Abstract
Neutrophil gelatinase-associated lipocalin (NGAL), a 25 kDa protein produced by injured nephron epithelia, is one of the most promising new markers of renal epithelial injury. In contrast to serum creatinine and urinary output, which are measures of kidney function, NGAL is specifically induced in the damaged nephron and then released into blood and urine, where it can be readily measured. Careful proof-of-concept studies using defined animal models have uncovered the sources and trafficking of NGAL in acute kidney injury (AKI) and have addressed the contributions of renal and non-renal sources. Clinical studies indicate that NGAL, unlike creatinine, is a marker responsive to tissue stress and nephron injury, but less so to adaptive hemodynamic responses. In certain clinical settings, NGAL is an earlier marker compared with serum creatinine. In addition, clinical studies have shown that NGAL is a powerful predictor of poor clinical outcomes, which can be used to risk stratify patients when combined with serum creatinine. NGAL has important limitations, including its responsiveness in systemic inflammation, which is partially uncoupled from its response to kidney injury and which needs to be considered when interpreting NGAL results clinically. This review covers the biology and pathophysiology of NGAL and summarizes the results of the growing body of clinical studies that have addressed the utility of NGAL in the early diagnosis of AKI, in the distinction of intrinsic AKI and in the prognostic assessment of broad patient populations.
Keywords: Acute kidney injury, Creatinine, NGAL
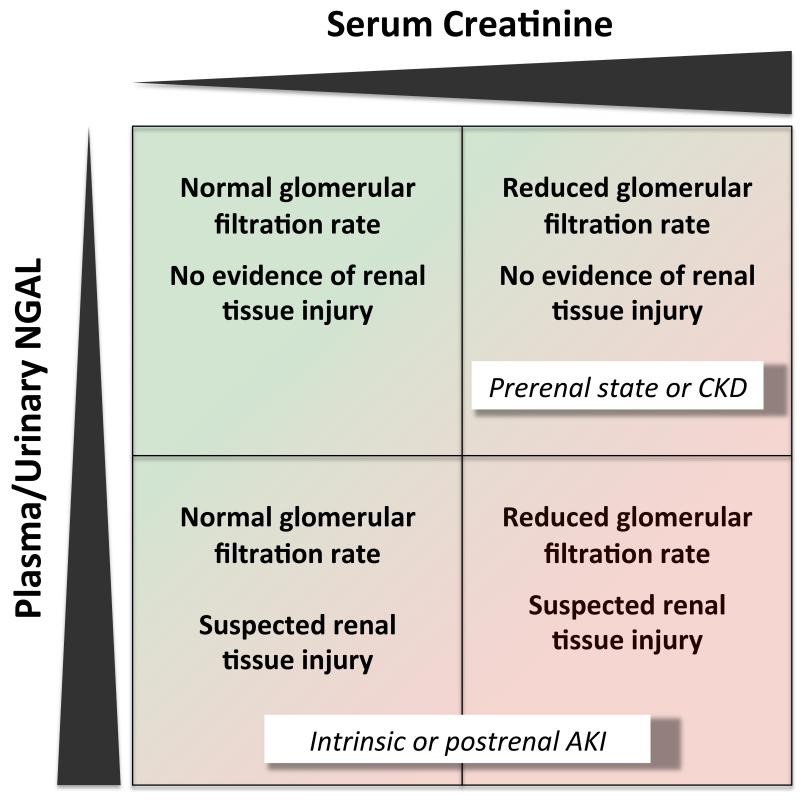
Acute kidney injury (AKI) is a common problem (Nash et al., 2002) and has high mortality rates (Uchino et al., 2005). Currently, the diagnosis of acute kidney injury relies on serum creatinine and urinary output, two markers of kidney function rather than kidney injury. It is widely acknowledged that creatinine in acute kidney injury has several limitations, including its delayed response and its responsiveness to purely hemodynamic adaptations of the glomerular filtration rate, often referred to as “prerenal state” (Jo et al., 2007), (Devarajan, 2007),(Cruz et al., 2011), (Bennett et al., 2008). Due to the delayed response of creatinine and the lack of specific symptoms, AKI is usually diagnosed late and an early specific therapy of intrinsic acute kidney injury is frequently unavailable. On the other hand, creatinine often reflects chronic kidney disease rather than acute kidney injury. Because of these shortcomings of serum creatinine (figure 1), more reliable biomarkers are needed for the diagnosis of acute kidney injury (Siew et al., 2011). A desirable biomarker should be non-invasive, detectable at very early stages of acute damage, specific for cellular damage and prognostically relevant. Many biomarkers are under investigation regarding their diagnostic and prognostic values. NGAL is one of them. This article’s purpose is to review biologic and physiologic data on NGAL, experimental models of NGAL in acute kidney injury and clinical studies on the diagnostic and prognostic utility of NGAL in patients with acute kidney injury.
