Abstract
BACKGROUND
Lowering blood pressure (BP) prevents stroke, however optimal target levels of blood reduction to prevent stroke recurrence are lacking. We hypothesized that targeting systolic BP of <130 mmHg would reduce stroke recurrence in patients with recent lacunar stroke.
METHODS
The Secondary Prevention of Small Subcortical Strokes (SPS3) was a multi-center international trial, involving 3020 patients with recent symptomatic MRI-defined lacunar infarcts randomized to two target levels of systolic BP: a) higher group 130–149 mm Hg vs. b) lower group <130 mm Hg, and followed for a mean of 3.7 years. The primary outcome was all recurrent stroke (including ischemic strokes and intracranial hemorrhages). The study is registered, NCT 00059306.
FINDINGS
Mean participant age was 63 years; after 1 year mean systolic BP was 138 mm Hg (95% CI 137 to 139) in the higher group and 127 mm Hg (95% CI, 126 to 128) in the lower group. At last study visit, the difference in systolic BP between groups averaged 11 mm Hg (±SD 16). The annualized rate of recurrent stroke in the higher target group was 2.77% (n=152) compared with 2.25% (n=125) in the lower target group (HR 0.81, 95% CI 0.64, 1.03, p-value 0.08). Similar trends were observed for reductions in disabling/fatal stroke (HR 0.81, 95%CI 0.53, 1.23, p-value 0.32) and in the composite outcome of stroke, myocardial infarct or vascular death (HR 0.84, 95%CI 0.68,1.01, p-value 0.10). Intracerebral hemorrhage was reduced by 63% in those assigned to the lower target group (HR 0.37 95% CI, 0.14, 0.89, p-value 0.03). Serious complications of BP lowering were in frequent, and not significantly different in frequency between groups.
INTERPRETATION
In patients with recent lacunar stroke, targeting asystolic BP of< 130 mm Hg did not significantly reduce all stroke, but markedly reduced intracerebral hemorrhage. The lower target was safe and well tolerated.
INTRODUCTION
Hypertension is the single most powerful and prevalent risk factor for stroke, particularly for stroke associated with cerebral small vessel disease. Blood pressure (BP) reduction is the most effective intervention to prevent stroke.1–3
Small subcortical brain infarcts, commonly known as lacunar strokes, comprise about 25% of ischemic strokes.4, 5 Most result from intrinsic disease of the small penetrating arteries. Despite their frequency and importance, no previous randomized trials have focused on secondary prevention of stroke in patients with MRI-defined lacunar stroke. Optimal target levels of BP control to prevent stroke recurrence in patients with cerebral small artery disease are lacking.6
The SPS3 trial tested two randomized interventions in a 2-by-2 factorial design in patients with recent symptomatic MRI-confirmed lacunar stroke: clopidogrel and aspirin vs. aspirin alone and two target levels of systolic BP. The results of the antiplatelet component have been published previously.7 The results of the BP target component are presented here.
METHODS
Details of the rationale, study design and participant characteristics of SPS3 have been described elsewhere.8, 9 In brief, SPS3 was a randomized, multicenter clinical trial conducted in 81 clinical centers in North America, Latin America, and Spain. Patients aged 30 years or older with a recent (≤180 days) symptomatic lacunar stroke who were without surgically-amenable ipsilateral carotid artery stenosis or major-risk cardioembolic sources were eligible and randomized, in a 2-by-2 factorial design, to both the antiplatelet intervention (double-blind) and to one of two target levels of systolic BP control; higher(130–149 mmHg) vs. lower (<130 mmHg). The blood pressure trial was open label using the PROBE study design.10 To avoid BP lowering in proximity to an acute stroke, participants were randomized at least two weeks after the qualifying stroke. Participants with a clinical lacunar syndrome were required to meet MRI criteria. Main exclusion criteria included disabling stroke (modified Rankin Scale ≥4), previous intracranial hemorrhage (excluding traumatic), or cortical ischemic stroke.7, 8 Participation required written informed consent and approval by local human research subjects committees. Randomization assignments, stratified by clinical center and baseline hypertensive status, were generated using a permuted-block design (variable block size) and protected from previewing.
Blood pressure management
Both normotensive and hypertensive patients were eligible for SPS3. Hypertension status was determined at study entry by BP measurements done at two consecutive visits prior to randomization. BP was measured three times at each visit, and the average of these three measurements was used to determine hypertensive status; BP medications were not discontinued.8, 11. Patients were classified as hypertensive if either or both were present: i) average BP from the two consecutive SPS3 visits was ≥140 mmHg systolic or ≥90 mmHg diastolic; ii) definite history of hypertension prior to the qualifying stroke and on antihypertensive medication at the time of visit. Following randomization, all patients were seen at least monthly for the first three months and on a quarterly basis there after for measurement of BP and adjustment of medications to reach assigned target. Participants who were not within their assigned target were seen at least monthly for blood pressure measurement and medication adjustments until their blood pressure was within the assigned target for two consecutive visits, after which they continued with the quarterly schedule. If at any subsequent quarterly visit their systolic blood pressure fell outside their assigned target, they returned for a blood pressure visit within one month. If that measurement was within the assigned target, they resumed the quarterly schedule. Sites were provided with an automated electronic device, the Colin 8800C.11 Blood pressure management was overseen at the clinical sites by a physician with special expertise in blood pressure control. If patients assigned to the higher target (130–149 mmHg) group were below target and on blood pressure lowering medications, the protocol required that antihypertensive medications be discontinued or their dose reduced unless prescribed for reasons other than blood pressure control. Those who were below target in the higher group and on no antihypertensive medications continued to be followed quarterly and if their systolic blood pressure increased, they were managed according their originally assigned target. Patients were designated “inactive” if they or their primary care physicians refused to have their blood pressure titrated to their assigned target per protocol. Patients were designated “failure to achieve assigned target” in the event of medical reasons that their blood pressure could not be managed into their assigned target or for patients who suffered intolerable side-effects of anti-hypertensive drugs, despite trying multiple agents. All participants were followed to a common end-study date, irrespective of active/inactive status or failing to achieve their target.
Antihypertensive medications were provided to study participants as prescribed by their local study physician. The medications available in the study formulary included at least one drug from major classes of antihypertensive medications, obtained and distributed by the Cooperative Studies Program Clinical Research Coordinating Center, Drug Distribution Center in Albuquerque, NM.
