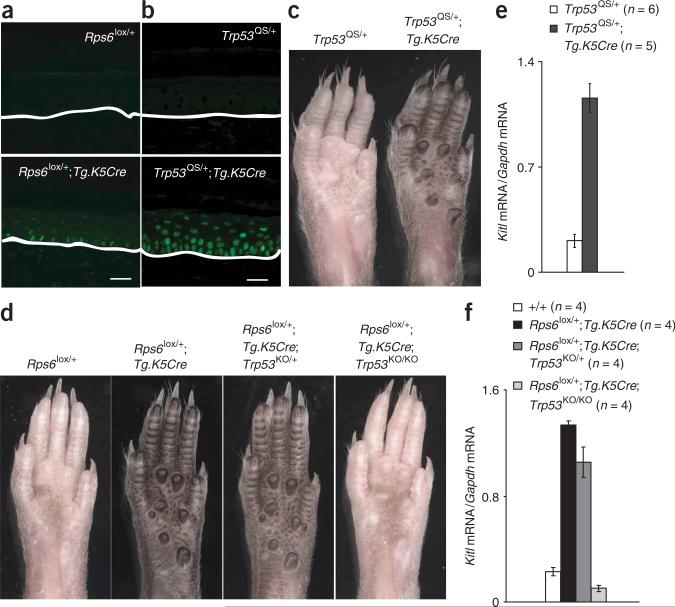
Figure 5.
p53 is sufficient and necessary to induce dark skin. (a,b) Immunofluorescence for p53 in footpad sections; white lines mark the dermal-epidermal junction. (c,d) Whole footpads from adult animals; all results shown are representative of at least three animals for each genotype. (e,f) Expression of Kitl mRNA in P30 footpad epidermis relative to Gapdh mRNA (mean ± s.e.m.) in animals of the indicated genotypes (two-tail P values are Trp53QS/+ versus Trp53QS/+; Tg.K5Cre/+ P = 0.00016; Rps6+/+ vs. Rps6lox/+; Tg.K5Cre P = 5 × 10–7; Rps6lox/+; Tg.K5Cre vs. Rps6lox/+; Tg.K5Cre; Trp53KO/KO P = 3.2 × 10–7). Scale bars: a,b, 25 μm.
To investigate a potential role for p53 more critically, we used a previously characterized conditional Trp53 allele generated by gene targeting embryonic stem cells, Trp53LSL-QS, in which expression of a mutant form of p53 (L25Q, W26S) is triggered by Cre recombinase36. The p53QS protein is compromised for transactivation but retains biological activity in apoptosis and growth suppression. Importantly, the mutant allele is controlled by endogenous Trp53 regulatory sequences—levels of Trp53QS mRNA are normal (after activation by Cre recombinase)—but the L25Q and W26S substitutions render the protein resistant to the E3 ubiquitin ligase Mdm2, leading to increased levels of p53QS protein.
In animals heterozygous for both Trp53LSL-QS and Tg.K5Cre (the latter to activate p53QS in keratinocytes; Fig. 5b), we observed markedly darkened footpads, ears, tail and hair (Fig. 5c and data not shown). We also found that Kitl mRNA expression is increased 5.5-fold in the footpad epidermis of Trp53LSL-QS/+; Tg.K5Cre/+ compared to control animals (Fig. 5d). Thus, activation of p53QS is sufficient to induce increased expression of Kitl mRNA and dark skin.
