Abstract
Background
Kidney failure predicts mortality in patients with cirrhosis. Identification of kidney failure etiology and recognition of those at the highest mortality risk remains a challenge.
Aims
We hypothesized that urinary Neutrophil Gelatinase-Associated Lipocalin (uNGAL) predicts mortality and identifies hepatorenal syndrome (HRS) in patients with cirrhosis.
Methods
Prospectively enrolled patients with cirrhosis were investigated by uNGAL immunoblot upon hospital admission. Kidney failure type was determined blinded to NGAL measurements.
Results
One hundred and eighteen patients were enrolled. Fifty-two (44%) patients had normal kidney function, 14 (12%) stable chronic kidney disease, 17 (14%) prerenal azotemia, 20 (17%) HRS, and 15 (13%) intrinsic acute kidney injury (iAKI). Patients with HRS had uNGAL levels intermediate between prerenal azotemia [median (IQR) 105 (27.5–387.5) v. 20 (15–45) ng/ml, p=0.004] and iAKI [325 (100–700), p<0.001]. Fifteen (13%) patients died. In unadjusted analysis, uNGAL predicted inpatient mortality (OR 2.00, 95% CI 1.36–2.94) and mortality or liver transplantation (OR 2.01, 95% CI 1.42–2.85). In multiple regression models, uNGAL>110 ng/ml (OR 6.05, 95% CI 1.35–27.2) and HRS (OR 6.71, 95% CI 1.76–25.5) independently predicted mortality, adjusting for age and serum creatinine>1.5 mg/dL.
Conclusions
uNGAL strongly predicts short term inpatient mortality in both unadjusted and adjusted models. Patients with HRS may have uNGAL levels intermediate between those with prerenal azotemia and iAKI. Further studies are needed to determine if uNGAL can improve discrimination of HRS from other types of acute kidney injury and predict short- and long-term cirrhosis outcomes.
Keywords: Acute kidney injury, biomarker, cirrhosis, hepatorenal syndrome, NGAL, mortality
Introduction
Acute kidney injury (AKI) in patients with cirrhosis is common and deadly. Up to 20% of hospitalized patients with cirrhosis develop AKI [1–5] and once AKI occurs there is a reported 4-fold increased risk of mortality.[4] In cirrhosis, AKI types include prerenal azotemia, hepatorenal syndrome (HRS) and intrinsic acute kidney injury (iAKI) but their effect on mortality risk varies. Unfortunately these forms of AKI are difficult to distinguish clinically as serum creatinine (sCr), the clinical standard to define kidney function, poorly discriminates AKI type in cirrhosis.[6–8] Recently, in an effort to improve the definition of AKI and to highlight the importance of non-HRS kidney dysfunction in cirrhosis, the Acute Dialysis Quality Initiative (ADQI) and the International Ascites Club (IAC) jointly published a consensus statement regarding AKI classification [9], incorporating the Risk, Injury, Failure, Loss and End stage disease (RIFLE) and the Acute Kidney Injury Network (AKIN) guidelines. IAC defined HRS [10] was classified as a specific form of AKI[11], but the need for validation of this classification and for new biomarkers of kidney dysfunction in cirrhosis were emphasized.
The lack of a kidney function biomarker that both rapidly and accurately discriminates HRS from other forms of AKI is the basis for the 48 hour diagnostic algorithm proposed by the IAC, which includes diuretic withdrawal and volume administration.[10] Not only does this delay AKI treatment but it potentially worsens portal pressure elevation in patients with HRS.[12–14] Although sCr is a non-specific marker of kidney dysfunction, the severity of sCr elevation is strongly associated with mortality in cirrhosis[4, 5, 15, 16] and is one of only three components in the Model for End Stage Liver Disease (MELD) score used to prioritize patients for liver transplant.[16] However, sCr likely does not completely describe the relationship between kidney function and mortality as different types of kidney failure portend different prognoses. Although HRS is clearly associated with excess mortality[17, 18], data in other forms of kidney dysfunction are lacking.
These limitations of sCr highlight the need for improved diagnostic methods to determine AKI type in cirrhosis. An ideal AKI biomarker would be specific, simple to measure, unaffected by the abnormalities that alter sCr in cirrhosis and would predict mortality as a function of AKI type and severity. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is a protein expressed by injured kidney tubular epithelia.[19–23] Urinary NGAL (uNGAL) levels rise exponentially early in the course of AKI, prior to sCr elevation,[19, 24–26] and can predict AKI in patients undergoing liver transplantation.[27–29] uNGAL levels are not impacted by volume status, diuretic use or prerenal azotemia.[25] In addition, non-progressive chronic kidney disease (CKD) does not induce NGAL expression.[25] Finally, growing evidence also suggests that elevated uNGAL levels independently predict clinical outcomes, including short term mortality.[25, 30, 31]
Therefore, we hypothesized that in patients with cirrhosis, uNGAL would discriminate type of AKI and predict clinical outcomes, including mortality. We tested these hypotheses in a prospective cohort of hospitalized patients with cirrhosis.