Outcome Events
Ischemic stroke was clinically defined as a focal neurological deficit persisting for greater than 24 hours, with absence of hemorrhage documented by neuro-imaging. Intracranial hemorrhages included intracerebral, subdural/epidural and subarachnoid locations as defined by neuro-imaging. Disabling strokes were those with modified Rankin scores of ≥3 assessed after 3 to 6 months due to recurrent stroke. Strokes were counted as fatal if death occurred within 30 days or if death after 30 days was attributable to the stroke. Secondary outcomes included acute myocardial infarct, defined by standard criteria consisting of a compatible clinical history combined with ECG and/or cardiac enzyme changes and requiring acute hospitalization and death was classified as vascular, non-vascular, or unknown. Safety outcomes were serious complications of hypotension and those related to the use of BP medications.
All reported efficacy outcomes were confirmed by a central adjudication committee that was unaware of treatment assignment.
Sample size estimates and statistical analysis
The initial sample size of 2500 patients was calculated assuming an average follow-up of three years, an estimated 3-year recurrent stroke rate of 21%, a 25% relative risk reduction in stroke by intensive BP control, a type I error of 0.05, and a 90% power. Sample size re-estimation, performed midway through the trial to assess power based on the currently observed overall event rate in the study, resulted in an increase in the sample size from 2500 to 3000 patients. Details of the sample size estimation were described elsewhere.12
We hypothesized that assignment to the lower target systolic BP would result in a reduction in stroke recurrence (the combination of ischemic strokes and all intracranial hemorrhages, including subdural hematomas). In addition, there were two pre-specified subgroup analyses: a) excluding participants who did not meet criteria for hypertension at entry, i.e. those who had systolic BP of <130mmHg while not taking BP lowering medications. These patients were randomized, but they were not treated with antihypertensive medications unless BP exceeded the assigned target range during follow-up; and b) censoring follow-up for the initial 6 months following randomization justified by maximal separation of the achieved BP requiring an average of 6 months of medication titration. Participants, who did not die or withdraw from the study during the first 6 months irrespective of whether or not they had an event during this time, were included.
The primary analyses used standard time-to-event methods with each treatment arm assessed using the log-rank test and Cox proportional hazards models to compute hazard ratios. Time to event was computed as time to first event if multiple events of the same type occurred, and for the composite endpoint of stroke, myocardial infarction, or vascular death. Patients not experiencing events were censored at the time of termination of study participation or death. The proportional hazards assumption was verified by assessing the interaction between time and the BP intervention group. Interactions between covariates and blood pressure intervention group were evaluated with the use of Cox models to determine if effect of intervention differed by specific subgroups. All analyses were based on the intention-to-treat principle.
The trial was monitored by an independent data and safety monitoring committee selected by the sponsor.
RESULTS
Between 2003 and 2011, 3020 participants were entered from North America (n=1960; 65%), Latin America (n=694; 23%) and Spain (n=366; 12%) and followed for a mean of 3.7 (range 0–8.6; ±SD 2) years(Supplementary materials. Figure 1). The mean participant age was 63 (±SD11) years, 63% were men, and history of hypertension, diabetes, and current tobacco smoking were present in 75%, 37% and 20%, respectively(Table 1). The median time from qualifying stroke to randomization was 62 days. At study entry, the average systolic BP was 144mm Hg (95%, CI143 to 145) in the higher group and 142 mm Hg (95%, CI 141 to 143) in the lower group with the same mean number of BP medications used by both groups (1.7, ±SD1.2). Of 3020 participants, 314 were normotensive at entry.
Table 1.
Patient characteristics
| Higher Target 130–149 mmHg (N=1519) | Lower Target ≤130 mmHg (N=1501) | |
|---|---|---|
|
| ||
| Mean age, years | 63 (11) | 63 (11) |
|
| ||
| Men, % | 65 | 61 |
|
| ||
| Blood pressure at entry, mean | ||
|
| ||
| -Systolic | 144 (19) | 142 (19) |
|
| ||
| -Diastolic | 79 (11) | 78 (10) |
|
| ||
| Body-mass index | 29 (8) | 29 (6) |
|
| ||
| History of hypertension, % | 75 | 75 |
|
| ||
| Diabetes, % | 36 | 37 |
|
| ||
| Ischemic heart disease, % | 11 | 10 |
|
| ||
| Prior clinical stroke or TIA, % | 14 | 16 |
|
| ||
| Current tobacco smoker, % | 20 | 21 |
|
| ||
| Qualifying event | ||
| - ischemic stroke, % | 99 | 98 |
| - TIA, % | 1 | 2 |
|
| ||
| Ethnicity/Race | ||
|
| ||
| -White, % | 50 | 52 |
|
| ||
| - Hispanic, % | 31 | 30 |
|
| ||
| - Black, % | 17 | 16 |
|
| ||
| - Other, % | 3 | 2 |
|
| ||
| Region | ||
|
| ||
| - North America, % | 65 | 65 |
|
| ||
| - Latin America, % | 23 | 23 |
|
| ||
| - Spain, % | 12 | 12 |
|
| ||
| Anti-hypertensive medications at study entry | ||
|
| ||
| Mean number | 17 (1.2) | 1.7 (1.2) |
|
| ||
| Anti-hypertensive medications at 1 yr^^ | ||
|
| ||
| Mean number | 1.8 (1.4) | 2.4 (1.3) |
|
| ||
| -thiazides, % | 43 | 58 |
|
| ||
| -ACEI/ARB, % | 63 | 80 |
|
| ||
| -Calcium Channel Blockers, % | 30 | 43 |
|
| ||
| -Beta blockers, % | 25 | 31 |
|
| ||
| -Other, % | 9 | 11 |
|
| ||
| Anti-hypertensive medication @ last visit** | ||
|
| ||
| Mean number | 1.8 (1.4) | 2.4 (1.4) |
|
| ||
| -thiazides, % | 38 | 54 |
|
| ||
| -ACEI/ARB, % | 60 | 78 |
|
| ||
| -Calcium Channel Blockers, % | 39 | 43 |
|
| ||
| -Beta blockers, % | 28 | 35 |
|
| ||
| -Other, % | 11 | 14 |
|
| ||
| Statins during follow up, % | 84 | 85 |
At one year mean number and all categories p<0.0001, except beta blockers (p=0.0008), and other, p=0.051. At last visit: mean number and all categories p<0.0001, except other p=0.042.
