Abstract
Background/Purpose
We observed that fibroblast growth factor receptors 1 and 2 (Fgfr1, Fgfr2) are expressed during abdominal wall development in mice and hypothesized that conditional mutation of these genes would result in abdomial wall defects.
Methods
Section in situ hybridizations were performed for Fgfr1 and Fgfr2 on wild-type embryos at embryonic day (E) 11.5 and E13.5. Conditional mutation of Fgfr1 and Fgfr2 was achieved with a tamoxifen inducible Cre at E8.5. Litters were harvested at E17.5, whole mount photographs were taken, and paraffin sections were generated and stained with hematoxylin and eosin.
Results
Fgfr1 was expressed in ectoderm, lateral plate mesoderm, and myoblasts, whereas Fgfr2 was expressed almost exclusively in the early dermis and ectoderm of the abdominal wall. Conditional mutation of both Fgfr2 alleles and one Fgfr1 allele resulted in omphalocele in 38.7% of mutants. Histologic examination in mutants demonstrated disruptions in dermal and muscle development.
Conclusions
Mutant embryos with omphalocele arising from mutation in Fgfr1 and Fgfr2 exhibit disruptions in the development of the secondary abdominal wall structures. These findings are consistent with a model of ventral abdominal wall development in which organization of the muscles and connective tissue (secondary abdominal wall structures) is influenced by positional information emanating from the primary abdominal wall.
Keywords: Omphalocele, Fgf receptors, Mutation, Mouse, Model, Patterning
The abdominal wall is composed of 4 pairs of muscles and their encasing fascia, which are symmetrically arranged in reference to the vertical anterior midline of the body. In mice and other nonhuman mammals, there is an additional outer layer of striated muscle termed the panniculus carnosus, which enables movement of the skin independent of the deeper muscles [1]. The formation of the abdominal wall occurs through several steps. The primary body wall forms out of lateral plate mesoderm and ectoderm, which form a simple sheet of tissue. After folding of primary body wall to join in the midline, myoblasts begin their dorsal to ventral migration out of the somite [2]. As myoblasts reach their target destinations along the perimeter of the abdominal wall, they organize into myofibers and give rise to 4 paired muscle groups (external oblique, internal oblique, transversus abdominus, and rectus abdominus) [3]. The distinct orientation of muscle fibers deviates most dramatically from the dorsal to ventral migration pathway in muscle pairs that are closest to the skin and the ventral midline such as the external oblique and the rectus abdominus [4]. In contrast, the transversus abdominus, which is the furthest away from the midline and the skin, organizes and orients its myofibers dorsoventrally (the direction along which the myoblasts migrated).
The unique patterning of ventral body wall structures like the muscles of the abdominal wall has led to an intriguing hypothesis: that organization and patterning are determined during the dorsal to ventral migration of myoblasts by positional signals emanating from within the primary body wall. According to this hypothesis, structures that directionally deviate the most from the ventral to dorsal migration pathway would be under the greatest influence of the primary body wall. The anatomical point or points where this directional deviation begins is a developmental boundary termed the lateral somitic frontier [5,6]. The organization and patterning of normal abdominal wall musculature are consistent with the lateral somitic frontier hypothesis. Simply put, structures closest to the ectoderm are under the greatest influence of the ectoderm during development and will deviate the most from the direction of myoblast migration.
Omphalocele, a congenital defect of the abdominal wall, appears to result from and arrest in secondary abdominal wall development. Patients with this defect are born with protrusion of varying amounts of the intestine and liver into a sac centered around the umbilicus. Although several causative genes have been identified [7,8], the developmental mechanism of this defect remains elusive. If omphalocele truly arises from an arrest in abdominal wall development, then some of the migrating myoblasts will not reach their target destinations; and some of the critical signals emanating from the primary abdominal wall necessary for patterning secondary abdominal wall structures (muscle, dermis, and connective tissue) will be absent. This has led us to postulate that mouse embryos with omphalocele should exhibit disruptions in secondary abdominal wall structures.
The fibroblast growth factors (Fgfs) are a large group of growth factors that act through membrane-bound tyrosine kinase receptors. Mutations in various combinations of Fgfs and their receptors have been shown to be causative in cleft palate defects [9–11]; and null mutation of fibroblast growth factor receptor (Fgfr) 2IIIb results in skin [12] and eyelid defects [13,14], the latter of which can be associated with omphalocele [15]. We have observed that Fgfr2IIIb null mutants can occasionally develop an omphalocele (unpublished data). These observations have led us to hypothesize that loss of Fgf signaling will result in omphalocele. We chose to test this hypothesis with a receptor-based approach.