We then asked whether p53 was required for dark skin caused by reduced Rps gene dosage. We crossed Rps6lox/+;Tg.K5Cre/+ animals to those carrying a Trp53 knockout allele37, Trp53KO, and assessed expression of Kitl mRNA and skin color. In Rps6lox/+; Tg.K5Cre/+ animals, deficiency for p53 (Trp53KO/KO) completely reversed the elevation of Kitl mRNA and dark skin (Fig. 5e,f). In fact, hemizygosity for p53 (Trp53KO/+) partially ameliorates dark skin; the pigmentary phenotype of Rps6lox/+; Tg.K5Cre/+; Trp53KO/+ animals is intermediate between that of Rps6lox/+; Tg.K5Cre/+ and Rps6lox/+;Tg.K5Cre/+;Trp53KO/KO animals (Fig. 5e). A similar effect of the Trp53KO allele was observed for Rps19Dsk3 and Rps20Dsk4 (Supplementary Fig. 5b,c). Thus, p53 is a critical link between reduced Rps gene dosage and Kitl-induced epidermal melanocytosis.
Finally, the Rps19Dsk3 allele affords the opportunity to examine the mechanism by which RPS19 mutations cause abnormalities in humans with Diamond-Blackfan syndrome. Although severely affected individuals have reduced birth weight, limb malformations and complete red cell aplasia, there is both variable expressivity and reduced penetrance; even within families, the same RPS19 mutation may cause overt Diamond-Blackfan syndrome, isolated macrocytosis and/or elevated erythrocyte adenosine deaminase activity, or be clinically silent7.
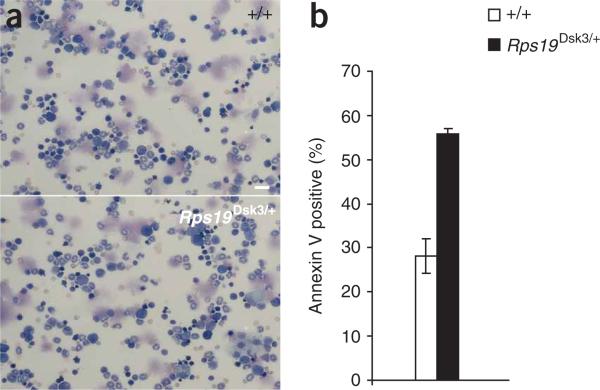
On both the original inbred (C3HeB/HeJ) and a mixed genetic background, we found that Rps19Dsk3 animals show a lower red blood cell count and growth retardation compared to nonmutant animals; we also observed reduced birth weight (~ 10–15% reduction) and a depressed reticulocyte count (~ 50% reduction) on the inbred background (Table 1 and Supplementary Table 2 online). We could not discern any differences in bone marrow cytology between mutant and nonmutant animals (Fig. 6a), and the erythrocyte phenotype was mild, with ~ 5% and ~ 10% reductions in red blood cell count at 8 weeks and 20 weeks of age, respectively (Supplementary Table 3 online). However, as has been described in humans with Diamond-Blackfan anemia38, we observed increased apoptosis in bone marrow progenitor cells39 in Rps19Dsk3/+ compared to nonmutant controls (Fig. 6b).
Table 1.
Effects of Rps19 and Trp53 mutations on erythrocytes and body weight
| Inbred (C3HeB/FeJ) |
Mixed |
|||||
|---|---|---|---|---|---|---|
| Trp53 genotype | +/+ | +/+ | +/+ | +/+ | KO/+ | KO/KO |
| Rps19 genotype | +/+ | Dsk3/+ | +/+ | Dsk3/+ | Dsk3/+ | Dsk3/+ |
| No. of animals (male:female) | 5 (3:2) | 6 (2:4) | 14 (7:7) | 16 (9:7) | 21 (11:10) | 8 (4:4) |
| RBC | 9.35 ± 0.16 | 8.67 ± 0.17 (P = 0.02)a | 9.81 ± 0.11 | 9.07 ± 0.10 (P = 0.00004)b | 9.68 ± 0.08 (P = 0.00001)c | 9.83 ± 0.17 (P = 0.0012)d |
| MCV | 50.9 ± 0.22 | 51.6 ± 0.20 (P = 0.04)a | 48.9 ± 0.28 | 49.8 ± 0.26 (P = 0.03)b | 48.8 ± 0.22 (P = 0.004)c | 49.0 ± 0.38 |
| Reticulocytes | 702 ± 43 | 297 ± 40 (P = 0.0002)a | n.d. | n.d. | n.d. | n.d. |
| No. of animals (male:female) | 10 (2:8) | 13 (7:6) | 3 (2:1) | 6 (4:2) | 9 (5:4) | |
| Weight (g) | 11.3 ± 0.23 | 9.54 ± 0.18 (P = 1.7 × 10–6)a | 13.6 ± 0.52 | 11.1 ± 0.35 (P = 0.007)b | 12.6 ± 0.28 (P = 0.003)c | n.d. |
Blood counts were obtained at 8 weeks of age; body weight was obtained at P21. All values are given as mean ± s.e.m. (with P values based on multiple regression in which litter and sex are factors); different cohorts of animals were used for the hematological and the weight studies.