Permanent discontinuation of BP therapy occurred in17% of the higher group participants and 16% of the lower group (p=0.20). Three percent of participants were lost to follow-up, with an additional number ending follow-up early for other reasons (8% withdrew consent, 5% site closure, 0.4% physician request, 2% other reasons).
Blood pressure management
At one year of follow up the achieved average systolic BPs were 138 mm Hg (95%, CI 137 to 139) and 127 mm Hg (95% CI, 126 to 128) for the higher and lower groups respectively with 75% of the higher group and 65% of the lower being in assigned target range. At the last study visit, the average systolic BP difference between the two groups was 11 mm Hg (±SD 16)(Figure 1). Those assigned to the lower target averaged a greater number of antihypertensive drugs (Table 1). The mean number of medications during the course of the study averaged 1.8 for the higher target vs. 2.4 for the lower target. (Figure 1).
Figure 1.
Systolic blood pressure by treatment group.
Outcomes
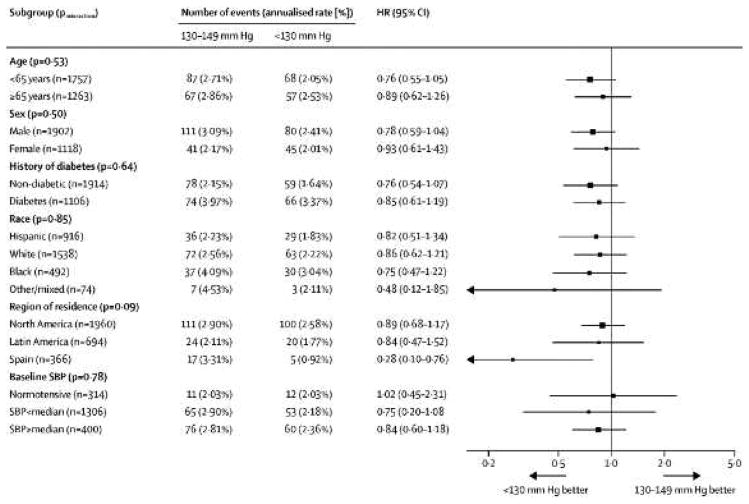
During follow-up, 277 first recurrent strokes occurred. Of first recurrent strokes, 86% (n=243) were ischemic and 14% (n=34) were intracranial hemorrhages. The annualized rate of recurrent stroke among those assigned to the higher target was 2.77% as compared with 2.25% in the lower target group (HR 0.81, 95% CI 0.64, 1.03 p-value 0.08) (Table 2, Figure 2). There was no heterogeneity of treatment effect on the primary outcome according to age, sex, or among the pre-specified subgroups (Figure 3). Non-significant trends of similar magnitude for higher vs. lower target BP were seen for disabling/fatal stroke (HR 0.81, 95%CI 0.53, 1.23 p-value 0.32) and for the composite outcome of stroke, myocardial infarct or vascular death (HR 0.84, 95%CI 0.68,1.01 p-value 0.10 ) The risk of intracerebral hemorrhage was significantly lower in those assigned to the lower target group (6 vs. 16 events) (HR 0.37 95% CI; 0.15, 0.95 p-value 0.03), while mortality rates were nearly identical. (Table 2).
Table 2.
Primary Outcomes
| Higher Target (n=1519) | Lower Target (n=1501) | HR (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| N | Rate (%/pt-yr) | N | Rate (%/pt-yr) | |||
| All stroke (ischemic & hemorrhage) | 152 | 2.77 | 125 | 2.25 | 0.81 (0.64, 1.03) | 0.08 |
| - ischemic stroke/unknown* | 131 | 2.4 | 112 | 2.0 | 0.84 (0.66, 1.09) | 0.19 |
| - intracranial hemorrhage | 21** | 0.38 | 13^^ | 0.23 | 0.61 (0.31, 1.22) | 0.16 |
| - intracerebral | 16 | 0.29 | 6 | 0.11 | 0.37 (0.15,095) | 0.03 |
| - subdural/epidural | 5 | 0.091 | 6 | 0.11 | 1.18 (0.36,3.88) | 0.78 |
| - other | 2 | 0.036 | 4 | 0.072 | 1.97 (0.36, 10.74) | 0.43 |
| Disabling/fatal stroke* | 49 | 0.89 | 40 | 0.72 | 0.81 (0.53, 1.23) | 0.32 |
| Myocardial infarct | 40 | 0.70 | 36 | 0.62 | 0.88 (0.56, 1.39) | 0.59 |
| Major vascular events** | 188 | 3.46 | 160 | 2.91 | 0.84 (0.68, 1.04) | 0.10 |
| Deaths (all) | 101 | 1.74 | 106 | 1.80 | 1.03 (0.79, 1.35) | 0.82 |
| -Vascular death | 41 | 0.70 | 36 | 0.61 | 0.86 (0.55, 1.35) | 0.52 |
| -Non-vascular | 35 | 0.60 | 40 | 0.68 | 1.12 (0.71, 1.76) | 0.62 |
| -Uncertain | 25 | 0.43 | 30 | 0.51 | 1.18 (0.69, 2.00) | 0.55 |
1 classified as both intracerebral and other; 1 classified as both intracerebral and subdural/epidural
1 classified as both intracerebral and subdural/epidural; 2 classified as both intracerebral and other
Figure 2.
Probability of patients experiencing a primary event by time after randomization. Primary events were all recurrent strokes, myocardial infarction, or vascular death. HR=hazard ratio.
Figure 3.
Primary outcome assessed by demographic and clinical subgroups. HR=hazard ratio. SBP=systolic blood pressure.
Subgroup analysis restricted to the 2706 participants classified as hypertensive at study entry showed a 20% reduction in recurrent stroke(HR 0.80, 95% CI 0.62, 1.02, p-value 0.07) (Figure 4). Censoring the first 6 months of follow up in all participants showed a nearly identical hazard ratio for recurrent stroke(HR 0.81, 95% CI 0.62, 1.06). BP lowering offered similar effects on stroke recurrence irrespective of stroke sub-type. The effect on lacunar infarcts, comprising 71% of the recurrent ischemic strokes was a 13% reduction (HR 0.87 (95% CI: 0.62, 1.22 p-value 0.41). There was no interaction between the antiplatelet and BP target interventions (p-value 0.46)
Figure 4.