Methods
Study Subjects
Consecutive adult patients with cirrhosis admitted to Columbia University Medical Center between January 2007 and September 2009 were eligible. The diagnosis of cirrhosis was based upon liver biopsy [available from our institution in 50 (42%) of patients] or a combination of imaging, laboratory and clinical evidence with physician documentation. Patients on chronic hemodialysis (HD), anuria for the first 24 hours (therefore unable to provide urine for NGAL measurement), urinary tract infection (urine WBC > 10 per high power field or positive urine culture), proteinuria > 500 mg/day, or urinary obstruction were excluded. This protocol was approved by the Columbia University Institutional Review Board and all subjects provided informed consent.
Study Design
Patients were prospectively followed from admission until discharge. Urine for uNGAL measurement was collected once at the time of admission and all samples were assayed simultaneously after kidney function adjudication. sCr was prospectively measured in all patients and adjudication of kidney function category was performed by an attending nephrologist (T.L.N.) and gastroenterologist (E.C.V.) blinded to uNGAL.
Definitions of Kidney Disease
Stable Chronic Kidney Disease (CKD)
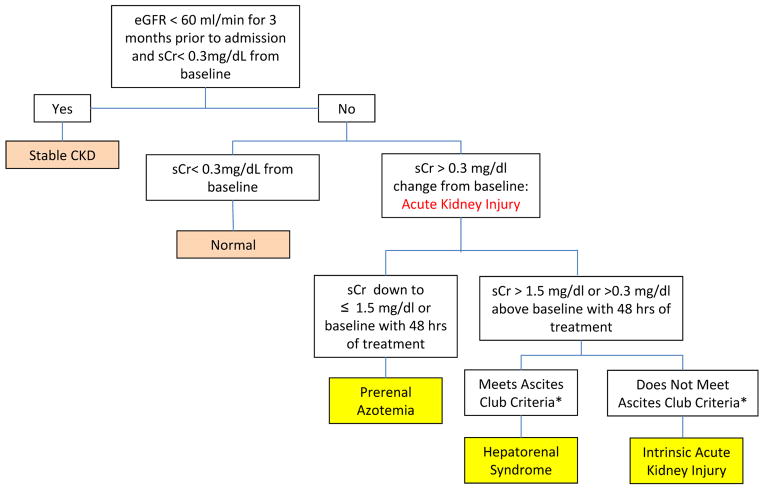
Estimated glomerular filtration rate (eGFR) < 60 ml/min over 3 months prior to hospital admission and < 0.3 mg/dL above baseline (Figure 1).
Fig. 1.
Algorithm for the categorization of kidney function.
- Presence of cirrhosis and ascites
- sCr >1.5 mg/dL (or 133 micromoles/L)
- No improvement of sCr (decrease equal to or less than 1.5 mg/dL) after at least 48 hours of diuretic withdrawal and volume expansion.
- Absence of shock, current or recent treatment with nephrotoxic drugs
- Absence of parenchymal kidney disease as indicated by proteinuria >500 mg/day, microhematuria (>50 RBCs/high power field, and/or abnormal renal ultrasound scanning
Normal Kidney Function
Stable sCr ≤ 1.5 mg/dL and < 0.3 mg/dL above baseline.
HRS
Defined by the IAC[10] and ADQI-IAC Working Group guidelines[9], including the presence of cirrhosis and ascites with an elevation of sCr > 1.5 mg/dL that failed to improve after 48 hours of diuretic withdrawal and volume expansion with albumin, in the absence of shock, nephrotoxic medications, or parenchymal kidney disease suggested by proteinuria > 500 mg/day, microhematuria (>50 red blood cells/high powered field) and/or abnormal kidney imaging. HRS type I (defined by the IAC as rapidly progressive with a doubling of the initial serum creatinine to a level greater than 2.5 mg/dl in less than 2 weeks) and HRS type II (defined by the IAC as moderate renal failure which follows a steady or slowly progressive course) were combined for analysis.
Prerenal azotemia
A transient increase in Scr to > 1.5 mg/dL and 0.3 mg/dL above baseline, with subsequent decreased in sCr to ≤ 1.5 mg/dL or to mean baseline creatinine within 48 hours of treatment with diuretic withdrawal and intravenous hydration.
iAKI
Defined as acute elevation in Scr to > 1.5 mg/dL and 0.3 mg/dL above baseline, not responding with 48 hours of volume resuscitation and not meeting the criteria for HRS.
Laboratory Measurements
Urine samples were centrifuged at 12,000 rpm for 10 minutes and the supernatant was stored at −80°C. uNGAL together with standards (0.3 to 3 ng) of human recombinant NGAL protein was quantified by immunoblot with nonreducing 4–20% gradient polyacrylamide gels (Bio-Rad Laboratories, Hercules, California) and monoclonal antibodies (1:10,000; Antibody Shop, BioPorto Diagnostics, Gentofte, Denmark). Immunoblots are required to document the monomeric form of uNGAL. Scr was assayed in the clinical laboratory by the Jaffe reaction. Urine creatinine was measured by colorimetric assay (BioAssay Systems, Hayward, CA) and was used to calculate eGFR with the Modification of Diet in Renal Disease (MDRD) formula.[32] Urine sodium was measured by ion-selective electrode assay (Olympus AU270, Center Valley, PA) and used to determine fractional excretion of sodium (FENa).