Figure 1.
Pathophysiological classification of AKI based on creatinine and NGAL
NGAL in acute kidney injury: from biology to pathophysiology
NGAL is a 25 kDa protein of the lipocalin family. Lipocalin proteins are composed of 8 β-strands that form a β-barrel enclosing a calyx (Flower et al., 2000). This NGAL structure was illustrated by crystallography (Goetz et al., 2002). The calyx binds and transports small molecules. NGAL was originally identified in neutrophils, but it is also expressed in kidney, liver and epithelial cells in response to various pathologic states, such as inflammation, infection, intoxication, ischemia, acute kidney injury and neoplastic transformation (Kjeldsen et al., 2000), (Goetz et al., 2000), (Cowland and Borregaard, 1997), (Nielsen et al., 1996), (Xu et al., 1995), (Mishra et al., 2003), (Mishra et al., 2005).
NGAL exists as a 25 kDa monomer, 45 kDa homodimer and conjugated to gelatinase as a 135 kDa heterodimeric form (Kjeldsen et al., 1993). The monomeric form and to some extent the heterodimeric forms are the predominant forms produced by tubular epithelial cells, whereas the homodimeric form is specific to neutrophils (Cai et al., 2010).
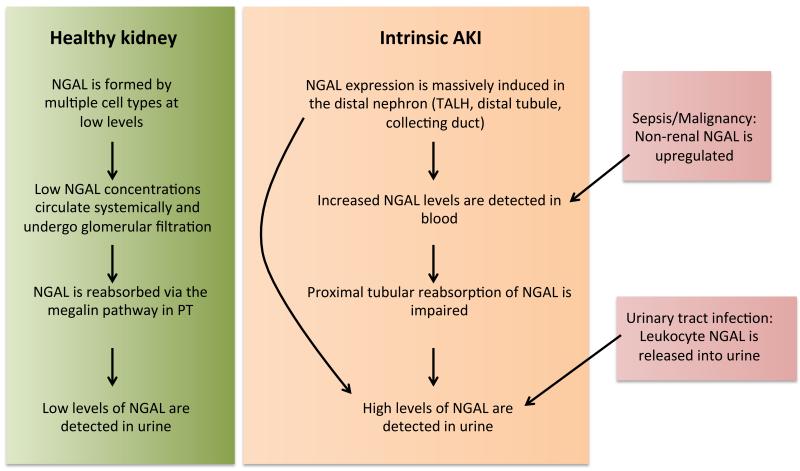
NGAL binds bacterial siderophores or endogenous compounds in mammals, including catechol, with high affinity (Cai et al., 2010), (Bao et al., 2010). NGAL inhibits bacterial growth by binding siderophores and it can effectively transport iron into cells, where the iron is released inducing downstream cellular responses (Yang et al., 2002), (Li et al., 2004). Processes induced by NGAL include bacteriostasis, anti-apoptotic effects, and an enhanced proliferation of renal tubules, which constitute possible pathways of NGAL-mediated kidney protection in acute injury. NGAL is expressed at low constant rates in multiple cell types. Accordingly, in healthy individuals, low NGAL levels are detectable in the systemic circulation. In the kidney, NGAL is filtered in the glomerulus and luminal NGAL is readily reabsorbed in proximal tubule by a megalin-dependent pathway (Hvidberg et al., 2005), (Mori et al., 2005).
Hence, at baseline only low levels of NGAL are detectable in urine. Immediately following acute kidney inury, NGAL is massively upregulated in the distal part of the nephron, i. e. thick ascending limb of Henle’s loop, distal tubule, and collecting duct. This leads to increased urinary and plasma NGAL levels, presumably resulting from both apical and basolateral secretion from nephron epithelia. Impaired proximal tubular reabsorption in the setting of proximal tubular injury may further potentiate increased NGAL levels in urine (Mori et al., 2005), (Schmidt-Ott, 2011), (Schmidt-Ott et al., 2007) (Figure 2). These characteristics may make NGAL superior or complementary to creatinine in the diagnosis of acute kidney injury (figure 1).
Figure 2.
Schematic model of NGAL sources and NGAL traffic in AKI
NGAL may originate from non-renal tissues, in particular in malignancy (Zhang et al., 2012), (Barresi et al., 2011) and sepsis (Wheeler et al., 2008), (Bagshaw et al., 2010), causing elevated NGAL levels in the absence of acute kidney injury. On the other hand, leucocyturia in the setting of urinary tract infections leads to increased urinary NGAL levels (Yilmaz et al., 2009). Hence, non-renal diseases may constitute important confounders, when NGAL levels are interpreted with regard to acute kidney injury. Tomonaga et al (Tomonaga et al., 2012) showed in a large and heterogenous primary care population that urinary NGAL levels are also dependent on gender, age, and liver function, and that they correlate with inflammatory parameters. Increased urinary NGAL levels were found in 6.5% of patients in the absence of AKI based on conventional criteria.