Fibroblast growth factor receptors 1 and 2 have been reported to be expressed at E14.5 in the mouse abdominal wall in the Genepaint database [16]. Germline mutation of either Fgfr1 or Fgfr2 results in early in utero lethality [17–19]. To examine the role of these genes in abdominal wall development while escaping the early lethality of mutation, we used a tamoxifen inducible Rose EsR-Cre conditional mutagenesis strategy [20,21]. We demonstrate that conditional mutation of Fgfr1 and Fgfr2 results in an omphalocele phenotype similar to the human defect that exhibits disruptions in patterning of secondary abdominal wall structures.
1. Methods
1.1. Animals
Institutional Animal Care and Use Committee approval for these studies was obtained from the University of Wisconsin School of Medicine and Public health (PFN protocol M02258). All animals were maintained in a clean facility with access to fresh food and water and kept on a 12-hour alternating light/dark cycle.
1.2. Section in situ hybridization
Wild-type breedings were set up. Noon of the day of the plug was considered embryonic day (E) 0.5. Females were euthanized by cervical dislocation on either E11.5 or E13.5. Embryos were harvested into cold phosphate-buffered saline (PBS) and fixed overnight in Bouin’s at 4°C. They were dehydrated through a series of escalating PBS/ethanol steps and embedded into paraffin. Sections were taken at 10-μm thickness and floated onto slides. Sections were dewaxed and rehydrated. Slides were treated with hydrogen peroxide, and section in situs were performed at 60°C for Fgfr1 and Fgfr2 [22,23] with antisense probes. Sections were stained overnight at 37°C, fixed with 4% Paraformaldehyde, and cover slipped with glycerol. Photographs were taken under a light microscope and reconstructed using the merge tool in Adobe Photoshop (San Jose, CA).
1.3. Generation of mutant fetuses
Fgfr2 [24] conditional homozygous males carrying 1 or 2 copies of Rosa EsR-Cre [20] were mated to females homozygous for conditional alleles of both Fgfr1 [25] and Fgfr2. Noon of the day of the plug was considered E0.5. Pregnant females were given a single dose of 0.1 mg/g tamoxifen (Sigma, St. Louis, MO) dissolved in peanut oil at E8.5 by oral gavage. Pregnant females were euthanized by cervical dislocation at E17.5 to E17.75, and the litters were harvested into cold PBS. Whole mount photographs were taken under a stereoscopic dissecting microscope. Tails were taken for genotyping for Fgfr1, Fgfr2, and general Cre by polymerase chain reaction [21,22,24,25].
1.4. Histology
The skin over the posterior thorax was opened, and Bouin’s was directly injected into the thoracic and abdominal cavities with a 1-mL syringe and a 30-gauge needle. Embryos were submerged in Bouin’s, fixed overnight at 4°C, dehydrated through a series of escalating PBS/ethanol steps, and embedded into paraffin. Sections were taken at 10-μm thickness and floated onto slides in a 50°C water bath. Sections were dewaxed and rehydrated, stained with hematoxylin and eosin, dehydrated, and cover slipped. Photomicrographs were taken at 20× and 400× using a standard light microscope.