Rps19+/+ versus Rps19Dsk3/+ on inbred background.
Rps19+/+ versus Rps19Dsk3/+ on mixed background.
Rps19Dsk3/+ versus Rps19Dsk3/+; Trp53KO/+.
Rps19Dsk3/+ versus Rps19Dsk3/+; Trp53KO/KO.
RBC, red blood cellcount; MCV, mean corpuscular volume; n.d., not done.
Figure 6.
Effect of Rps19Dsk3 on bone marrow. (a) Representative photomicrographs of bone marrow aspirates (stained with Wright-Giemsa) from animals of the indicated genotypes. Scale bar, 40 μm. (b) The number of annexin V–positive bone marrow progenitor cells (lineage-cKit+) in nonmutant and Rps19Dsk3/+ animals (± s.e.m.) at 8 weeks of age; n = 3 for each genotype. Rps19Dsk3/+ differed significantly from Rps19+/+ (P = 0.013 based on a two-tail t-test).
Finally, in a cross with animals carrying the Trp53KO allele, we found that reduced dosage of Trp53 rescued both the erythrocyte and body weight phenotypes caused by Rps19Dsk3 (Table 1 and Supplementary Table 3). Thus, like dark skin, p53 is a critical and necessary link from reduced Rps gene dosage to both tissue-specific (epidermal melanocytosis, erythrocytic hypoplasia) and whole-animal (growth retardation) phenotypes.
DISCUSSION
Although hemizygosity for genes encoding different ribosomal protein subunits has been recognized for decades to cause reduced cellular and organismal growth, Rps and Rpl mutations have more recently been discovered to cause markedly specific phenotypes, including cancer in zebrafish40, white spotting in mice3 and anemia in humans4–6. These observations have led to hypotheses for an ‘extraribosomal’ function of certain ribosomal protein subunits, as it is not clear how a general impairment of translation in every cell of the body might have tissue-specific manifestations. Our work demonstrates that mutations of Rps6, Rps19 and Rps20 each give rise to the same phenotype—dark skin caused by epidermal melanocytosis—and act through a common pathway that depends on accumulation of p53 and increased expression of Kitl. These findings demonstrate, on an organismal level, that p53 serves not only as a guardian for DNA damage but also as a sensor of ribosome integrity, provides a mechanistic explanation for both the pleiotropy and tissue specificity that accompany reduced dosage of ribosomal protein genes in many different organisms, and has implications for understanding both normal human variation and human disease.