Randomised trials of long-term blood-pressure lowering for secondary stroke prevention.
Adverse events
Serious complications of BP lowering were infrequent (<2%) but more often seen among those assigned to the lower target (23 vs. 15 events) (HR 1.53, 95% CI 0.80–2.93) (Table 3). Syncope was the most common (0.5% vs. 0.8% among those assigned lower vs. higher targets, respectively, p-value 0.14), but did not result in permanent sequelae. Neither association was statistically significant. Symptoms potentially related to BP reduction reported at each follow up were similar in both groups (Table 3).
Table 3.
Safety outcomes
| Higher Target (N =1519) | Lower Target (N =1501) | HR (95%CI) | p value | |||
|---|---|---|---|---|---|---|
| N | Rate (%/pt-yr) | N | Rate (%/pt-yr) | |||
| Serious complications of hypotension | 15 | 0.26 | 23 | 0.40 | 1.53 (0.80, 2.93) | 0.20 |
| -orthostatic syncope | 5 | 0.087 | 11 | 0.19 | 2.18 (0.76, 6.27) | 0.14 |
| -stroke associated with hypotension | 1 | 0.017 | 2 | 0.034 | 2.00 (0.18, 22.09) | 0.57 |
| -MI associated with hypotension | 0 | N/A | 0 | N/A | N/A | N/A |
| -fall with injury secondary to hypotension | 0 | 0.00 | 3 | 0.052 | N/A | N/A |
| -other | 11 | 0.19 | 9 | 0.15 | 0.82 (0.34, 1.97) | 0.65 |
| Serious complications related to antihypertensive medications | 0 | 0.00 | 1* | 0.017 | N/A | N/A |
| Side effects related to blood pressure management (ever reported), % | N | % | N | % | OR (95% CI) | p value |
| -unsteadiness when standing | 355 | 24 | 375 | 26 | 1.09 (0.92, 1.29) | 0.31 |
| -blurred vision when standing | 103 | 7 | 85 | 6 | 0.82 (0.61, 1.11) | 0.19 |
| -dizziness when standing up | 304 | 21 | 324 | 22 | 1.10 (0.92, 1.31) | 0.30 |
| -lightheadedness when standing | 236 | 16 | 222 | 15 | 0.94 (0.77, 1.15) | 0.54 |
| -palpitations when standing | 24 | 0.41 | 21 | 0.36 | 0.86 (0.48, 1.55) | 0.62 |
Bradycardia requiring hospitalization.
DISCUSSION
Lowering systolic BP to a target of <130mmHg in patients with recent lacunar stroke resulted in statistically non-significant trends toward reductions in all stroke, disabling/fatal stroke, and major vascular events. These effects were of clinically important magnitude, and assignment to the lower target was associated with few serious side-effects. The relative effects of assignment to the lower target were consistent among major participant subgroups, including subjects with diabetes mellitus, Hispanics, and regardless of systolic BP at entry (Figure 3). Excluding patients who were normotensive at entry (10% of participants), recurrent stroke was reduced by 20% (p-value 0.07) among those assigned to the lower BP target relative to those in the higher BP target group (Figure 4).
It is a general construct that “lower is better” for chronic BP management after stroke, but optimal clinical practice requires that benefit and disutility associated with specific targets be defined. Results of PROGRESS showed that lowering BP in stroke survivors was associated with an important reduction of 28% in stroke recurrence. The mean achieved systolic BP at the end of the study was 138 mmHg, but the optimal target of BP control was not established.2 Similar to the recent ACCORD trial,13 the SPS3 trial explored the efficacy and safety of targeting systolic BPs below 130 mmHg and, uniquely, in patients with MRI-defined lacunar stroke attributed to small vessel disease.
The results of the SPS3 BP target trial are best considered in the context of prior trials of long-term BP lowering in patients with prior brain ischemia1–3, 14–19(Supplementary material Table 1). While SPS3 tested target levels (not specific antihypertensive agents) and explored BP lowering in patients with well-defined ischemic stroke subtype, the magnitude of the reduction in stroke observed in SPS3, although not statistically significant, is strongly supported by these previous trials testing BP lowering after stroke.1–3
The trial protocol was based on achieving the assigned target of systolic BP, and the use of specific antihypertensive agents was not specified. Those assigned to the lower target used an average of 2.4 antihypertensive medications, with a different distribution of medication categories between groups (Table 1). The mean achieved difference of systolic BP over the course of the trial was 11mmHg; based on previous studies, it was anticipated that this difference would result in about a 30% reduction in recurrent stroke. The observed reduction of 19% (95% CI −3, 36%) was smaller than the hypothesized 25%. This could be due to chance or the specific patient population tested.2, 20 While the confidence interval for the observed 19% reduction (95% CI −3% to 36%) includes the hypothesized 25% reduction, it also spans 0% and thus is not statistically significant. Intracerebral hemorrhage was reduced by 63%, consistent with the known sensitivity of this stroke subtype to strict blood pressure control. 15 The intracerebral hemorrhage results indicate that the NNT to prevent one intracerebral hemorrhage at four years (about the average follow-up in SPS3) is 175.
The SPS3 blood pressure trial had limitations. First, the observed stroke rate was about 50% of anticipated. The relatively low stroke recurrence rate is similar to that seen in recent trials for prevention of recurrent stroke; this may be the result of good blood pressure control in both treatment arms, the frequent use of statins, and high adherence to antiplatelet therapy.21–23 Second, the assignment to BP targets was not blinded which could potentially have introduced bias. However stroke endpoints were confirmed by a blinded central adjudication committee, as commonly done in large hypertension trials.24 Third, while the study tested treatment targets and not the effect of specific blood pressure agents, it would be difficult to exclude if any of the results are due to mechanisms beyond the effects of lowering BP. Finally, not all patients reached their assigned target at any point during follow-up (4.6% in the higher and 4.9% in the lower group), similar to what was observed in other trials testing blood pressure targets and reflecting clinical realities of blood pressure management.13, 24 A major strength of the SPS3 trial is that BP lowering was tested in a well-defined and homogenous cohort of stroke patients.