Statistical Analysis
The primary outcomes were inpatient mortality and the diagnosis of kidney function category. Secondary outcomes included a composite of mortality and liver transplant, initiation of HD, a composite of mortality and HD and admission to the intensive care unit (ICU). Continuous variables including biomarker levels were compared across renal function groups by nonparametric testing (ranksum), and categorical variables by Χ2.
Univariate and multiple logistic regression models were used to evaluate relationships between uNGAL and clinical outcomes. As biomarker levels were not clearly normally distributed, receiver operating characteristic (ROC) curves were generated to determine biomarker (uNGAL, sCr and FENa) cutoffs which maximized their sensitivity and specificity in the prediction of mortality. These cutoffs were used in multiple logistic regression modeling of clinical outcomes. Additional predictors of clinical outcomes tested include age, MELD score, etiology of liver disease, renal function group, admission serum total bilirubin, international normalized ratio (INR), and the presence of ascites or varices. In addition, pre-specified interactions between biomarkers (uNGAL, sCr, FeNa) and HRS or iAKI as kidney failure type were tested. All variables which predicted mortality with p<0.2 or thought to be central to the analysis were included in the multivariable model building. Variables that were no longer significant at p<0.05 or not essential to the analysis were sequentially removed. As MELD was calculated directly from sCr values, these two variables were not included in the same multivariable models (R2=0.5).
Relationships between uNGAL and outcomes were evaluated using both uNGAL level and the uNGAL/urinary creatinine ratio, which were highly correlated (R2 = 0.91), and hence we report only the uNGAL level. Statistics were performed using Stata 10.0 (College Station, TX).
Results
Cohort Characteristics
One-hundred eighteen patients were enrolled. The median age was 56 years, 61% were male and 45% had hepatitis C virus (HCV) (Table 1). Complications of cirrhosis were common, including ascites (70%), esophageal varices (52%) and hepatocellular carcinoma (15%). Median MELD on admission was 18, 19% had a baseline eGFR < 60 ml/min, and median length of stay was 6 days. Patients with infections other than urinary tract infection were included. Twenty-five (21%) had active infection on admission (8 spontaneous bacterial peritonitis [SBP], 4 cholangitis/cholecystitis, 5 cellulitis, 3 pneumonia, 2 colitis, 1 osteomyelitis, 1 abdominal abscess and 1 necrotizing fasciitis), 6 complicated by bacteremia (Table 1). All patients with HRS had a diagnostic paracentesis on admission, but only two were consistent with SBP. Six HRS patients had infections in the month prior to admission that were completely treated by the time of presentation. uNGAL did not differ significantly in patients with or without infection [median (IQR) 50 (20–250) ng/ml v. 30 (10–75) ng/ml, respectively, p=0.10].
Table 1.
Patient characteristics at enrollment upon hospital admission.
| Whole Cohort n=118 |
Normal n=52 |
Stable CKD n=14 |
Prerenal n=17 |
HRS n=20 |
iAKI n=15 |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Age, median (IQR) | 56 (49–62) | 54.5 (46–60.5) | 59 (53–71) | 55 (50–67) | 57 (54.5–64.5 | 55 (45–63) |
|
| ||||||
| Male gender, n (%) | 72 (61) | 37 (71) | 7 (50) | 9 (53) | 8 (40) | 11 (73) |
|
| ||||||
| Etiology of Cirrhosis, n (%) | ||||||
| Hepatitis C (HCV) | 53 (45) | 23 (44) | 6 (43) | 8 (47) | 11 (55) | 5 (33) |
| Alcohol | 22 (19) | 8 (15) | 2 (14) | 6 (35) | 2 (10) | 4 (27) |