NGAL Sources and Trafficking: Animal models
Renal ischemia-reperfusion injury in mice and rats induces a marked up-regulation of NGAL expression in ischemic injured kidneys within 3 hours of injury, whereas serum creatinine displays milder responses. In fact, increased creatinine levels are only observed following profound bilateral renal ischemia, while creatinine remains unchanged in unilateral or mild bilateral ischemia (Mishra et al., 2003). Maximum expression levels of NGAL mRNA in injured kidneys were elevated more than 1000-fold after 24 to 48 hours (Schmidt-Ott et al., 2006). Urinary NGAL protein was undetectable in urine before ischemia and became evident within 2-3 hours of clinically significant ischemia. Time of occurrence, intensity and duration of urinary NGAL expression correlated with those of the ischemic event. Maximal NGAL levels following AKI displayed up to 1000-fold increases in urine (from 0.04 to 40 mg/ml) and 300-fold increases in blood (from 0.1 to 30 mg/ml) (Mishra et al., 2003), (Schmidt-Ott et al., 2006). Administration of exogenous recombinant NGAL in a mouse model of ischemia-reperfusion injury resulted in a protection of the kidney from ischemic damage (Mori et al., 2005).
In a mouse model of cisplatin-induced AKI, expression of NGAL protein measured by Western blot was induced in the kidney within 3 hours of high dose cisplatin administration in a dose- and duration-dependent manner (Mishra et al., 2003), (Mishra et al., 2004). By immunofluorescence, NGAL was detectable predominantly in proximal tubule cells, although this is likely a reflection of NGAL endocytosis in proximal tubular cells rather than proximal tubular synthesis of NGAL. In sharp contrast, changes in serum creatinine were not evident until 96 hours after cisplatin (Mishra et al., 2004).
In a rat model of acute toxin-induced kidney injury, NGAL mRNA expression was significantly up-regulated in tubular epithelia 3-12 h after lipopolysaccharide application (Han et al., 2012). Both plasma and urinary NGAL levels increased in parallel. In addition, urinary NGAL was the most sensitive indicator of gentamicin nephrotoxicity, compared to other biomarkers, with significant changes occurring within 24 hours, before any changes in serum creatinine were evident (Hoffmann et al., 2010).
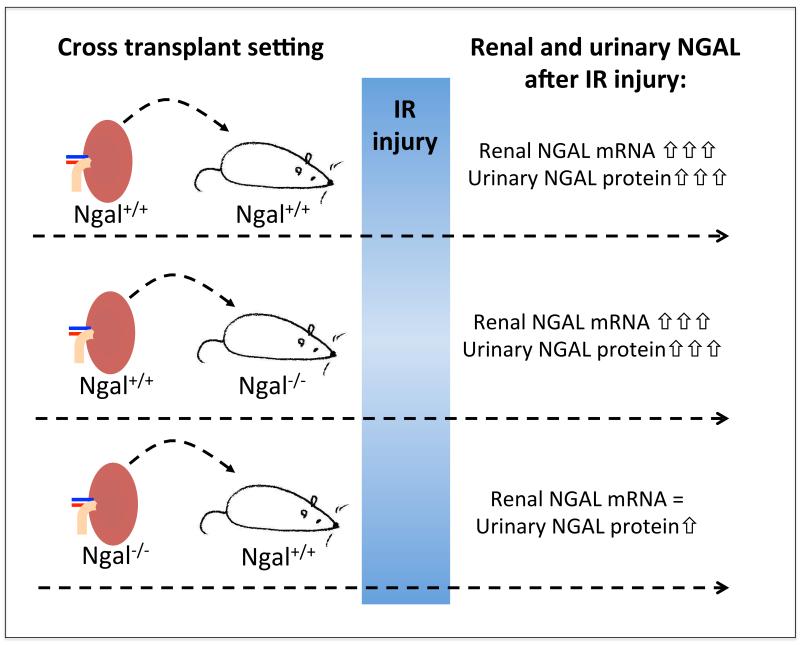
In all animal models, NGAL levels in the acutely injured kidney and urinary NGAL levels reflected acute kidney injury more accurately when compared with serum creatinine. Since the temporal resolution in these studies was limited due to the requirement of repeated intermittent urine and blood sampling, Paragas et al. (Paragas et al., 2011) developed a novel genetic mouse model to non-invasively analyze NGAL expression In this study, an NGAL reporter mouse was generated by knocking reporter genes into the genomic locus encoding NGAL. This facilitated reporter expression mirroring normal endogenous NGAL expression and allowed its non-invasive detection. Acute renal injury was induced by ischemia reperfusion and nephrotoxins. The time course of luminescent reporter expression was visualized in living mice. The luminescence was 10-fold increased 3–6h after renal artery clamping with peak expression (~25–80 fold) 12 hours after ischemia. The intensity of the response depended on the ischemic dose. Importantly, non-ischemic kidneys and extra-renal tissues showed no luminescence. Kidney NGAL reporter expression and urinary NGAL levels were strictly correlated, both temporally and in the intensity of their responses, implying that the protein originated from the kidney.