In conclusion, the results of the SPS3 BP trial, although not showing a statistically significant reduction, are congruent with findings of prior trials of BP lowering after stroke and support a treatment target of systolic BP <130mmHg for most patients with recent lacunar stroke. Whether these results from a cohort with recent lacunar strokes due mainly to cerebral small vessel disease apply to patients with strokes from other mechanisms requires additional trials.
Supplementary Material
Acknowledgments
Funding: SPS3 was an investigator-initiated trial funded by a cooperative agreement with the National Institute of Neurological Disorders and Stroke of United States (U01 NS38529-04A1).
THE SPS3 STUDY GROUP
CLINICALSITES INORDER OF PARTICIPANTS ENROLLED (n=number of participants)
United States, 50 sites (n=1677)
University of Texas Health Science Center at San Antonio, San Antonio, TX: Oscar Benavente, MD, Robert Hart, MD, Pablo Pergola, MD, Santiago Palacio, MD, Irma Castro, Arlene Farias, Ana Roldan, MD, MS (108); Boston University, Boston, MA: Carlos Kase, MD, Irene Gavras, MD, Helena Lau, RN, Matt Ogrodnik, Nancy Allen, RN (92); Mayo Clinic Rochester, Stroke Center, Rochester, MN: Irene Meissner, MD, John Graves, MD, Deb Herzig, RN, Jody Covalt, RN (90); University of California San Diego, San Diego, CA: Brett Meyer, MD, Christy Jackson, MD, Paul Gamble, MD, Nancy Kelly, RN, Janet Warner, RN, Jo Bell, RN (82); Mayo Clinic Scottsdale, Scottsdale, AZ: Bart Demaerschalk, MD, Michael Hogan, MD, Daniel Wochos, MD, Judith Wieser, RN, Barbara Cleary, RN, Lori Wood, RN (74); Metrohealth Medical Center, Cleveland, OH: Joseph Hanna, MD, Thomas Zipp, MD, Scott Bailey, RN, Dana Cook, RN, Alice Liskay, RN, Dana Simcox, RN, Joan Kappler, RN (70); Berman Center for Clinical Research, Minneapolis, MN: David Anderson, MD, Richard Grimm, MD, Donna Brauer, RN (68); University of Kentucky, Lexington, KY: Creed Pettigrew, MD, Anand Vaishnov, MD, Peter Sawaya, MD, Anna Fowler, RN, Nedda Hughes, PA, Johnya Rice, RN, Kathy Vanderpool, RN (64); St Louis University, St. Louis, MO: Salvador Cruz-Flores, MD, H Douglas Walden, MD, Eve Holzemer, RN (57); Wayne State Univ. School of Medicine, Detroit, MI: Sunitha Santhakumar, MD, Renee Van Stavern, MD, Seemant Chatuverdi, MD, John Flack, MD, Flicia Mada, RN, David Wiseman, RN, Elizabeth Berlow, RN, Julie Klinker, RN (57); The Methodist Hospital, Houston, TX: David Chiu, MD, Addison Taylor, MD, Larry Katz, PhD (57); University of Arizona, Tucson, Tucson, AZ: Bruce Coull, MD, Lien Howard, MD, Mina Malekniazi, RN, Melissa Van Skiver, Denise Bruck, Stacey Redman, RN (54); St. John’s Mercy Medical Center, St. Louis, MO: William Logan, MD, David Carpenter, MD, Sally Schroer, RN (52); Henry Ford Hospital, Detroit, MI: Angelo Katramados, MD, Brian Silver, MD, Jerry Yee, MD, Krisy Aiello, RN, Kathleen Wilson, RN, Sharon McCarthy, RN (51); Melbourne Internal Med Assoc MIMA, Melbourne, FL: Bhuvaneswari Dandapani, MD, C Peter Spies, MD, Carole Vasile, RN, Betty Anthony, RN, Jennifer Ferguson, Sharon Krubel, RN, Amanda Synman, Natalie Andrews (50); Vanderbilt University Medical Center, Nashville, TN: Howard Kirshner, MD, Craig Sussman, MD, Diane Brown, RN (46); Columbia University Medical Center, New York, NY: Mitch Elkind, MD, Russell John Crew, MD, Jai Radhakrishan, MD, Tania E. Corporan, MD, Julisa Diaz, MD, Rebeca Aragon, BS (45); The Ohio State University, Columbus, OH: Andrew Slivka, MD, Dan Spetie, MD, Julie Agriesti, Peggy Notstein, RN (41); University of South Alabama, Mobile, AL: Dean Naritoku, MD, Richard Zweifler, MD, Michael Culpepper, MD, Mel Parnell, RN, Robin Yunker, RN, Kelly Boots, RN, Renay Drinkard, RN, Rachel Backlin (39); University of Rochester, Rochester, NY: Curtis Benesch, MD, John Bisognano, MD, Ann Leonhardt, RN, Justine Zentner, RN, Molly Hildreth, LVN (34); University of Texas Southwestern, Dallas, TX: Mark Johnson, MD, Yinghui Liu, MD, Robert Goldsteen, MD, April Blair, MSW, Gregg Wright, Naomie Gathua, RN (33); Medical College of Wisconsin, Milwaukee, WI: Diane Book, MD, Sunu Eapen, MD, Clarence Grimm, MD, Barbara Blaney, Stephanie Rozman, Linda Gaertner, RN, Erin Bradenburg, Laura Loomis, RN, Jolene Monarch- Cotton, RN, Jean Ravavelli-Meyer, RN, Anna Golembieski, RN (33); University of Rochester Medical Center, Rochester, NY: Scott W. Burgin, MD, Joshua Hollander, MD, Walter Polashenski, MD, Patricia Wallace, RN, Cheryl Weber, RN (32); Oregon Health & Science University, Portland, OR: Helmi Lutsep, MD, Don Girard, MD, Kali Seisler, Megan Cingel, Megan Ross, Rachel Stone, Darren Larsen, RN, Ann Doherty, RN (30); Wake Forest University, Winston-Salem, NC: David Lefkowitz, MD, Levy Pavel, MD, Nancy Buchheimer, Sara Vaughn, BA, Emily Smith, Jean Satterfield, RN (29); University of Washington, Seattle, WA: David Tirschwell, MD, Christine Logar, MD, Michael Ryan, MD, Glenn Schubert, MPH, Patricia Tanzai, RN (27); Cooper Health System Dept of Medicine, Camden, NJ: Tom Mirsen, MD, Susan