2. Results
2.1. Fgfr1 and Fgfr2 expression in abdominal wall development
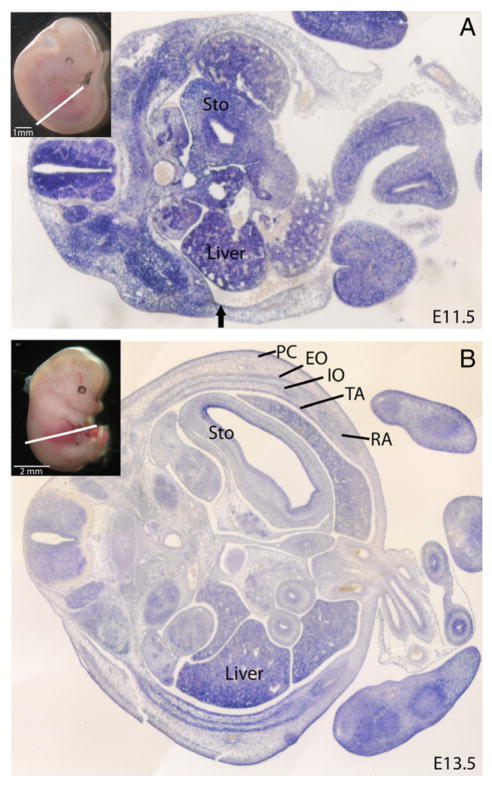
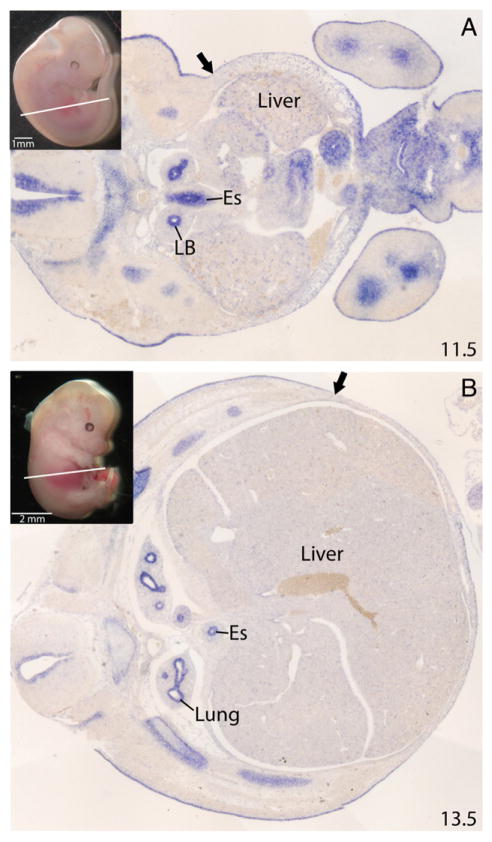
We examined the expression of Fgfr1 and Fgfr2 in wild-type mouse embryos during abdominal wall development. Section in situ hybridizations were performed at E11.5 and E13.5, 4 and 2 days, respectively, before abdominal wall closure (E15.5–16.0). These receptors demonstrated both distinct and overlapping patterns of expression. Fgfr1 was expressed in both the primary and secondary abdominal wall at E11.5 (Fig. 1A). At E13.5, it was present in all of the developing muscles of the abdominal wall, with the strongest expression in the lateral muscles (external oblique and internal oblique and transversus abdominus) and the weakest expression in the ventral regions of the rectus muscle. Fgfr1 was also expressed in the dermis and panniculus carnosus, but expression was limited or absent in the connective tissue and ribs overlying the external obliques (Fig. 1B). In contrast, Fgfr2 expression was limited to the ectoderm and early dermis of the secondary abdominal wall and in the mesoderm of the primary abdominal wall at E11.5 (Fig. 2A). At E13.5, expression was again limited to the dermis, ectoderm, and the ribs but not in the muscles or other connective tissue structures of the abdominal wall (Fig. 2B).
Fig. 1.
Expression of Fgfr1 in abdominal wall development. A, In situ in mouse at E11.5. Areas of staining blue indicate expression of Fgfr1. Inset demonstrates the plane of section. Arrow indicates boundary between primary abdominal wall (right of arrow) and secondary abdominal wall (left of arrow). B, In situ in mouse at E13.5. PC indicates panniculus carnosus; EO, external oblique; IO, internal oblique; TA, transversus abdominus; RA, rectus abdominus; Sto, stomach.
Fig. 2.
Expression of Fgfr2 in abdominal wall development. A, In situ in mouse at E11.5. Arrow indicates boundary between primary abdominal wall (right of arrow) and secondary abdominal wall (left of arrow). B, In situ in mouse at E13.5. Inset demonstrates the plane of section. Arrow indicates proximal limit of dermal staining. LB indicates lung bud; Es, esophagus.