| HCV and alcohol | 13 (11) | 6 (12) | 3 (21) | 1 (6) | 2 (10) | 1 (7) |
| Cryptogenic/NAFLD | 12 (10) | 6 (12) | 2 (14) | 2 (12) | 2 (10) | 0 (0) |
| HBV | 4 (3) | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (13) |
| Autoimmune hepatitis | 5 (4) | 2 (4) | 1 (7) | 0 (0) | 2 (10) | 0 (0) |
| Other | 9 (8) | 5 (10) | 0 (0) | 0 (0) | 1 (5) | 3 (20) |
|
| ||||||
| Race/Ethnicity, n (%) | ||||||
| Non-Hispanic White | 34 (29) | 13 (25) | 3 (21) | 8 (47) | 7 (35) | 3 (20) |
| Hispanic White | 53 (45) | 27 (52) | 8 (57) | 6 (35) | 7 (35) | 5 (33) |
| Black | 16 (14) | 6 (12) | 3 (21) | 2 (12) | 1 (5) | 4 (27) |
| Other | 15 (13) | 6 (12) | 0 (0) | 1 (6) | 5 (25) | 3 (20) |
|
| ||||||
| Complications of cirrhosis, n (%) | ||||||
| Ascites | 83 (70) | 28 (54) | 9 (64) | 15 (88) | 20 (100) | 11 (55) |
| Esophageal varices | 61 (52) | 34 (65) | 5 (36) | 8 (47) | 9 (45) | 5 (33) |
| Hepatocellular carcinoma | 18 (15) | 6 (12) | 4 (29) | 4 (24) | 2 (10) | 2 (13) |
| TIPS | 15 (13) | 6 (12) | 2 (15) | 1 (6) | 3 (15) | 3 (20) |
|
| ||||||
| Laboratories, median (IQR) | ||||||
| Total bilirubin (mg/dL) | 3.35 (1.5–7) | 2.55 (1.35–4.8) | 1.45 (0.8–2.6) | 4.3 (2.7–9.4) | 5.4 (1.6–10.55) | 6.7 (1.2–9.1) |
| INR | 1.53 (1.23–1.9) | 1.43 (1.23–1.68) | 1.23 (1.08–1.44) | 1.71 (1.56–1.93) | 1.93 (1.7–2.25) | 1.45 (1.16–2.39) |
|
| ||||||
| MELD, median (IQR) | 18 (13–24) | 14.5 (10.5–18) | 14.5 (12–16) | 22 (19–25) | 25 (22.5–34) | 24 (19–35) |
|
| ||||||
| Baseline eGFR<60, n (%) | 22 (19) | 0 (0) | 14 (100) | 1 (6) | 4 (24) | 3 (23) |
|
| ||||||
| Active infection on admission, n (%) | 25 (21) | 11 (21) | 2 (14) | 3 (18) | 2 (10) | 7 (47) |
|
| ||||||
| Length of stay (days), median (IQR) | 6 (3–11) | 4 (2–8) | 5 (3–10) | 7 (3–17) | 14.5 (6–18) | 6 (4–14) |
|
| ||||||
| Mortality, n (%) | 15 (13) | 1 (2) | 1 (7) | 0 (0) | 9 (60) | 4 (27) |
Overall, 52 (44%) patients had normal kidney function, 14 (12%) had stable CKD, and 52 (44%) had AKI. Among these 52 AKI patients, 17 (14%) were diagnosed with prerenal azotemia, 20 (17%) with HRS and 15 (13%) with iAKI.
Relationships between Biomarkers and Type of Kidney Function
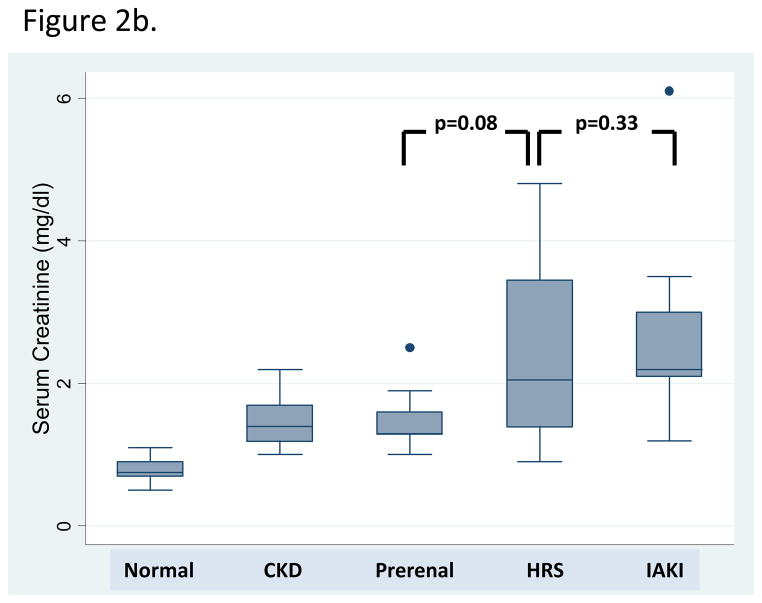
Although sCr levels were elevated in patients with kidney dysfunction, sCr poorly differentiated AKI type (Table 2, Figure 2). sCr was significantly higher in patients with iAKI compared to those with normal kidney function and stable CKD, but statistically similar to those with prerenal azotemia and HRS. sCr was significantly higher in patients with HRS compared to prerenal azotemia but similar to patients with iAKI. In addition, FENa was significantly higher in patients with iAKI than in patients either with HRS or prerenal azotemia. However, mean FENa in all groups was < 1%, the range classically associated with volume depletion and not tubular injury; therefore making iAKI detection by FENa alone non-specific. FENa levels did not discriminate patients with HRS from those with prerenal azotemia.
Table 2.
Levels of kidney function biomarkers by adjudicated categories of kidney function
| Adjudicated Kidney Function Category | |||||
|---|---|---|---|---|---|
| Biomarker, Median (IQR) | Normal n=52 |
Stable CKD n=14 |
Prerenal n=17 |
HRS n=20 |
iAKI n=15 |
| uNGAL (ng/mL) | 20 (10–47.5) | 50 (10–70) | 20 (15–45) | 105 (27.5–387.5)** | 325 (100–700)*** |
| sCr (mg/dL) | 0.75 (0.7–0.9)*** | 1.6 (1.5–1.7) | 1.3 (1.3–1.6) | 2.05 (1.4–3.45)** | 2.2 (2.1–3) |
| FENa (%) | 0.28 (0.11– 0.81)* | 0.66 (0.27– 2.0)* | 0.22 (0.10– 0.77) | 0.17 (0.09–0.33) | 0.7 (0.27–2.7)* |
Values significantly different from that for prerenal azotemia with
p<0.05,
p≤0.01 or
p≤0.001.