McAllister, MD, Arnaud Bastien, MD, Patricia Niblack, MLT (26); St. Joseph’s Hospital and Medical Center, Phoenix, AZ: James Frey, MD, Carol Darbonne, BS (25); Marshfield Clinic Dept. of Neurology, Marshfield, WI: Percy Karanjia, MD, Narayana Murali, MD, Richard Dart, MD, Kathleen Mancl, CCRP (24); Sutter Neuroscience Institute, Sacramento, CA,: Richard Atkinson, MD, Roger Lieberman, MD, Teresa Carter, RN, Pat Zrelak, RN, Nola Kenney, RN (22); Ruan Neurology Clinic and Research Center, Des Moines, IA: Michael Jacoby, MD, David Jones, MD, Jeffrey De Francisco, MD, Theresa Hamm, RN (21); University of Miami, Miller School, Miami, FL: José Romano, MD, Gustavo Ortiz, MD, Maria del Carmen Lichtenberger, MALS (17); Johns Hopkins Bayview, Baltimore, MD: Rafael Linas, MD, Steven Kravet, MD, Janice Alt, RN (16); University Hospitals Case Medical Center, Cleveland, OH: Sophia Sundararajan, MD, Mahboob Rahman, MD, Tom Horvath, David Korosec, RN, Chris Murphy, RN (16); Helen Hayes Hospital, West Haverstraw, NY: Jason Greenberg, MD, Laura Lennihan, MD, Marjorie King, MD, Laura Tenteromano, RN (15); Emory University, Atlanta, GA: Michael Frankel, MD, Joyce Doyle, MD, Janet Braimah, RN (13); Research Foundation of SUNY, Buffalo, NY: Lorainne Pereira, MD, Marilou Ching, MD, Robert Sawyer, MD, Kathy Parkes, RN, Cheryl Conover (9); Florida Neurovascular Institute, Tampa, FL: Erfan Albakri, MD, German Ramirez, MD, Stephenie Segal, RN, Kathy Taylor, RN, Judy Jackson (9); University of Illinois at Chicago, Chicago, IL: Kathy Helgason, MD, Maureen Hillmann, RN (2); Spartanburg Regional Medical Center, Spartanburg, SC: Robert Ringel, MD, Nicholas Fleming, MD, Bunny Mckown, RN (1); Cedars Sinai Medical Center, Los Angeles, CA: Stanley Cohen, MD, Robert Jenders, MD, Ravinder Singh, MD, Minh-Thu La, PhD, Khanhphong Trinh (9); North General Hospital, New York, NY: Jesse Weinberger, MD, Lewis Wright, MD, Dorothy Burch, RN (9); Mt. Sinai School of Medicine, New York, NY: Ronnie Horowitz, MD, Steven Atlas, MD, Sandra Augustine, RN (9); University of Washington at St. Louis, St. Louis, MO: Renee Van Stavern, MD, Angela Brown, MD, Jannie Serna, RN, Jill Newgent, RN, Julie Naylor, Laura Carpenter (5); University of California-San Francisco Fresno, Fresno, CA: Tanya Warwick, MD, Steven Stoltz, MD, Rebekah Garcia, CCRP (4); Loyola University, Maywood, IL: Michael Scheck, MD, Linda Chadwick, RN (4); Indiana University, Indianapolis, IN: James Fleck, MD, Myron Weinberger, MD, Alison Sears, RN (2); Colorado Neurological Institute, Denver, CO: Chris Fanale, MD, Lenden Neeper, CCRC, Paula Fisk, CRC (2); Nevada Neuroscience Institute at Sunrise, Las Vegas, NV: Stanley Cohen, MD, Daniel Sabry, MD, Ron Phoenix, RN (1); Stanford University, Stanford, CA: David Tong, MD, Madelleine Garcia, RN (1).
Canada, 8 sites (n = 283)
Hôpital de L’Enfant-Jesus de CHA, Quebec City, QC: Ariane Mackey, MD, Jovette Morin, MD, Annette Haché, RN, Claudette Lessard, (48); McGill University - Montreal General, Montreal, QC: Robert Côté, MD, Laurence Green, MD, Lisa Wadup, LVN, Anne-Marie Fontaine, RN (42); Centre for Clinical Research, Halifax, NS: Gordon Gubitz, MD, Rosario Rebello, MD, Tim Dean, MD, Yvette Reidy, RN (41); The Ottawa Hospital Civic Campus, Ottawa, ON: Mukul Sharma, MD, Grant Stotts, MD, Heather Clark, MD, Melodie Mortensen, RN, Jenniffer Sauve, RN, Doris Sharma, RN, Michelle Savage, RN (37); McGill University – Jewish General Hospital, Montreal, QC: Jeffrey Minuk, MD, Luc Trudeau, MD, Claudia Schanz, RN (37); Charles LeMoyne Hospital, Greenfield Park, QC: Leo Berger, MD, Sylvain Brunet, MD, Johanne Pontbriand, RN, Martine Mainville, Denise Racicot, RN (36); University of Calgary, Calgary, AB: Michael; Hill, MD, Karyn Fischer, RN, Andrea Cole-Kaskayne, RN, Carol Keeney, RN (35); University of Alberta Stroke Research NACTRC, Edmonton, AB: Ashfaq Shuab, MD, Khurshid Khan, MD, Naeen Dean, MD, Frederika, Herbert, RN, Karen Kastelic, RN (7)
Peru, 1 site(n =186)
Hospital Sabogal, Lima, Perú: Edwin Javier Pretell, MD, José Valdivia, MD, Marissa Pretell (186)
Ecuador, 1site(n=171
Hospital-Clínica-Kennedy, Guayaquil: Oscardel Brutto, MD, Rocio Santibáñez, MD, Joffre Lara, MD, Mauricio Zambrano (171)
Mexico, 4 sites (n = 165)
Instituto Nacional de Neurología y Neurocirugía, México City: Antonio Arauz, MD, G Amin Cervantes, MD, Adolfo Leyva, MD, Itzel Camacho, MD (92); Hospital Civil/México, Guadalajara, JAL: José Luis Ruiz Sandoval, MD, Eduardo Salcido Vásquez, MD, Carmen Ruiz (31); Instituto Nacional de Ciencias Médicas y Nutrición, México City: Carlos Cantú Brito, MD, Margarita Fernández, MD (26); Universidad Autónoma de Nuevo León, Monterrey, NL: Juan Fernándo Góngora-Rivera, MD, Juan Manuel Escamilla, MD, Joaquín Moxica, MD, Genny Arciniega, MD, Wendy Joana Gonzalez (16)
Chile,2 sites(n =127)
Universidad Católica, Santiago: Hospital Naval, Viñadel Mar: Gonzalo Matamala, MD, Helmut Goecke, MD, Marcela Parra, RN, Jessica Pozo, RN (69); Hospital Clinico Universidad Católicade Chile, Santiago: Jorge Tapia, MD, Ivan Esteban Godoy, MD, Marcela Valdes, RN (58)