The connection between epidermal melanocytosis and p53 is surprising, as the typical consequences of p53 activation—apoptosis and cell cycle arrest—might be expected to cause hypo- rather than hyperpigmentation. However, our experiments with the conditional Rps6 allele indicate that p53 acts in keratinocytes rather than melanocytes to cause dark skin; indeed, our results suggest that p53 activation in developing pigment cells causes the white-spotting phenotype observed in Dsk3 and Dsk4 mutants, as would be predicted for cell-autonomous action of p53. It has been demonstrated previously that hemizygosity for Rps6 causes p53-dependent apoptosis in early mouse development32 and in adult T cells33. Because mutations in Rps19 and Rps20 have similar effects, p53 likely plays a general role in sensing ribosome integrity, and may provide a critical link between many ribosomal protein alterations and their phenotypic effects, as in Diamond-Blackfan anemia (see below). We note, however, that the causal link between p53 and Kitl in the pathogenesis of dark skin probably does not apply to the erythroid defect, as bone marrow transplantation can cure Diamond-Blackfan anemia7, but not deficiency for Kitl41. Thus, the action of Kit ligand on hematopoietic cells is cell non-autonomous, whereas the defect in Diamond-Blackfan anemia is cell-autonomous.
Although the connection between disease phenotypes and p53 generally focuses on loss-of-function mutations, dark skin likely represents a novel endpoint for normal p53 action14. From this perspective, we note that Cui et al.42 have also implicated p53 in hyperpigmentation caused by chronic exposure to low levels of UVB irradiation. These investigators postulated that the tanning response is mediated by p53-dependent activation of Pomc, thereby promoting changes in pigment synthesis and/or melanocyte differentiation. However, our results suggest that a role for p53 in tanning may also depend on Kitl, consistent with the observation of Slominski et al.43 that deficiency of Pomc has no effect on pigmentation in a defined genetic background. By contrast, Cui et al.42 report that p53 mutant mice show a relatively pale skin color. From this perspective, Trp53 is perhaps a ‘better’ candidate gene for pigmentary variation than Pomc; indeed, Beckman and colleagues44,45 have suggested previously that clinal variation in TP53 haplotypes underlies climatic adaptation in humans. Thus, the same selective pressures responsible for defense against the damaging effects of UV radiation may also contribute to basal differences in pigmentary phenotype.
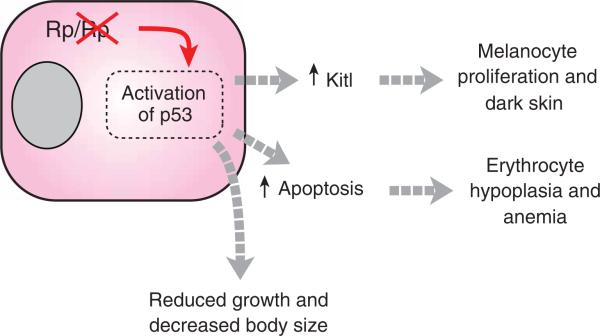
Although p53 is poised to respond to stress signals in many different cell types, our results suggest that variation in the type of response accounts for both the tissue-specificity and the pleiotropy associated with reduced ribosomal protein dosage, with increased expression of Kitl in keratinocytes causing dark skin, and cell cycle arrest and/or apoptosis in the bone marrow causing red cell aplasia (Fig. 7). Even reduced body size in Rps19Dsk3 mutant mice was rescued by p53 deficiency; thus, most if not all the consequences of hemizygosity for ribosomal protein genes may be due to activation of p53-dependent pathways. In Drosophila Minute mutants, a reduced rate of development and altered body size has long been ascribed to a general impairment of cellular protein synthesis2,13,46; however, our results suggest an alternative and easily testable explanation that involves activation of Dmp53, which encodes the Drosophila homolog of p53.
Figure 7.
Pathophysiology of mutations affecting ribosomal proteins (Rp). As described in the text, reduced dosage of Rps6, Rps19 or Rps20 triggers stabilization and/or activation of p53, which gives rise to a pleiotropic phenotype whose components depend on the sensitivity and response of individual cell types and on specific downstream targets of p53. All of the phenotypes can be rescued by deficiency for Trp53.