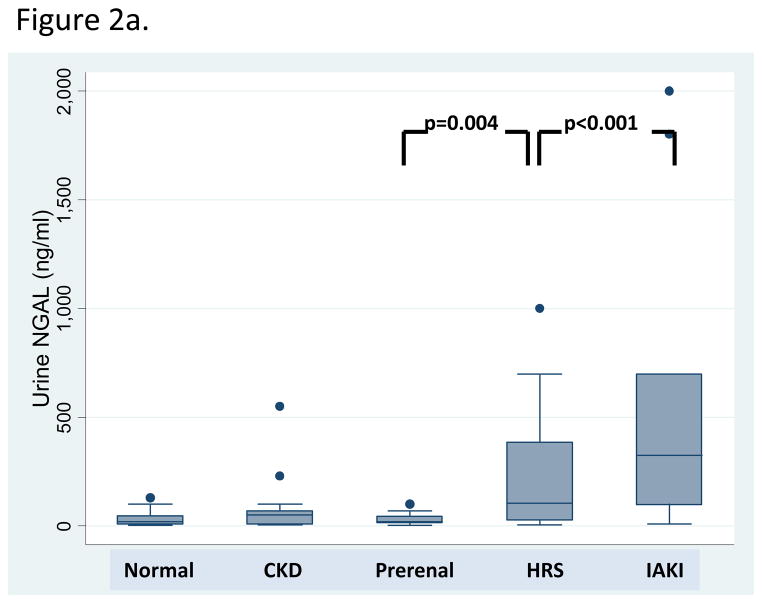
Fig. 2.
Box plot representation of (A) uNGAL and (B) serum creatinine biomarker levels across kidney function groups.
In contrast, uNGAL levels discriminated type of AKI. uNGAL levels were highest in patients with iAKI, significantly different from patients with normal kidney function, stable CKD, prerenal azotemia and HRS. In HRS, uNGAL was intermediate between, and significantly different from, patients either with iAKI (p<0.001) or prerenal azotemia (p=0.004). In patients with prerenal azotemia, uNGAL levels were low and equivalent to levels in patients with normal kidney function and stable CKD; no patient with prerenal azotemia had a uNGAL level > 100 ng/mL.
Receiver operator characteristic curve (ROC) analysis was performed to test each biomarker’s ability to identify patients with iAKI. uNGAL and sCr similarly discriminated iAKI (AUC 0.86 v. 0.89, respectively, p=0.6). uNGAL discriminated iAKI better than FENa (AUC 0.71, p=0.1), though this difference was not significant. At a cutoff of 110 ng/ml, uNGAL was 88% sensitive and 85% specific for the diagnosis of non-prerenal AKI.
Prediction of Clinical Outcomes
Fifteen (13%) patients died in the hospital, 8 (7%) underwent inpatient liver transplant, 13 (11%) required HD and 26 (22%) required intensive care unit admission (Table 3). Mortality was significantly higher for patients with HRS (60%, p<0.001) or iAKI (27%, p=0.02) compared to the rest of the cohort. Median (IQR) uNGAL [300 (50–600) ng/ml v. 30 (10–70) ng/ml, p=0.004] and sCr [2.5 (1.4–3.5) mg/dl v. 1 (0.8–1.6) ng/dl, p<0.001] were significantly greater in patients who died compared to those who survived until discharge, respectively.
Table 3.
Univariate logistic regression of biomarkers for the prediction of clinical outcomes.
| OR (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Biomarker | Mortality n=15 |
Mortality or Liver Transplant n=22 |
Dialysis n=13 |
Mortality or Dialysis n=18 |
Intensive Care Unit n=26 |
| uNGAL (per ng/mL) | 2.00 (1.36–2.94) | 2.01 (1.42–2.85) | 1.66 (1.14–2.41) | 1.91 (1.34–2.74) | 1.58 (1.17–2.12) |
| Scr (per mg/dL) | 2.95 (1.68–5.61) | 3.69 (2.04–6.68) | 2.50 (1.47–4.26) | 3.65 (2.00–6.67) | 2.11 (1.33–3.34) |
| MELD | 1.51 (1.07–1.24) | 1.18 (1.10–1.28) | 1.10 (1.04–1.17) | 1.16 (1.08–1.25) | 1.08 (1.02–1.13) |
| FENa (%) | 0.32 (0.07–1.48) | 1.07 (0.92–1.25) | 1.13 (0.95–1.34) | 1.10 (0.94–1.29) | 1.05 (0.90–1.22) |
In univariate logistic regression, uNGAL, sCr and MELD were associated with poor clinical outcomes, while FENa was not (Table 3). In multiple logistic regression analysis, uNGAL at a cutoff of 110 ng/ml and a diagnosis of HRS were independent predictors both of inpatient mortality and of the composite of inpatient mortality and liver transplant (Table 4). In contrast, sCr > 1.5 mg/dL and MELD > 17 did not independently predict mortality.
Table 4.