Argentina, 5sites(n =45)
Centro Neurológico, Buenos Aires: Conrado Estol, MD, Cecilia Peralta, MD, Adriana Ellenberg, MD, Daniela Chezzio, MD (14); Hospital Británico, Buenos Aires: Manuel M. Fernández Pardal, MD, Hernan Trimachi, MD, Pablo Bonardo, MD, Julieta Mazziotti, MD (12); Hospital Ramos Mejía, Buenos Aires: Raul Carlos Rey, MD, Gustavo Caruso, MD, Luciana Melamud, MD, Sandra Lepera, MD, Ana Paula Stilman (7); Instituto FLENI, Buenos Aires: Sebastian Ameriso, MD, Ramiro Sánchez, MD, Guadalupe Bruera, MD, Javier Moschini, MD, Maria J. Ramírez (8); Hospital Universitario Austral, Buenos Aires: José A. Bueri, MD, Sebastián Sevilla, MD, Brunode Ambrosi, MD, Gabriela Marinsalta (4)
Spain, 10sites(n =366)
Hospital Bellvitge, Barcelona: Francisco Rubio, MD, Yurek, Krupinski, MD, Ana Carvajal, MD (66); Hospital del Mar, Barcelona: Jaume Roquer, MD, Ana Oliveras Serrano, MD, Jordi Jiménez Conde, MD, Ana Rodríguez, MD, Gemma Romeral, RN (58); Hospital Del Sagrat Cor, Barcelona: Adrià Arboix, MD, Antoni Pelegrí, MD, Lorena Blanco (54); Hospital Parc Taulí, Barcelona: David Cánovas Vergé, MD, Jordi Estela Herrero, MD, Ana Gómez, RN, Lorena Blanco (51); Hospitalde Girona Dr. Josep Trueta, Girona: Joaquín Serena Leal, MD, Mar Castellanos, MD, Verónica Cruz, Mercè Cepeda, RN (45); Hospital Universitario German Triasi Pujol, Barcelona: Meritxell Gomis, MD, Juan Arenillas, MD, Antonio Dávalos, MD, Ana Suñol, RN, Silvia Reverté, RN (27); Hospitalde la Santa Creui Sant Pau, Barcelona: José Lluis Martí-Villalta, MD, Sergio Martínez, MD, Rebeca Marín, RN (25);Hospital Clínico Universitariode Santiagode Compostela, Santiagode Compostela: José Castillo Sánchez, MD, Miguel Blanco González, MD, Manuel Rodríguez, MD, Isabel Jiménez, Jaime Rodríguez, RN (23); Hospital La Paz, Madrid: Exuperio Díez Tejedor, MD, Patricia Martínez, MD, Blanca Fuentes, MD (15); Hospital Generalde Cataluña, Barcelona: Lluis Soler Singla, MD, Ernest Balaguer, MD, Joan Izquierdo, MD, Cristina Soler, Maria Armenteros. (2)
COORDINATING CENTER: (University of British Columbia, Vancouver, BC, Canada and University of Texas Health Science Center at San Antonio, TX, USA)
Oscar Benavente, MD (PI), Robert Hart, MD (Co-PI), Pablo Pergola, MD (Hypertension Co-PI), Ana Roldan, MD, MS (Study Manager for sites in Latin America and Spain), Marie-France Benavente, RN, BScN (Study Manager for sites in North America), Carole White, RN, PhD (Study Manager for site sin North America), Camilla Robu, MBA (Project Administrator), Che Kelly, RN, MEd (Project Administrator), Robert Talbert, PharmD (Pharmacology Consultant), Eduardo Martinez (Project Data Manager); Neuroradiology Core: Carlos Bazan, MD, Gabriela Pergola, MD; Neuropsychology Core: Lesly Pearce, MS, Raymond Costello, PhD, Claudia Jacova, PhD, Luisa Camelia, PhD, Crystal Mendoza, MA, Brandy Pratt, B. Kin, Steve Holliday, PhD
STATISTICAL AND DATA MANAGEMENT CENTER: (University of Alabama at Birmingham, AL, USA)
Leslie McClure, PhD (PI), Christopher Coffey, PhD (co-PI), Jeff Szychowski, PhD, George Howard, PhD, Charles Katholi, PhD, Yu Zhang, MS, Kalyani Peri, MS, Charles All corn, Richard Mailhot, LisaIrby, Fekisha Guyton, MPA, MaryJo Sewell
DRUG DISTRIBUTION CENTER: (VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center, Albuquerque, NM, USA)
Robert Ringer, RPh, BCNP, Dennis Raisch, PharmD, Dave Hunt, MS
REGIONAL COORDINATING CENTERS
Spain: Francisco Rubio, MD; Clara M. Rosso, MD; Ariadna Martin; Mireia Sanllorente Argentina: Celso Arabetti, MD; José Luis Fernández, MD; Maria Julia Cremona, Ana Capece, MD
EXECUTIVE COMMITTEE
Oscar Benavente, MD (Chair), Robert Hart, MD, Marie Benavente, RN, BScN, Christopher Coffey, PhD, Robin Conwit, MD, Leslie McClure, PhD, Pablo Pergola, MD, Ana Roldan, MD, MS, Jeff Szychowski, PhD, Robert Talbert, PharmD
STEERING COMMITTEE
Oscar Benavente, MD (Chair), David Anderson, MD, Antonio Arauz, MD, Marie Benavente, RN, BScN, Christopher Coffey, PhD, Robin Conwit, MD, Robert Côté, MD, Bart, Demaerschalk, MD, Oscardel Brutto, MD, Mitchell Elkind, MD, Robert Hart, MD, Carlos Kase, MD, Leslie McClure, PhD, Claudia Moy, PhD, Pablo Pergola, MD, Ana Roldan, MD, Mukul Sharma, MD MS, Jeff Szychowski, PhD, Robert Talbert, PharmD, Carole White, RN, PhD
PUBLICATIONS COMMITTEE
Carole White, RN, PhD (Chair), Oscar Benavente, MD, Curtis Benesch, MD, Christopher Coffey, PhD, Robin Conwit, MD, John Graves, MD, Richard Grimm, MD, Gordon Gubitz, MD, Steve Holliday, PhD, Helena Lau, RN, MSPH, Claudia Moy, PhD, Lesly Pearce, MS, Jeff Szychowski, PhD
EVENTS ADJUDICATION COMMITTEE
Maria Aguilar, MD (Chair), David Sherman, MD (Former Chair), Elaine Nasco, (Events Coordinator), Joe Blackshear, MD, Mina Chung, MD, Pierre Fayad, MD, Brian Gage, MD, Clay Johnston, MD, Walter Kernan, MD, Enrique Leira, MD, Jose Merino, MD, Gustavo Roman, MD, Cathy Sila, MD, Gene Sung, MD, Carlvan Walraven, MD, Richard Zweifler, MD