2.2. Conditional mutation of a single copy of Fgfr1 and both copies of Fgfr2 induced after E8.5 results in omphalocele
Mutation of Fgfr1 and Fgfr2 was induced using a tamoxifen inducible Rosa Esr-Cre system through the method of intragastric injection [21]. Fgfr2 conditional homozygous males carrying 1 or 2 copies of Rosa EsR-Cre were mated to females homozygous for conditional alleles of both Fgfr1 and Fgfr2. Pregnant females were then injected with a single dose of 0.1 mg/g Tamoxifen dissolved in peanut oil. Pregnant females were euthanized at E17.5 to E17.75 and the litters were harvested. A total of 9 litters were generated. The average litter size was 4.67 indicating a high incidence of in utero death. There were a total of 31 genotypic mutants (fetuses carrying a copy of Rosa Esr-Cre) and 11 genotypically normal fetuses (without a copy of Rosa Esr-Cre) (Table 1). Of the genotypic mutants, 12 (38.7%) had an omphalocele (Fig. 3C) and 19 (61.3%) had a normal-appearing abdominal wall (Fig. 3B). All genotypically normal fetuses had a normal abdominal wall (Fig. 3A). In addition, conditional homozygous mutation of Fgfr1 or Fgfr2 alone did not result in omphalocele (data not shown).
Table 1.
Incidence of omphalocele in Fgfr1/2 mutants
| Litter | Genotypic mutant with normal abdomen R1c/+; R2c/c; EsrCre | Genotypic mutant with omphalocele R1c/+; R2c/c; EsrCre | Genotypically normal with normal abdomen R1c/+; R2c/c | Total |
|---|---|---|---|---|
| 1 | 2 | 0 | 0 | 2 |
| 2 | 2 | 1 | 1 | 4 |
| 3 | 1 | 0 | 1 | 2 |
| 4 | 1 | 3 | 3 | 7 |
| 5 | 2 | 4 | 3 | 9 |
| 6 | 0 | 4 | 1 | 5 |
| 7 | 1 | 6 | 0 | 7 |
| 8 | 2 | 1 | 2 | 5 |
| 9 | 1 | 0 | 0 | 1 |
| 12 | 19 | 11 | 42 | |
| Mutants (%) | 38.71% | 61.29% |
Fig. 3.
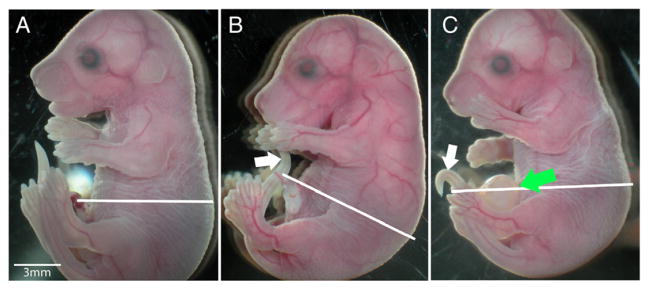
Mutation of Fgfr1 and Fgfr2 results in omphalocele. (A) wild-type fetus at E17.5, (B) genotypic Fgfr1/Fgfr2 mutant fetus at E17.5 without omphalocele, and (C) genotypic Fgfr1/Fgfr2 mutant fetus at E17.5 with omphalocele. Green arrow indicates omphalocele. White lines indicate planes of section for Fig. 4A–I. White arrow bent tail typical of Fgfr2 mutant indicating some level of recombination.
2.3. Mutant fetuses with omphalocele exhibit disruptions in the organization of secondary abdominal structures
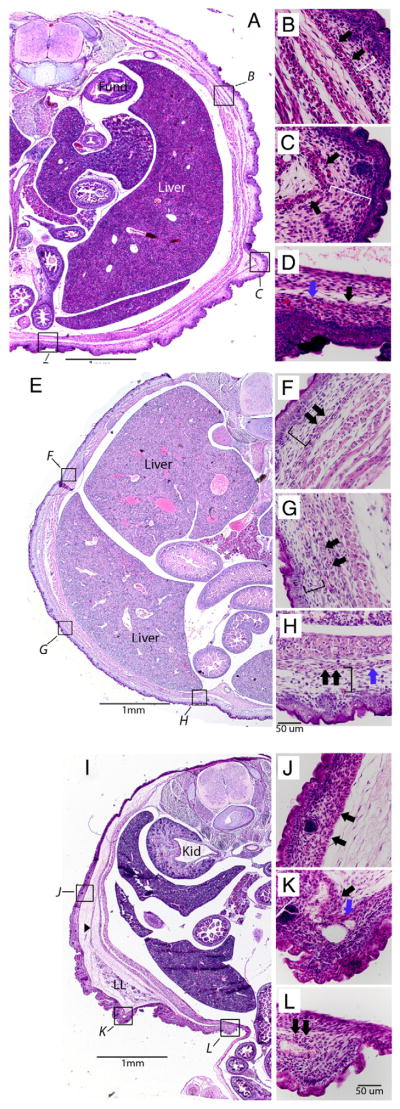
We examined the histology of the wild-type fetuses as well as genotypic mutant fetuses with and without omphalocele at E17.5. Wild-type fetuses exhibit an even distribution and thickness of the dermis dorsally to ventrally (Fig. 4A). Underlying the dermis is a loose layer of connective tissue (Fig. 4B, C; white brackets), which becomes denser and more compact in the area overlying the rectus abdominus (Fig. 4D). The panniculus carnosus can be seen as a continuous thin layer of muscle originating near the dorsal midline (Fig. 4A), running the circumference of the abdominal wall (Fig. 4A, C; black arrows) and ending near the ventral midline (Fig. 4D, blue arrow). In the genotypic mutant without omphalocele, the dermis remains evenly distributed dorsally to ventrally (Fig. 4E). The connective tissue layer underlying the dermis remains loose and noncompact (Fig. 4F–H, black brackets), and the panniculus carnosus is continuous from the dorsal midline to the ventral midline (Fig. 4F–G, black arrows; Fig. 4H, blue arrow). In contrast, the genotypic mutant with omphalocele exhibits alterations in the patterning and organization of several structures in the abdominal