Multiple logistic regression models to predict inpatient mortality and the composite of inpatient mortality and liver transplantation.
| For Prediction of Mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 0 (* Rp2=0.22) | Model 1 (Rp2=0.23) | Model 2 (Rp2=0.24) | Model 3 (Rp2=0.31) | Model 4 (Rp2=0.32) | ||||||
| OR (95%CI) | p | OR (95%CI) | P | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| Age (yr) | 1.03 (0.98–1.09) | 0.25 | 1.03 (0.97–1.09) | 0.40 | 4.05 (0.98–1.11) | 0.15 | 1.03 (0.96–1.10) | 0.46 | 1.02 (0.95–1.09) | 0.56 |
| uNGAL >110 (ng/mL) | 12.5 (3.67–42.5) | <0.001 | 8.02 (2.09–30.8) | 0.002 | 8.60 (2.38–31.0) | 0.001 | 7.11 (1.78–28.3) | 0.005 | 6.05 (1.35–27.2) | 0.02 |
| Scr > 1.5 (mg/dL) | -- | 1.98 (0.93–10.0) | 0.41 | -- | -- | 1.74 (0.36–8.44) | 0.50 | |||
| MELD > 17 | -- | -- | 3.39 (0.58–19.76) | 0.18 | 1.61 (0.23–11.02 | 0.63 | -- | |||
| HRS | -- | -- | -- | 6.10 (1.43–26.0) | 0.014 | 6.71 (1.76–25.5) | 0.005 | |||
| For Prediction of Mortality or Liver Transplantation | ||||||||||
| Model 0 (Rp2=0.23) | Model 1 (Rp2=0.24) | Model 2 (Rp2=0.29) | Model 3 (Rp2=0.34) | Model 4 (Rp2=0.36) | ||||||
| OR (95%CI) | P | OR (95%CI) | p | OR (95%CI) | P | OR (95%CI) | p | OR (95%CI) | p | |
| Age (yr) | 1.02 (0.97–1.07) | 0.38 | 1.02 (0.97–1.07) | 0.45 | 1.04 (0.98–1.10) | 0.16 | 1.01 (0.95–1.07) | 0.78 | 1.02 (0.96–1.09) | 0.45 |
| uNGAL >110 (ng/mL) | 14.7 (4.93–44.0) | <0.001 | 11.0 (2.79–43.5) | 0.001 | 9.10 (2.92–28.4) | <0.001) | 10.0 (2.31–43.7) | 0.002 | 8.26 (2.41–28.3) | 0.001 |
| Scr > 1.5 (mg/dL) | -- | 1.58 (0.40–6.29) | 0.51 | -- | 1.17 (0.27–5.11) | 0.83 | -- | |||
| MELD > 17 | -- | -- | 6.74 (1.25–36.4) | 0.03 | -- | 2.58 (0.61–21.0) | 0.16 | |||
| HRS | -- | -- | -- | 8.58 (2.32–31.8) | 0.001 | 6.04 (159–22.9) | 0.008 | |||
Rp2 = pseudo R-squared
Discussion
This is the first longitudinal study to investigate uNGAL measurement for the prediction of mortality and AKI type in hospitalized patients with cirrhosis. These data suggest that a single uNGAL measurement obtained at hospitalization has the potential to assist in determining type of kidney dysfunction, perhaps informing patient management and improving outcomes. Our findings confirm that similar findings on other AKI populations[25, 27–29, 31] can be applied to patients with cirrhosis. uNGAL levels were significantly different in each category of AKI: highest in iAKI, intermediate in HRS and low in prerenal disease. Furthermore, uNGAL levels in patients with prenrenal azotemia were similar to those with normal kidney function and stable CKD. In contrast, sCr was not different between patients with iAKI and HRS and although FENa was significantly higher in patients with iAKI than in patients with either HRS or prerenal azotemia, FENa did not discriminate HRS from prerenal azotemia.
Our investigation also confirms prior studies demonstrating that a single uNGAL measurement on hospital admission independently predicted poor clinical outcomes including short-term inpatient mortality, in uni- and multivariable models. This is again in contrast to traditional measurements of kidney and liver disease severity including sCr and MELD score. If confirmed, uNGAL could improve risk stratification for patients admitted to the hospital with cirrhosis, perhaps leading to early ICU admission, transplant evaluation, and prompt initiation of HRS therapy.
This study did not strictly apply or validate the ADQI-IAC working group guidelines for AKI types, though the IAC HRS diagnostic criteria and criteria similar to the AKIN methods were used. These guidelines continue to classify HRS and prerenal disease as forms of AKI but endorsed the need for future study of “the epidemiology in terms of incidence and prevalence” of AKI in cirrhosis and for the identification of “better markers for renal dysfunction in cirrhosis such as…NGAL.” We adopted the term iAKI to differentiate HRS and prerenal disease from other forms of AKI, and thus evaluate the prevalence of these discrete syndromes, understand their individual impact on mortality and determine whether uNGAL could be used to differentiate these disease states. Our data demonstrate that hospitalized patients with cirrhosis are at a high risk for AKI (44% of the cohort, 29% of these with iAKI and 38% with HRS). Similar to limited previous reports[4], these data suggest that non-HRS types of AKI are common in patients with cirrhosis and further studies are needed to determine inciting factors of AKI in this population.