INDEPENDENT MEDICAL MONITOR
Barney Stern, MD, University of Maryland, MD, USA
NIH-NINDS PROJECT OFFICERS
Robin Conwit, MD, Claudia Moy, PhD
DATA AND SAFETY MONITORING BOARD
K. Michael Welch, MD (Chair), William Clarke, PhD, Jeffrey Cutler, MD, Karen Furie, MD, Karen Johnston, MD, Matthew Mayo, PhD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gueyffier F, Boissel JP, Boutitie F, Pocock S, Coope J, Cutler J, et al. Effect of antihypertensive treatment in patients having already suffered from stroke. Gathering the evidence. The INDANA (INdividual Data ANalysis of Antihypertensive intervention trials) Project Collaborators. Stroke. 1997;28(12):2557–62. doi: 10.1161/01.str.28.12.2557. [DOI] [PubMed] [Google Scholar]
- 2.Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358(9287):1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 3.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34(11):2741–8. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 4.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32(12):2735–40. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 5.Sacco S, Marini C, Totaro R, Russo T, Cerone D, Carolei A. A population-based study of the incidence and prognosis of lacunar stroke. Neurology. 2006;66(9):1335–8. doi: 10.1212/01.wnl.0000210457.89798.0e. [DOI] [PubMed] [Google Scholar]
- 6.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(1):227–76. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 7.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367(9):817–25. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, et al. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke. 2011;6(2):164–75. doi: 10.1111/j.1747-4949.2010.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White CL, Szychowski JM, Roldan A, Benavente MF, Pretell EJ, Del Brutto OH, et al. Clinical Features and Racial/Ethnic Differences among the 3020 Participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) Trial. J Stroke Cerebrovasc Dis. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansson L, Hedner T, Dahlof B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press. 1992;1(2):113–9. doi: 10.3109/08037059209077502. [DOI] [PubMed] [Google Scholar]
- 11.Pergola PE, White CL, Graves JW, Coffey CS, Tonarelli SB, Hart RG, et al. Reliability and validity of blood pressure measurement in the Secondary Prevention of Small Subcortical Strokes study. Blood Press Monit. 2007;12(1):1–8. doi: 10.1097/MBP.0b013e3280858d5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClure LA, Szychowski JM, Benavente OR, Coffey CS. Sample size re-estimation in an on-going NIH-sponsored clinical trial: the Secondary Prevention of Small Subcortical Stroke trial experience. Contemp Clin Trials. 2012;33:1088–93. doi: 10.1016/j.cct.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Effect of antihypertensive treatment on stroke recurrence. Hypertension-Stroke Cooperative Study Group. JAMA. 1974;229(4):409–18. doi: 10.1001/jama.1974.03230420021019. [DOI] [PubMed] [Google Scholar]
- 15.Chapman N, Huxley R, Anderson C, Bousser MG, Chalmers J, Colman S, et al. Effects of a perindopril-based blood pressure-lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke. 2004;35(1):116–21. doi: 10.1161/01.STR.0000106480.76217.6F. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson S, Olofsson B-O, Wester P-O. Atenolol in secondary prevention after stroke. Cerebrovasc Dis. 1995;5:21–5. [Google Scholar]
- 17.Group PC. Post-Stroke Antihypertensive Treatment Study. Chinese Medical Journal. 1995;108(9):710–7. [PubMed] [Google Scholar]
- 18.Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359(12):1225–37. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Healey JS, Pogue J, Chrolavicius S, Flather M, Hart RG, et al. Irbesartan in patients with atrial fibrillation. N Engl J Med. 364(10):928–38. doi: 10.1056/NEJMoa1008816. [DOI] [PubMed] [Google Scholar]
- 21.Bousser MG, Amarenco P, Chamorro A, Fisher M, Ford I, Fox KM, et al. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet. 377(9782):2013–22. doi: 10.1016/S0140-6736(11)60600-4. [DOI] [PubMed] [Google Scholar]
- 22.Hong KS, Yegiaian S, Lee M, Lee J, Saver JL. Declining stroke and vascular event recurrence rates in secondary prevention trials over the past 50 years and consequences for current trial design. Circulation. 2011;123(19):2111–9. doi: 10.1161/CIRCULATIONAHA.109.934786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359(12):1238–51. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson L, Zanchetti A. The Hypertension Optimal Treatment (HOT) Study--patient characteristics: randomization, risk profiles, and early blood pressure results. Blood Press. 1994;3(5):322–7. doi: 10.3109/08037059409102281. [DOI] [PubMed] [Google Scholar]
- 25.Schrader J, Luders S, Kulschewski A, Hammersen F, Plate K, Berger J, et al. Morbidity and Mortality After Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES) Stroke. 2005;36(6):1218–26. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.