By clamping of a segmental artery it was possible to directly compare NGAL expression in damaged and intact regions of the same kidney. NGAL expression appeared specifically in tubular segments of injured nephrons, but no NGAL induction was observed in non-ischemic zones. The study by Paragas et al. also examined whether urinary NGAL originates in the kidney. To address this, kidney cross-transplants between NGAL knockout (NGAL−/−) and wild type mice (NGAL+/+) were performed (Figure 3). After renal artery clamping there was a 230-fold and 185-fold induction of NGAL mRNA in ischemic NGAL+/+ kidneys transplanted into NGAL+/+ hosts and NGAL+/+ kidneys transplanted into NGAL−/− hosts, respectively. Conversely, there was only a 6-fold NGAL mRNA induction in ischemic NGAL−/− kidneys transplanted into NGAL+/+ hosts presumably deriving from host cells that invaded the kidney. Consistently, urinary NGAL protein levels were elevated to a similar degree following ischemia of NGAL+/+ kidneys transplanted to NGAL+/+ or NGAL−/− hosts. However, only a mild increase of urinary NGAL was observed after ischemia of NGAL−/− kidneys transplanted into NGAL+/+ hosts, indicating that some extra-renal source contributed to urinary NGAL levels. However, the increase was substantially smaller when compared to urinary NGAL levels after ischemia of an NGAL+/+ kidney, suggesting that the majority of urinary NGAL is derived from the ischemic kidney.
Figure 3.
Elucidation of the sources of urinary NGAL by cross-transplant experiments between mice genetically deficient in NGAL (NGAL−/−) and NGAL-expressing wildtype mice (NGAL+/+).
NGAL and its clinical utility as a biomarker of AKI
In clinical medicine, diagnosis of acute kidney injury is currently based on serum creatinine and urine output, but these markers have major limitations. First, they do not differentiate between structural kidney damage and purely functional hemodynamic triggers of a reduced GFR. Second, the diagnosis is delayed until creatinine has accumulated, which inevitably delays treatment. NGAL could help in resolving these shortcomings due to its ability to identify tissue damage rather than renal dysfunction and due to its rapid induction in response to AKI. Numerous studies have addressed the potential clinical utility of NGAL in AKI. All studies combined include more than 7000 patients.
The studies show a remarkable heterogeneity regarding the patient populations studied, the biofluids analyzed, the assays used to measure NGAL levels, the definitions of clinical endpoints, and the reported test characteristics (Table 1). Most of these studies are monocentric, while four studies were multicentric (Nickolas et al., 2012), (Parikh et al., 2011), (Perry et al., 2010), (Wheeler et al., 2008) and another two studies were performed within a framework of multicenter trials (Koyner et al., 2012), (Trachtman et al., 2006). AKI diagnosis was made according to current diagnostic criteria based on postoperative creatinine dynamics (>50% or > 0.3 mg/dl increases of creatinine).
Table 1.