The mechanism by which patients with HRS have intermediate uNGAL levels remains uncertain. HRS physiology is classically thought to be an extreme prerenal state [33, 34] with severe renovascular vasoconstriction and decreased GFR, but normal intrinsic kidney function. Kidney function can return to normal after improvement of hepatic hemodynamics (i.e. TIPS, surgical portal-caval shunts and liver transplant),[6, 7, 35–40] or after transplantation of the kidney into a recipient with normal hepatic function.[6, 8] However, pathologic investigations have reported subtle kidney tubular and glomerular damage in HRS kidneys, some seen only with electron microscopy,[11, 41] perhaps resulting from the cellular changes associated with chronic activation of angiotensin-aldosterone signaling.[9] It is conceivable that profound renovascular constriction may cause sub-clinical tubular damage in at least a subset of nephrons, not detectable by urinary sodium, which is not sensitive enough to detect mild or patchy tubular epithelial damage. These findings contrast with prerenal azotemia, which while also demonstrating low FENa, expressed lower amounts of uNGAL, either reflecting the less severe end of a clinical spectrum that includes HRS or a qualitatively distinct entity. uNGAL elevation in patients with HRS is unlikely to be due to urine hyperconcentration as correction for urine creatinine did not alter the relationships we report.
This study has several limitations. sCr inaccurately measures kidney function in cirrhosis,[6–8] and there is no widely available technique to accurately measure GFR in these patients. This lack of a reliable gold standard may have affected our ability to differentiate types of kidney failure in the absence of kidney biopsy, which is rarely performed in this population given the risk of complications. In addition, there is no consensus on how HRS fits into the RIFLE [11] and AKIN [41] criteria. Our criteria utilized the standardized IAC definition of HRS but as a sCr of 1.5 mg/dL may be elevated for some patients, it is possible that patients with abnormal kidney function may have been classified as normal. Although difficulties in classification of kidney injury in cirrhosis are acknowledged limitations of our study and the field of AKI diagnostics in cirrhosis, utilizing standardized definitions based upon the IAC classification was thought to be essential in this first description of uNGAL in cirrhosis. This study was also limited by small sample size, especially in the important HRS and iAKI groups. This limitation likely affected our ability to detect significant differences in diagnostic test characteristics between biomarkers and larger studies for more robust data are needed to verify our findings. A larger study may also have allowed us to evaluate HRS types I and II individually. It is possible that uNGAL elevation is more prominent in type I than in type II HRS, due to more severe renal vasosconstriction. Lastly, active infection may lead to elevated uNGAL levels. However, we excluded patients with urinary tract infection, only two of our HRS patients had infection (SBP) at admission and uNGAL levels were statistically similarly between patients with current or recent infections compared to non-infected patients. The utility of uNGAL in the setting of infection must be further studied.
In conclusion, uNGAL differentiates AKI type and predicts mortality in patients with cirrhosis. uNGAL also independently predicts clinical outcomes including short-term inpatient mortality. These findings, if confirmed in larger cohorts, could lead to the development of biomarker algorithms to rapidly identify patients with HRS and more accurately predict prognosis.
Acknowledgments
Grant Support: R01 DK073462 - IRON LIPOCALIN NGAL IN KIDNEY DISEASE
Footnotes
Disclosures: Abbott Labs and Biosite Inverness have licensed NGAL from Columbia University for the detection of AKI and CKD.
References
- 1.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–2077. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 2.Hampel H, Bynum GD, Zamora E, El-Serag HB. Risk factors for the development of renal dysfunction in hospitalized patients with cirrhosis. Am J Gastroenterol. 2001;96:2206–2210. doi: 10.1111/j.1572-0241.2001.03958.x. [DOI] [PubMed] [Google Scholar]
- 3.Terra C, Guevara M, Torre A, Gilabert R, Fernandez J, Martin-Llahi M, et al. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005;129:1944–1953. doi: 10.1053/j.gastro.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 4.du Cheyron D, Bouchet B, Parienti JJ, Ramakers M, Charbonneau P. The attributable mortality of acute renal failure in critically ill patients with liver cirrhosis. Intensive Care Med. 2005;31:1693–1699. doi: 10.1007/s00134-005-2842-7. [DOI] [PubMed] [Google Scholar]
- 5.Wu CC, Yeung LK, Tsai WS, Tseng CF, Chu P, Huang TY, et al. Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis. Clin Nephrol. 2006;65:28–33. doi: 10.5414/cnp65028. [DOI] [PubMed] [Google Scholar]
- 6.Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201–205. [PubMed] [Google Scholar]
- 7.Papadakis MA, Arieff AI. Unpredictability of clinical evaluation of renal function in cirrhosis. Prospective study. Am J Med. 1987;82:945–952. doi: 10.1016/0002-9343(87)90156-2. [DOI] [PubMed] [Google Scholar]
- 8.Cholongitas E, Shusang V, Marelli L, Nair D, Thomas M, Patch D, et al. Review article: renal function assessment in cirrhosis - difficulties and alternative measurements. Aliment Pharmacol Ther. 2007;26:969–978. doi: 10.1111/j.1365-2036.2007.03443.x. [DOI] [PubMed] [Google Scholar]