wall. First, the thickness of the dermis increases dramatically from the dorsal region of the body wall to the ventral midline (Fig. 4I). Second, the connective tissue directly underlying the dermis is very dense and cellular compared with the 2 other embryos (Fig. 4J–L). Directly underlying this dense layer is the panniculus carnosus without an interceding loose layer of connective tissue (Fig. 4J–K). Third, the panniculus carnosus becomes discontinuous well before the midline (Fig. 4K, blue arrow) and remains so up to the midline (Fig. 4L, black arrows indicate 2 isolated islands of muscle in the presumed location of the panniculus carnosus). Finally, underlying the panniculus carnosus is an additional layer of loose connective tissue. In the embryos with omphalocele, this layer is much thicker than that of either wild-type embryos or genotypic mutants without omphalocele. Taken together, the omphalocele phenotype appears to be associated with disruptions in secondary abdominal wall structures (dermis, muscle, and connective tissue) that are in closest proximity to the skin and ventral midline. In contrast, the remaining abdominal wall structures that lie farther away from that ectoderm appear normal in the omphalocele embryo.
Fig. 4.
Histologic analyses of wild-type and mutant mice at E17.5. A, Transverse section (20×) of wild-type mouse. Boxes with italic letters correspond to high-magnification photomicrographs (400×). B, Posterior abdominal wall. C, Lateral abdominal wall. D, Ventral abdominal wall. Black arrows indicate panniculus carnosus. White brackets indicate loose connective tissue space between panniculus carnosus and dermis. Blue arrow indicates ventral most point of panniculus carnosus. E, Photomicrograph of transverse section (20×) of a genotypic Fgfr1; Fgfr2; Cre mutant without omphalocele. F, Posterior abdominal wall. G, Lateral abdominal wall. H, Ventral abdominal wall. Black brackets indicate loose connective tissue space between panniculus carnosus and dermis. I, Transverse section (20×) of a genotypic Fgfr1; Fgfr2; Esr-Cre mutant with omphalocele. J, Posterior abdominal wall. K, Lateral abdominal wall. L, Ventral abdominal wall. Black arrowhead in 4I indicates connective tissue to lower limb anlagen. Black arrows in 4L indicate isolated islands of panniculus carnosus. Fund indicates fundus; PC, panniculus carnosus; LL, lower limb; Kid, kidney.
3. Conclusions
In this study, we examine the role of Fgfr1 and Fgfr2 in the formation of the abdominal wall. We postulated that Fgfr1 and Fgfr2 would play a significant role in abdominal wall development. Our gene expression data indicated that both receptors are expressed in partially overlapping patterns in the abdominal wall before reduction of the physiologic hernia at E15.5 to E16.0. Conditional mutagenesis of these receptors resulted in a gross morphologic phenotype similar to human omphalocele. Our examination of the histology of the defect indicates that development and organization of the panniculus carnosus, dermis, and the interceding loose connective tissue layer between these 2 structures is disrupted. These disruptions were most severe closest to the ventral midline. The results are consistent with a developmental model in which positional information emanates from the outer tissue layers of primary abdominal wall (possibly the ectoderm) to direct the development and organization of secondary abdominal wall structures including the muscles, connective tissue, and dermis.