Clinical studies addressing the diagnostic and prognostic utility of NGAL
| Reference | Sample size |
Clinical setting | NGAL assay | Biofluid | AUC-ROC | Diagnostic/prognostic outcome |
|---|---|---|---|---|---|---|
| Mishra 2005 (15) |
71 | Children after cardiac surgery | WB, ELISA | Urine Plasma |
0.99 0.91 |
Prediction of AKI |
| Dent 2007 (36) | 120 | Children after cardiac surgery | Triage | Plasma | 0.96 | Prediction of AKI |
| Bennett 2008 (6) |
196 | Children after cardiac surgery | ARCHITECT | Urine | 0.95 | Prediction of AKI |
| Wagener 2006 (37) |
81 | Adults after cardiac surgery | WB | Urine | 0.74 (3 hours) 0.80 (18 hours) |
Prediction of AKI |
| Perry 2010 (32) |
879 | Adults after cardiac surgery | Triage | Plasma | 0.64 (after surgery) 0.74 (on day 3) |
Prediction of AKI |
| Haase-Fielitz 2009 (38) |
100 | Adults after cardiac surgery | Triage | Plasma | 0.80-0.89 | Prediction of AKI |
| Koyner 2010 (39) |
123 | Adults after cardiac surgery | ELISA | Urine | 0.88 (6 hours) | Prediction of AKI |
| Parikh 2011 (31) |
1219 | Adults after cardiac surgery | ARCHITECT Triage |
Urine Plasma |
0.67 0.70 |
Prediction of AKI |
| Hirsch 2007 (50) |
91 | Children after contrast agent | ELISA | Urine Plasma |
0.92 0.91 |
Prediction of AKI |
| Bachorzewska- Gajewska 2006 (51) |
35 | Adults after contrast agent | ELISA | Urine Plasma |
NA NA |
Prediction of AKI |
| Wheeler 2008 (33) |
143 | Critically ill septic children | ELISA | Plasma | 0.68 | Prediction of AKI |
| Zappitelli 2007 (45) |
140 | Critically ill children | ELISA | Urine | 0.78 | Prediction of AKI |
| Cruz 2010 (46) | 301 | Critically ill adults | Triage | Plasma | 0.78 | Prediction of AKI |
| De Geus 2011 (44) |
632 | Critically ill adults | Triage | Urine Plasma |
0.76-0.80 | Differentiation of sustained AKI from non-sustained AKI and no AKI |
| Bagshaw 2010 (48) |
82 | Critically ill adults with established AKI septic vs non septic |
ARCHITECT Triage |
Urine Plasma |
0.77 | Prediction of AKI progression and RRT |
| Singer 2011 (41) |
145 | Hospitalized adults with established AKI | ARCHITECT | Urine | 0.87 | Diagnosis of intrinsic AKI (versus prerenal AKI) |
| Hall 2011(42) | 249 | Hospitalized adults with established AKI | ELISA | Urine | 0.75 (prediction model of conv. Parameter + biomarker) |
Prediction of worsening AKI or in- hospital death |
| Koyner 2012 (34) TRIBE-AKI |
380 | Hospitalized adults with established AKI | ARCHITECT Triage |
Urine Plasma |
0.79 (adjusted) 0.83 (adjusted) |
Prediction of worsening AKI |
| Nickolas 2012 (30) |
1635 | Adult patients admitted to ER | WB, ARCHITECT |
Urine | 0.81 | Diagnosis of intrinsic AKI (versus prerenal AKI, stable CKD or normal function) |
| Nickolas 2008 (40) |
635 | Adult patients admitted to ER | WB | Urine | 0.95 | Diagnosis of intrinsic AKI (versus prerenal AKI, stable CKD or normal function) |
| Chen 2012 (43) |
150 | Adult patients admitted to coronary care unit |
ELISA | Plasma Urine |
0.83 0.80 |
Prediction of AKI onset and 6-mo mortality |
| Trachtman 2006 (35) |
34 | Children with D+HUS | ELISA | Urine Plasma |
NA NA |
Prediction of RRT |
| Yilmaz 2009 (49) |
89 | Children with UTI without AKI | ELISA | Urine | 0.99 | Diagnosis of UTI in absence of AKI |
Several studies investigated cardiac surgery patients (Bennett et al., 2008), (Mishra et al., 2005), (Parikh et al., 2011), (Dent et al., 2007), (Wagener et al., 2006), (Haase-Fielitz et al., 2009), (Koyner et al., 2010). The advantage of this setting is that the time point of injury is known. Most of these studies showed NGAL both in the blood and urine to be a decent predictor of acute kidney injury, although AUC-ROCs varied substantially.
In children undergoing cardiopulmonary bypass NGAL measurements in urine and blood showed remarkable promise in identifying patients at risk of AKI early (Bennett et al., 2008), (Mishra et al., 2005), (Dent et al., 2007). These studies showed a delay of 1-3 days in the diagnosis of AKI, when serum creatinine was used as a gold standard marker. There was a significant increase of NGAL levels measured by Western blotting, ELISA, and standardized clinical platforms (Abbott ARCHITECT and Alere Triage device) two hours after surgery both in the urine and blood. Both urine and plasma NGAL predicted AKI independently, with areas under the receiver operating characteristic curves (AUC-ROCs) of 0.95-0.998 for the urine NGAL and 0.91-0.96 for the plasma NGAL.
A number of prospective observational studies on NGAL were performed on adult patients after cardiac surgery (Parikh et al., 2011), (Perry et al., 2010), (Koyner et al., 2012), (Wagener et al., 2006), (Haase-Fielitz et al., 2009), (Koyner et al., 2010). Increased urinary NGAL levels were observed as early as 1 to 3 hours after surgery, while peak concentrations of NGAL in urine and plasma were reached 6 h after the operation (Parikh et al., 2011), (Wagener et al., 2006), (Koyner et al., 2010). Depending on the NGAL assay, the time point reported and the definition of AKI, the predictive ability of NGAL was markedly different among these studies. AUC-ROC of urinary NGAL varied from 0.74 (Wagener et al., 2006) to 0.88 (Koyner et al., 2010). AUC-ROC of plasma NGAL varied from 0.64 (Perry et al., 2010) to 0.89 (Haase-Fielitz et al., 2009).