In some respects, the erythroid defect we observed in Dsk3 mutant mice, albeit mild, is surprising, given previous work47,48 on animals carrying an Rps19 targeted allele that show no erythroid abnormalities. However, this study47,48 found that the nontargeted Rps19 allele in their model showed compensatory upregulation, which is quite unusual and apparently not the case for the Dsk3 mutation. This suggests that mice carrying additional, and perhaps multiple, missense alleles of Rps genes may show severe erythroid defects, providing an additional model for the human condition. Regardless, tissue-specific variation in the activation of, or response to, p53, is a plausible mechanism to account for Diamond-Blackfan syndrome in humans, and suggests new avenues for both diagnosis and treatment. For example, efforts to identify mutations in the ~ 75% of affected individuals that do not have mutations in RPS19 could be expanded beyond ribosomal protein subunit genes to include components of the p53 pathway, and pharmacologic approaches titrated to control p53 activation, expression or downstream effectors in bone marrow may prove to be helpful for treating individuals with red cell aplasia or other bone marrow failure syndromes.
Of the 15 Dsk mutants identified in the original screen16, 5 were associated with epidermal melanocytosis; the 3 that have yet to be identified and characterized (Dsk6, Dsk8 and Dsk11) are very similar to each other and to Dsk3 and Dsk4. These and other additional dark skin mutants may provide a sensitive means to learn more about the control and targets of p53 in an organismal context.
METHODS
Animals and animal experiments
We obtained mice carrying KitWv from The Jackson Laboratory, Dct-lacZ from M. Shin (Fox Chase Cancer Center) and I. Jackson (MRC Human Genetics Unit), Rps6lox from G. Thomas (University of Cincinnati)11, Tg.K5Cre from S. Artandi (Stanford University) and J. Jorcano (Epithelial Biomedicine Division CIEMAT)24 and Trp53KO from T. Jacks (Massachusetts Institute of Technology)37.
For the studies of Kit signaling (Fig. 4), we injected P2 animals intraperitoneally with PBS or 25 μg of monoclonal rat antibody to mouse cKit (ACK2, Chemicon). We obtained measurements of hematologic parameters at 8 weeks of age from retroorbital blood samples. For the studies of hematopoietic apoptosis, we flushed bone marrow from femurs of 8-week-old animals with RPMI containing 10% FCS, and we stained thin-layer cell preparations from a cytocentrifuge with Wright-Giemsa. Lineage-negative, cKit-positive bone marrow progenitor cells were identified and analyzed by immunostaining and FACS analysis for cKit, Gr-1, Mac-1, CD-3, CD-4, CD-8, B220 and TER-119 as previously described39. We used annexin V-FITC staining (CalBiochem) as a marker for apoptotic cells. Three animals of each genotype were analyzed. All experiments were carried out under a protocol approved by the Stanford Administrative Panel on Laboratory Animal Care.
Genetics
Generation of Dsk3/+ and Dsk4/+ mice on an isogenic C3HeB/FeJ background15 and preliminary linkage studies were described previously16. For high-resolution mapping, (C3H3B/FeJ – Dsk × CAST/Ei)BC1 backcross progeny were generated and screened as described17, using SSLP and/or SSCP markers as indicated (Fig. 2a and Supplementary Fig. 2a). Physical distances and coordinates reflect the mm8 assembly as presented by the UCSC genome browser49. All mutations were confirmed with sequencing or restriction digestion of PCR amplicons. For Dsk3, markers M1, M2, M7 and M23 represent single-strand conformation polymorphisms apparent from amplicons corresponding, respectively, to 7:24,200,000–24,200,100, 7:25,300,000–25,300,100, 7:24,300,000–24,300,100 and 7:24,900,000–24,900,100.
Rps19Dsk3 and Rps20Dsk4 were genotyped by direct sequencing (Dsk3) or by MspI digestion (Dsk4) of PCR-amplified genomic DNA. The KitW-v (ref. 18), Rps6lox (ref. 33), Tg.K5Cre24, Tg.MitfCre25, Trp53KO (ref. 37) and Trp53QS (ref. 36) mutations were genotyped as described previously.