It is unclear how the observed changes in abdominal wall development mechanistically relate to the formation of omphalocele. Our in situ data demonstrate that the only anatomical region of overlap in the expression of Fgfr1 and Fgfr2 is in the early ectoderm and dermis. Interestingly, the most dramatic effects on development in embryos with omphalocele aside from the omphalocele itself are in the dermis and the tissues directly underlying it. Our observation suggests that dermis and possibly the ectoderm are repositories for information that directs the organization of the underlying connective tissue layers and panniculus carnosus. Mutation of Fgfr1 and Fgfr2 would then appear to disrupt the transmission of information from these structures critical for organizing and patterning the adjacent secondary abdominal wall structures. This adds a wrinkle to the lateral somitic frontier hypothesis. The original hypothesis favors the lateral plate mesoderm as the primary repository for positional information. Our data suggest that there is a least some type of gradient at work in either the ectoderm and/or the mesoderm adjacent to the ectoderm and that a disruption in development of that area alters the gradient and subsequent positional information. Further experiments will be necessary to provide additional supporting evidence for this hypothesis.
Other genetic animal models of omphalocele exhibit similar findings to ours. For example, Msx-1/Msx2 mutant mice manifest a discontinuity of the panniculus carnosus [26]; and Alx-4 mutant mice demonstrate a loss of the loose connective tissue layer between the dermis and panniculus carnosus [27]. Two other models exhibit findings that support our hypothesis that the ectoderm and/or dermis is critical in directing development of the underlying tissue layers. Hoxb4 mutants, which develop the murine equivalent of pentalogy of Cantrell, exhibit a thickening of the ventral dermis in the area of the defect [28] similar to our model; and Rock-1 mutants have a defect in keratinocyte function [15].
One of the pitfalls of this model is the incomplete penetrance of the phenotype. We suspect that the lack of complete penetrance is arising from 2 factors. First, tamoxifen inducible Cres are frequently mosaic in nature [21]. Second, there may be either additional Fgf receptors or receptors from other families such as the transforming growth factor receptors that compensate by up-regulating their expression in the absence of Fgfr1 and Fgfr2. Further studies will be required to sort this out.
In summary, we report a new genetic animal model for omphalocele that exhibits disruptions in the patterning of secondary abdominal wall structures closest to the skin and ventral midline. This findings are supportive of a hypothesis in which positional information stored within the primary body wall or possibly the ectoderm may be critical in directing organization of secondary abdominal wall structures.
Acknowledgments
The authors would like to thank Karen Williams in the Department of Surgery and UW-SMPH for her assistance in preparation of the figures for this manuscript.
Footnotes
Papers Presented at the 41st Annual Meeting of the American Pediatric Surgical Association, Orlando, FL, May 16–19, 2010.
This research was generously supported by a grant from the American College of Surgeons (PFN) Faculty Research Fellowship 2006–2008.