- 9.Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011 doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 10.Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cereda JM, Roulot D, Braillon A, Moreau R, Koshy A, Lebrec D. Reduction of portal pressure by acute administration of furosemide in patients with alcoholic cirrhosis. J Hepatol. 1989;9:246–251. doi: 10.1016/0168-8278(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Pagan JC, Salmeron JM, Feu F, Luca A, Gines P, Pizcueta P, et al. Effects of low-sodium diet and spironolactone on portal pressure in patients with compensated cirrhosis. Hepatology. 1994;19:1095–1099. doi: 10.1002/hep.1840190506. [DOI] [PubMed] [Google Scholar]
- 14.Okumura H, Aramaki T, Katsuta Y, Satomura K, Akaike M, Sekiyama T, et al. Reduction in hepatic venous pressure gradient as a consequence of volume contraction due to chronic administration of spironolactone in patients with cirrhosis and no ascites. Am J Gastroenterol. 1991;86:46–52. [PubMed] [Google Scholar]
- 15.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 17.Gines A, Escorsell A, Gines P, Salo J, Jimenez W, Inglada L, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 18.Pageaux GP, Ducos J, Mondain AM, Costes V, Picot MC, Perrigault PF, et al. Hepatitis C virus genotypes and quantitation of serum hepatitis C virus RNA in liver transplant recipients: relationship with severity of histological recurrence and implications in the pathogenesis of HCV infection. Liver Transpl Surg. 1997;3:501–505. doi: 10.1002/lt.500030504. [DOI] [PubMed] [Google Scholar]
- 19.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 20.Martensson J, Bell M, Oldner A, Xu S, Venge P, Martling CR. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med. 2010;36:1333–1340. doi: 10.1007/s00134-010-1887-4. [DOI] [PubMed] [Google Scholar]
- 21.Parravicini E, Nemerofsky SL, Michelson KA, Huynh TK, Sise ME, Bateman DA, et al. Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late onset culture-positive sepsis in very low birth weight infants. Pediatr Res. 2010;67:636–640. doi: 10.1203/PDR.0b013e3181da75c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasilewska A, Zoch-Zwierz W, Taranta-Janusz K, Michaluk-Skutnik J. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of cyclosporine nephrotoxicity? Pediatr Nephrol. 2010;25:889–897. doi: 10.1007/s00467-009-1397-1. [DOI] [PubMed] [Google Scholar]
- 23.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 24.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 25.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 27.Wagener G, Minhaz M, Mattis FA, Kim M, Emond JC, Lee HT. Urinary neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant. 2011;26:1717–1723. doi: 10.1093/ndt/gfq770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldkamp T, Bienholz A, Kribben A. Urinary neutrophil gelatinase-associated lipocalin (NGAL) for the detection of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant. 2011;26:1456–1458. doi: 10.1093/ndt/gfr146. [DOI] [PubMed] [Google Scholar]
- 29.Portal AJ, McPhail MJ, Bruce M, Coltart I, Slack A, Sherwood R, et al. Neutrophil gelatinase--associated lipocalin predicts acute kidney injury in patients undergoing liver transplantation. Liver Transpl. 2010;16:1257–1266. doi: 10.1002/lt.22158. [DOI] [PubMed] [Google Scholar]
- 30.Yang HN, Boo CS, Kim MG, Jo SK, Cho WY, Kim HK. Urine Neutrophil Gelatinase-Associated Lipocalin: An Independent Predictor of Adverse Outcomes in Acute Kidney Injury. Am J Nephrol. 31:501–509. doi: 10.1159/000309841. [DOI] [PubMed] [Google Scholar]
- 31.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 33.Ochs A, Rossle M, Haag K, Hauenstein KH, Deibert P, Siegerstetter V, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for refractory ascites. N Engl J Med. 1995;332:1192–1197. doi: 10.1056/NEJM199505043321803. [DOI] [PubMed] [Google Scholar]
- 34.Iwatsuki S, Popovtzer MM, Corman JL, Ishikawa M, Putnam CW, Katz FH, et al. Recovery from “hepatorenal syndrome” after orthotopic liver transplantation. N Engl J Med. 1973;289:1155–1159. doi: 10.1056/NEJM197311292892201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassinello C, Moreno E, Gozalo A, Ortuno B, Cuenca B, Solis-Herruzo JA. Effects of orthotopic liver transplantation on vasoactive systems and renal function in patients with advanced liver cirrhosis. Dig Dis Sci. 2003;48:179–186. doi: 10.1023/a:1021763221337. [DOI] [PubMed] [Google Scholar]
- 36.McDonald FD, Brennan LA, Turcotte JG. Severe hypertension and elevated plasma renin activity following transplantation of “hepatorenal donor” kidneys into anephric recipients. Am J Med. 1973;54:39–43. doi: 10.1016/0002-9343(73)90081-8. [DOI] [PubMed] [Google Scholar]
- 37.Koppel MH, Coburn JW, Mims MM, Goldstein H, Boyle JD, Rubini ME. Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. Evidence for the functionalnature of renal failure in advanced liver disease. N Engl J Med. 1969;280:1367–1371. doi: 10.1056/NEJM196906192802501. [DOI] [PubMed] [Google Scholar]
- 38.Mandal AK, Lansing M, Fahmy A. Acute tubular necrosis in hepatorenal syndrome: an electron microscopy study. Am J Kidney Dis. 1982;2:363–374. doi: 10.1016/s0272-6386(82)80096-6. [DOI] [PubMed] [Google Scholar]
- 39.Kanel GC, Peters RL. Glomerular tubular reflux--a morphologic renal lesion associated with the hepatorenal syndrome. Hepatology. 1984;4:242–246. doi: 10.1002/hep.1840040212. [DOI] [PubMed] [Google Scholar]
- 40.Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004;66:1–9. doi: 10.1111/j.1523-1755.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 41.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]