Histology
Tissues for histology were prepared as previously described16, as were morphometric measurements of skin darkness and Xgal-positive cells17. For LacZ staining of embryonic and adult tissues, E15.5 embryos, adult footpads and adult tails were fixed in 4% paraformaldehyde and immersed in X-gal overnight at room temperature. Immunofluorescence for p53 was carried out with rabbit anti-p53 antisera (Novocastra Laboratories) after antigen retrieval using 0.01M citrate buffer, pH 6 in a pressure cooker. We carried out dermal–epidermal separations by immersing tissues in 2 M NaBr at 37 °C. All photomicrographs are representative of at least three animals of each genotype.
Gene expression and statistics
RNA for qRT-PCR and expression array experiments was isolated from the footpad epidermis using Trizol (Invitrogen) and purified using RNeasy (Qiagen). We pooled multiple (2–4) footpads for each replicate and used at least three replicates for each genotype and/or time point. For qRT-PCR, we treated 1 μg of RNA with DNaseI before reverse transcription with Superscript III (Invitrogen). Diluted cDNA (fivefold) was amplified using the LightCycler FastStart DNA Master Plus SYBR Green I System (Roche). Primer sequences for Kitl, Pomc, Edn1 and Gapdh are available on request.
We used the Illumina Sentrix system (MouseRef-8, BD-26–201) for gene expression profiling, as previously described50. Differentially expressed genes were ranked according to a t statistic, with associated P values corrected for multiple testing according to Benjamini and Hochman29. Statistical analyses of physiological and histological phenotypes were carried out with either a two-sample t-test, or with multiple regression in which sex and litter were factors, as appropriate.
Supplementary Material
ACKNOWLEDGMENTS
We thank G. Thomas (University of Cincinnati) and S. Volarevic (University of Cincinnati) for Rps6lox mice, I. Jackson (MRC Human Genetics Unit) and M. Shin (Fox Chase Cancer Center) for Dct-lacZ mice, S. Artandi (Stanford University) and J. Jorcano (Epithelial Biomedicine Division CIEMAT) for Tg.K5Cre mice, A. Alizadeh (Stanford University) for Tg.MitfCre mice and T. Jacks (Massachusetts Institute of Technology) for Trp53KO mice. We thank P. Khavari and U. Francke for their careful review of the work, H. Manuel for technical support and B. Glader for advice regarding Diamond-Blackfan anemia. K.A.M. and C.Y.P. are supported by Mentored Clinical Scientist Development Investigator Awards from the National Institutes of Health. G.S.B. is supported by a Research Project Grant from the National Institutes of Health. Part of this work was supported by a grant from the German Human Genome Project (DHGP) and the National Genome Research Network (NGFN 01GR0430) to M.H.d.A.
Footnotes
References
- 1.Schultz J. The Minute reaction in the development of Drosophila melanogaster. Genetics. 1929;14:366–419. doi: 10.1093/genetics/14.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambertsson A. The minute genes in Drosophila and their molecular functions. Adv. Genet. 1998;38:69–134. doi: 10.1016/s0065-2660(08)60142-x. [DOI] [PubMed] [Google Scholar]
- 3.Oliver ER, Saunders TL, Tarle SA, Glaser T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development. 2004;131:3907–3920. doi: 10.1242/dev.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Draptchinskaia N, et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat. Genet. 1999;21:169–175. doi: 10.1038/5951. [DOI] [PubMed] [Google Scholar]
- 5.Cmejla R, Cmejlova J, Handrkova H, Petrak J, Pospisilova D. Ribosomal protein S17 gene (RPS17) is mutated in Diamond-Blackfan anemia. Hum. Mutat. 2007;28:1178–1182. doi: 10.1002/humu.20608. [DOI] [PubMed] [Google Scholar]