It needs to be considered that the diagnostic ability of a biomarker in clinical studies is dependent on the gold standard method used to detect the diagnostic outcome, i. e. AKI. A serum-creatinine-based diagnosis of AKI may be a poor measure of true kidney injury, especially when considering the marked responsiveness of serum creatinine to hemodynamic changes (“prerenal AKI”). To circumvent this issue, several studies have attempted to identify intrinsic AKI (as opposed to “prerenal” AKI) in patients based on clinical criteria, such as the recovery of creatinine to hemodynamic corrections (Nickolas et al., 2012), (Nickolas et al., 2008), (Singer et al., 2011). In these studies, the AUC-ROC for urinary NGAL for the diagnosis of intrinsic AKI ranged from 0.81-0.95, suggesting that NGAL may be better in identifying true damage to the nephron than predicting AKI by conventional creatinine-based criteria. However, these studies have the limitation of usually having to depend on clinical criteria of intrinsic kidney damage rather than renal biopsy results.
Additional data have demonstrated remarkable associations of NGAL levels with poor clinical outcomes, including worsening of AKI, need of renal replacement therapy, and mortality (Nickolas et al., 2012), (Trachtman et al., 2006), (Nickolas et al., 2008), (Singer et al., 2011), (Hall et al., 2011), (Chen et al., 2012). Importantly, patient populations stratified by serum creatinine were effectively subdivided into high-risk and low-risk populations, when NGAL levels were added (Nickolas et al., 2012), (Haase et al., 2011). Urinary NGAL predicted a composite outcome of dialysis initiation or death during hospitalization. NGAL also identified a substantial subpopulation with low serum creatinine at hospital admission, but who were at risk of adverse events. This creatinine-negative, NGAL-positive patient population, which has no AKI by conventional criteria, may represent a novel group of patients exhibiting “subclinical” AKI. AKI is independently associated with length of hospital stay (Chertow et al., 2005) and therefore makes treatment more costly. Early recognition of AKI based on NGAL may facilitate earlier and thus more cost-effective treatment than standard strategy (Shaw et al., 2011).
Studies focusing on critically ill patients (Zappitelli et al., 2007), (Cruz et al., 2010), (De Geus et al., 2011), (Bagshaw et al., 2010) also found NGAL to be increased in cases of AKI. Both, in critically ill children and adults in the intensive care unit (ICU) urinary NGAL was a decent diagnostic parameter of AKI with an AUC-ROC of 0.78-0.88 (Zappitelli et al., 2007), (De Geus et al., 2011). Plasma NGAL in the ICU population discriminated AKI with an AUC-ROC of 0.78-0.86 (Cruz et al., 2010), (De Geus et al., 2011).
The picture in critically ill septic patients was somewhat less clear, since sepsis is an important inducer of blood and urinary NGAL expression even in the absence of changes in creatinine. In sepsis, NGAL originates not only from the injured kidney but also from leucocytes and liver. Bagshaw et al (Bagshaw et al., 2010) found plasma and urinary NGAL levels to be substantially higher in patients with septic AKI compared to non-septic AKI. This fact may limit the diagnostic utility of NGAL in septic patients. These data were confirmed by Wheeler et al (Wheeler et al., 2008). In this multicenter study diagnostic value of plasma NGAL measured in septic patients was limited with AUC-ROC for prediction of AKI of 0.68. Yilmaz et al (Yilmaz et al., 2009) discovered increased NGAL levels measured by ELISA in children with urinary tract infections in the absence of AKI. In this study patients with urinary tract infections (UTI) had urinary NGAL levels of 91 ng/ml vs 12 ng/ml in patients without UTI. At present, the relevance of possible NGAL confounders such as sepsis or UTI remains unclear. Recently Martensen et al (Mårtensson et al., 2012) proposed to combine two different enzyme-linked immunosorbent assays to calculate the amount of the monomeric NGAL isoform, which is typically found in AKI, and to eliminate the detection of dimeric NGAL, which is typical of urinary tract infections. The combination of ELISA-Tests they used identified monomeric NGAL with an AUC-ROC of 0.92 and reduced the confounding effect of leukocyturia. However, this approach will need to be validated in larger cohorts to estimate the improvement in diagnosing AKI or intrinsic nephron injury.