References
- 1.Kaufman MH. The atlas of mouse development. San Diego (Calif): Elsevier Academic Press; 2008. [Google Scholar]
- 2.Christ B, Jacob M, Jacob HJ. On the origin and development of the ventrolateral abdominal muscles in the avian embryo. An experimental and ultrastructural study. Anat Embryol (Berl) 1983;166(1):87–101. doi: 10.1007/BF00317946. [DOI] [PubMed] [Google Scholar]
- 3.Shoenwolf GC, Bleyl SB, Brauer PR, et al. Larsen’s human embryology. Elsevier-Churchill Livingstone; 2009. pp. 222–32. [Google Scholar]
- 4.Netter FH. Atlas of human anatomy. Teterboro (NJ): Icon Learning Systems; 2003. pp. 241–4. [Plates] [Google Scholar]
- 5.Nowicki JL, Takimoto R, Burke AC. The lateral somitic frontier: dorso-ventral aspects of anterio-posterior regionalization in avian embryos. Mech Dev. 2003;120:227–40. doi: 10.1016/s0925-4773(02)00415-x. [DOI] [PubMed] [Google Scholar]
- 6.Burke AC, Nowicki JL. A new view of patterning domains in the vertebrate mesoderm. Dev Cell. 2003;4:159–65. doi: 10.1016/s1534-5807(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 7.Perveen R, Lloyd IC, Clayton-Smith J, et al. Phenotypic variability and asymmetry of Reiger syndrome associated with PITX2 mutations. Invest Ophthalmol Vis Sci. 2000;41(9):2456–60. [PubMed] [Google Scholar]
- 8.Eggenschwiler J, Ludwig T, Fisher P, et al. Mouse mutant embryos over expressing IGF-II exhibit phenotypic features of the Beckwith-Wiedemann and Simpson-Golabi-Behmel syndromes. Genes Dev. 1997;11(23):3128–42. doi: 10.1101/gad.11.23.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice R, Spencer-Dene B, Connor EC, et al. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J Clin Invest. 2004;113:1692–700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley BM, Mansilla MA, Ma J, et al. Impaired FGF signaling contributes to cleft lip and palate. Proc Natl Acad Sci U S A. 2007;104:4512–7. doi: 10.1073/pnas.0607956104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley BM, Murray JC. Sequence evaluation of FGF and FGFR gene conserved non-coding elements in non-syndromic cleft lip and palate cases. Am J Med Genet A. 2007;143:3228–34. doi: 10.1002/ajmg.a.31965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petiot A, Conti FJ, Grose R, et al. A crucial role for Fgfr2-IIIb signaling in epidermal development and hair follicle patterning. Development. 2003;130:5493–501. doi: 10.1242/dev.00788. [DOI] [PubMed] [Google Scholar]
- 13.De Moerlooze L, Spencer-Dene B, Revest J, et al. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signaling during mouse organogenesis. Development. 2000;127:483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Guo H, Xu X, et al. Fibroblast growth factor receptor 2 (Fgfr2) plays an important role in eyelid and skin formation and patterning. Dev Dyn. 2001;222:471–83. doi: 10.1002/dvdy.1205. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu Y, Thumkeo D, Keel J, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–53. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 17.Deng CX, Wynshaw-Boris A, Shen MM, et al. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 2004;8:3045–57. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi TP, Harpal K, Henkemeyer M, et al. Fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–44. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- 19.Arman E, Haffner-Krausz R, Chen Y, et al. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci U S A. 1998;95:5082–7. doi: 10.1073/pnas.95.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–22. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park EJ, Sun X, Nichol P, et al. System for tamoxifen-inducible expression of cre-recombinase from the Foxa2 locus in mice. Dev Dyn. 2008;237:447–53. doi: 10.1002/dvdy.21415. [DOI] [PubMed] [Google Scholar]
- 22.Trokovic R, Trokovic N, Hernesniemi S, et al. Fgfr1 is independently required in both mid- and hindbrain for sustained response to isthmic signals. EMBO J. 2003;22(8):1811–23. doi: 10.1093/emboj/cdg169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burns RC, Fairbanks TJ, Sala F, et al. Requirement for fibroblast growth factor 10 or fibroblast growth factor receptor 2-IIIb signaling for cecal development in mouse. Dev Biol. 2004;265(1):61–74. doi: 10.1016/j.ydbio.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Qiao W, Li C, et al. Generation of Fgfr1 conditional knockout mice. Genesis. 2002;32(1):85–6. doi: 10.1002/gene.10028.abs. [DOI] [PubMed] [Google Scholar]
- 25.Yu K, Xu J, Liu Z, et al. Conditional inactivation of Fgf receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bode growth. Development. 2003;130(13):3063–74. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Yamada G. Ventral abdominal wall dysmorphogenesis of Msx1/Msx2 double-mutant mice. Anat Rec A Discov Mol Cell Evol Biol. 2005;284(1):424–30. doi: 10.1002/ar.a.20180. [DOI] [PubMed] [Google Scholar]
- 27.Qu S, Niswender KD, Ji Q, et al. Polydactyly and ectopic ZPA formation in Alx-4 mutant mice. Development. 1997;124(20):3999–4008. doi: 10.1242/dev.124.20.3999. [DOI] [PubMed] [Google Scholar]
- 28.Manley NR, Barrow JR, Zhang T, et al. Hoxb2 and hoxb4 act together to specify ventral body wall formation. Dev Biol. 2001;237(1):130–44. doi: 10.1006/dbio.2001.0365. [DOI] [PubMed] [Google Scholar]