- 6.Gazda HT, et al. Ribosomal protein S24 gene is mutated in Diamond-Blackfan anemia. Am. J. Hum. Genet. 2006;79:1110–1118. doi: 10.1086/510020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis SR, Lipton JM. Diamond blackfan anemia: a disorder of red blood cell development. Curr. Top. Dev. Biol. 2008;82:217–241. doi: 10.1016/S0070-2153(07)00008-7. [DOI] [PubMed] [Google Scholar]
- 8.Gazda HT, Sieff CA. Recent insights into the pathogenesis of Diamond-Blackfan anaemia. Br. J. Haematol. 2006;135:149–157. doi: 10.1111/j.1365-2141.2006.06268.x. [DOI] [PubMed] [Google Scholar]
- 9.Flygare J, Karlsson S. Diamond-Blackfan anemia: erythropoiesis lost in translation. Blood. 2007;109:3152–3154. doi: 10.1182/blood-2006-09-001222. [DOI] [PubMed] [Google Scholar]
- 10.Leger-Silvestre I, et al. Specific role for yeast homologs of the Diamond Blackfan anemia-associated Rps19 protein in ribosome synthesis. J. Biol. Chem. 2005;280:38177–38185. doi: 10.1074/jbc.M506916200. [DOI] [PubMed] [Google Scholar]
- 11.Volarevic S, et al. Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science. 2000;288:2045–2047. doi: 10.1126/science.288.5473.2045. [DOI] [PubMed] [Google Scholar]
- 12.Angelini M, et al. Missense mutations associated with Diamond-Blackfan anemia affect the assembly of ribosomal protein S19 into the ribosome. Hum. Mol. Genet. 2007;16:1720–1727. doi: 10.1093/hmg/ddm120. [DOI] [PubMed] [Google Scholar]
- 13.Morata G, Ripoll P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 14.Vousden KH, Lane DP. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 15.Hrabe de Angelis MH, et al. Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nat. Genet. 2000;25:444–447. doi: 10.1038/78146. [DOI] [PubMed] [Google Scholar]
- 16.Fitch KR, et al. Genetics of dark skin in mice. Genes Dev. 2003;17:214–228. doi: 10.1101/gad.1023703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Raamsdonk CD, Fitch KR, Fuchs H, de Angelis MH, Barsh GS. Effects of G-protein mutations on skin color. Nat. Genet. 2004;36:961–968. doi: 10.1038/ng1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackenzie MA, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev. Biol. 1997;192:99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- 19.Hirobe T. Histochemical survey of the distribution of the epidermal melanoblasts and melanocytes in the mouse during fetal and postnatal periods. Anat. Rec. 1984;208:589–594. doi: 10.1002/ar.1092080414. [DOI] [PubMed] [Google Scholar]
- 20.Mayer TC. The migratory pathway of neural crest cells into the skin of mouse embryos. Dev. Biol. 1973;34:39–46. doi: 10.1016/0012-1606(73)90337-0. [DOI] [PubMed] [Google Scholar]
- 21.Cable J, Jackson IJ, Steel KP. Mutations at the W locus affect survival of neural crest-derived melanocytes in the mouse. Mech. Dev. 1995;50:139–150. doi: 10.1016/0925-4773(94)00331-g. [DOI] [PubMed] [Google Scholar]
- 22.Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992;115:1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- 23.Fromont-Racine M, Senger B, Saveanu C, Fasiolo F. Ribosome assembly in eukaryotes. Gene. 2003;313:17–42. doi: 10.1016/s0378-1119(03)00629-2. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez A, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- 25.Alizadeh A, Fitch KR, Niswender CM, McKnight GS, Barsh GS. Melanocytelineage expression of Cre recombinase using Mitf regulatory elements. Pigment Cell Melonoma Res. 2008;21:63–69. doi: 10.1111/j.1755-148X.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- 26.Hirobe T. Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes. Pigment Cell Res. 2005;18:2–12. doi: 10.1111/j.1600-0749.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 27.Kunisada T, et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa S, et al. In utero manipulation of coat color formation by a monoclonal antic-kit antibody: two distinct waves of c-kit-dependency during melanocyte development. EMBO J. 1991;10:2111–2118. doi: 10.1002/j.1460-2075.1991.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Statist. Soc. Ser. B. Methodological. 1995;57:289–300. [Google Scholar]