In contrast media induced AKI, significant NGAL increases in plasma and urine were reported 2 hours after contrast administration both in children (Hirsch et al., 2007), and adults (Bachorzewska-Gajewska et al., 2006). Diagnostic value of urinary and plasma NGAL in children were excellent with AUC-ROCs of 0.92 and 0.91 respectively (Hirsch et al., 2007).
Different studies have used various methodologies to measure NGAL levels, including experimental assays such as ELISAs and Western blots (Mishra et al., 2005), (Nickolas et al., 2012), (Wagener et al., 2006) as well as standardized clinical laboratory platforms, such as the Triage Assay for plasma NGAL (Parikh et al., 2011), (Koyner et al., 2012), (Haase-Fielitz et al., 2009), (Cruz et al., 2010), (De Geus et al., 2011), (Bagshaw et al., 2010) or the ARCHITECT assay for urinary NGAL (Bennett et al., 2008), (Nickolas et al., 2012), (Parikh et al., 2011), (Koyner et al., 2012), (Singer et al., 2011), (Bagshaw et al., 2010). The timepoint of sampling also varied among the studies (Perry et al., 2010), which further complicates interpretation and comparison of results of different studies. It is still debated whether urinary NGAL levels should be corrected for urinary creatinine or urinary osmolality to correct for urinary concentration. Direct comparison has suggested that correction of NGAL levels for urinary creatinine yielded similar test characteristics compared to uncorrected NGAL levels (Singer et al., 2011).
Expectedly, cut-off levels and test characteristics varied among the studies, which prohibits direct comparisons. Nickolas et al 2012 (Nickolas et al., 2012) showed closely correlating NGAL levels measured by either Western blots (to identify the AKI-specific monomeric form) or the ARCHITECT clinical platform. However, the best method of measurement and the optimal cut-off-levels depending on clinical setting remain to be identified.
Difficulties in interpreting results due to different settings, sampling time points, measurement methods and cut offs make additional studies necessary before NGAL can be recommended as a routine parameter in clinical practice.
Acknowledgements
We would like to thank the Stiftelsen Nordisk Fysiologi, SNF and the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) for their generous support for the AP Symposium on “Hemodynamic Mechanisms in Acute Kidney Injury”. This work was supported by grants to KMSO and DNM from the Deutsche Forschungsgemeinschaft.
Footnotes
Conflict of interest statement
Columbia University has licensed uNGAL to Abbott Laboratories for use in the diagnosis of AKI. K. Budde received research funds and/or honoraria from AiCuris, Pfizer, Novartis, Astellas, Roche, Hexal, Bristol-Myers Squibb, Veloxis Pharma, Effimune Pharma and Siemens. K.M. Schmidt-Ott has a consultation agreement with Abbott; and has participated on the advisory boards for Tardis Medical Consultancy. Other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am. J. Nephrol. 2006;26:287–292. doi: 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, D’amico G, Goldsmith D, Devarajan P, Bellomo R. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010;36:452–461. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- Bao G, Clifton M, Hoette TM, Mori K, Deng S-X, Qiu A, Viltard M, Williams D, Paragas N, Leete T, Kulkarni R, Li X, Lee B, Kalandadze A, Ratner AJ, Pizarro JC, Schmidt-Ott KM, Landry DW, Raymond KN, Strong RK, Barasch J. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat. Chem. Biol. 2010;6:602–609. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi V, Reggiani-Bonetti L, Di Gregorio C, Vitarelli E, Ponz De Leon M, Barresi G. Neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9) prognostic value in stage I colorectal carcinoma. Pathol. Res. Pract. 2011;207:479–486. doi: 10.1016/j.prp.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010;5:2229–2235. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-H, Chang C-H, Lin C-Y, Jenq C-C, Chang M-Y, Tian Y-C, Hung C-C, Fang J-T, Yang C-W, Wen M-S, Lin F-C, Chen Y-C. Acute kidney injury biomarkers for patients in a coronary care unit: a prospective cohort study. PLoS ONE. 2012;7:e32328. doi: 10.1371/journal.pone.0032328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- Cruz DN, De Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, Piccinni P, Ronco C. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36:444–451. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DN, Goh CY, Palazzuoli A, Slavin L, Calabrò A, Ronco C, Maisel A. Laboratory parameters of cardiac and kidney dysfunction in cardio-renal syndromes. Heart Fail Rev. 2011;16:545–551. doi: 10.1007/s10741-011-9231-9. [DOI] [PubMed] [Google Scholar]
- De Geus HRH, Bakker J, Lesaffre EMEH, Le Noble JLML. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am. J. Respir. Crit. Care Med. 2011;183:907–914. doi: 10.1164/rccm.200908-1214OC. [DOI] [PubMed] [Google Scholar]
- Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–212. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim. Biophys. Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Willie ST, Armen RS, Bratt T, Borregaard N, Strong RK. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry. 2000;39:1935–1941. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling C-R, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J. Am. Coll. Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery--a prospective cohort study. Crit. Care Med. 2009;37:553–560. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- Hall IE, Coca SG, Perazella MA, Eko UU, Luciano RL, Peter PR, Han WK, Parikh CR. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol. 2011;6:2740–2749. doi: 10.2215/CJN.04960511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Li Y, Liu M, Li Y, Cong B. Renal neutrophil gelatinase associated lipocalin expression in lipopolysaccharide-induced acute kidney injury in the rat. BMC Nephrol. 2012;13:25. doi: 10.1186/1471-2369-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, 3rd, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr. Nephrol. 2007;22:2089–2095. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Fuchs TC, Henzler T, Matheis KA, Herget T, Dekant W, Hewitt P, Mally A. Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology. 2010;277:49–58. doi: 10.1016/j.tox.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–777. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta. 2000;1482:272–283. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR. Biomarkers predict progression of acute kidney injury after cardiac surgery. J. Am. Soc. Nephrol. 2012;23:905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O’Connor MF, Devarajan P, Bonventre JV, Murray PT. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154–2165. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-Y, Ram G, Gast K, Chen X, Barasch K, Mori K, Schmidt-Ott K, Wang J, Kuo H-C, Savage-Dunn C, Garrick MD, Barasch J. Detection of intracellular iron by its regulatory effect. Am. J. Physiol., Cell Physiol. 2004;287:C1547–1559. doi: 10.1152/ajpcell.00260.2004. [DOI] [PubMed] [Google Scholar]
- Mårtensson J, Xu S, Bell M, Martling C-R, Venge P. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clin. Chim. Acta. 2012;413:1661–1667. doi: 10.1016/j.cca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am. J. Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann. Intern. Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, Elger A, Maarouf O, Sola-Del Valle DA, O’Rourke M, Sherman E, Lee P, Geara A, Imus P, Guddati A, Polland A, Rahman W, Elitok S, Malik N, Giglio J, El-Sayegh S, Devarajan P, Hebbar S, Saggi SJ, Hahn B, Kettritz R, Luft FC, Barasch J. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J. Am. Coll. Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paragas N, Qiu A, Zhang Q, Samstein B, Deng S-X, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin C-S, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat. Med. 2011;17:216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J. Am. Soc. Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TE, Muehlschlegel JD, Liu K-Y, Fox AA, Collard CD, Shernan SK, Body SC. Plasma neutrophil gelatinase-associated lipocalin and acute postoperative kidney injury in adult cardiac surgical patients. Anesth. Analg. 2010;110:1541–1547. doi: 10.1213/ANE.0b013e3181da938e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ott KM. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury--where do we stand today? Nephrol. Dial. Transplant. 2011;26:762–764. doi: 10.1093/ndt/gfr006. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Mori K, Kalandadze A, Li J-Y, Paragas N, Nicholas T, Devarajan P, Barasch J. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr. Opin. Nephrol. Hypertens. 2006;15:442–449. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- Shaw AD, Chalfin DB, Kleintjens J. The economic impact and cost-effectiveness of urinary neutrophil gelatinase-associated lipocalin after cardiac surgery. Clin Ther. 2011;33:1713–1725. doi: 10.1016/j.clinthera.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J. Am. Soc. Nephrol. 2011;22:810–820. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, Luft FC, Schmidt-Ott KM. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405–414. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga Y, Szucs T, Ambühl P, Nock S, Risch M, Risch L. Insights on urinary NGAL obtained in a primary care setting. Clin. Chim. Acta. 2012;413:733–739. doi: 10.1016/j.cca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, Jain A, Bullington N, Devarajan P. Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediatr. Nephrol. 2006;21:989–994. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit. Care Med. 2008;36:1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Pauksen K, Venge P. Serum measurements of human neutrophil lipocalin (HNL) discriminate between acute bacterial and viral infections. Scand. J. Clin. Lab. Invest. 1995;55:125–131. doi: 10.3109/00365519509089604. [DOI] [PubMed] [Google Scholar]
- Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Mol. Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Sevketoglu E, Gedikbasi A, Karyagar S, Kiyak A, Mulazimoglu M, Aydogan G, Ozpacaci T, Hatipoglu S. Early prediction of urinary tract infection with urinary neutrophil gelatinase associated lipocalin. Pediatr. Nephrol. 2009;24:2387–2392. doi: 10.1007/s00467-009-1279-6. [DOI] [PubMed] [Google Scholar]
- Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fan Y, Mei Z. NGAL and NGALR overexpression in human hepatocellular carcinoma toward a molecular prognostic classification. Cancer Epidemiol. 2012;36:e294–299. doi: 10.1016/j.canep.2012.05.012. [DOI] [PubMed] [Google Scholar]