- 30.Ellisen LW, et al. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol. Cell. 2002;10:995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 31.Barak Y, Juven T, Haffner R, Oren M. mdm2 expression is induced by wild type p53 activity. EMBO J. 1993;12:461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panic L, et al. Ribosomal protein S6 gene haploinsufficiency is associated with activation of a p53-dependent checkpoint during gastrulation. Mol. Cell. Biol. 2006;26:8880–8891. doi: 10.1128/MCB.00751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulic S, et al. Inactivation of S6 ribosomal protein gene in T lymphocytes activates a p53-dependent checkpoint response. Genes Dev. 2005;19:3070–3082. doi: 10.1101/gad.359305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loonstra A, et al. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc. Natl. Acad. Sci. USA. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan EL, et al. Homozygous K5Cre transgenic mice have wavy hair and accelerated malignant progression in a murine model of skin carcinogenesis. Mol. Carcinog. 2007;46:49–59. doi: 10.1002/mc.20192. [DOI] [PubMed] [Google Scholar]
- 36.Johnson TM, Hammond EM, Giaccia A, Attardi LD. The p53QS transactivation-deficient mutant shows stress-specific apoptotic activity and induces embryonic lethality. Nat. Genet. 2005;37:145–152. doi: 10.1038/ng1498. [DOI] [PubMed] [Google Scholar]
- 37.Jacks T, et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 38.Miyake K, et al. RPS19 deficiency leads to reduced proliferation and increased apoptosis but does not affect terminal erythroid differentiation in a cell line model of Diamond-Blackfan anemia. Stem Cells. 2008;26:323–329. doi: 10.1634/stemcells.2007-0569. [DOI] [PubMed] [Google Scholar]
- 39.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 40.Amsterdam A, et al. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2004;2:e139. doi: 10.1371/journal.pbio.0020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCulloch EA, Siminovitch L, Till JE, Russell ES, Bernstein SE. The cellular basis of the genetically determined hemopoietic defect in anemic mice of genotype Sl-Sld. Blood. 1965;26:399–410. [PubMed] [Google Scholar]
- 42.Cui R, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 43.Slominski A, et al. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology. 2005;146:1245–1253. doi: 10.1210/en.2004-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sjalander A, Birgander R, Kivela A, Beckman G. p53 polymorphisms and haplotypes in different ethnic groups. Hum. Hered. 1995;45:144–149. doi: 10.1159/000154275. [DOI] [PubMed] [Google Scholar]
- 45.Beckman G, et al. Is p53 polymorphism maintained by natural selection? Hum. Hered. 1994;44:266–270. doi: 10.1159/000154228. [DOI] [PubMed] [Google Scholar]
- 46.Marygold SJ, et al. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 2007;8:R216. doi: 10.1186/gb-2007-8-10-r216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsson H, et al. Erythropoiesis in the Rps19 disrupted mouse: analysis of erythropoietin response and biochemical markers for Diamond-Blackfan anemia. Blood Cells Mol. Dis. 2006;36:259–264. doi: 10.1016/j.bcmd.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Matsson H, et al. Targeted disruption of the ribosomal protein S19 gene is lethal prior to implantation. Mol. Cell. Biol. 2004;24:4032–4037. doi: 10.1128/MCB.24.9.4032-4037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karolchik D, et al. The UCSC Genome Browser Database. Nucleic Acids Res. 2003;31:51–54. doi: 10.1093/nar/gkg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strehlow AN, Li JZ, Myers RM. Wild-type huntingtin participates in protein trafficking between the Golgi and the extracellular space. Hum. Mol. Genet. 2007;16:391–409. doi: 10.1093/hmg/ddl467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.