Abstract
Alzheimer's disease (AD) and Parkinson's disease (PD) are among the most common neurodegenerative disorders affecting older populations. AD is characterized by impaired memory and cognitive decline while the primary symptoms of PD include resting tremor, bradykinesia and rigidity. In PD, mild cognitive changes are frequently present, which could progress to dementia (PD dementia (PDD)). PDD and AD dementias are different in pathology although the difference in microstructural changes remains unknown. To further understand these diseases, it is essential to understand the distinct mechanism of their microstructural changes. We used diffusion tensor imaging (DTI) to investigate white matter tract differences between early stage individuals with AD (n=14), PD (n=12), PDD (n=9), and healthy non-demented controls (CON) (n=13). We used whole brain tract based spatial statistics (TBSS) and a region of interest (ROI) analysis focused on the substantia nigra (SN). We found that individuals with PDD had more widespread white matter degeneration compared to PD, AD, and CON. Individuals with AD had few regional abnormalities in the anterior and posterior projections of the corpus callosum while PD and CON did not appear to have significant white matter degeneration when compared to other groups. ROI analyses showed that PDD had the highest diffusivity in the SN and were significantly different from CON. There were no significant ROI differences between CON, PD, or AD. In conclusion, global white matter microstructural deterioration is evident in individuals with PDD, and DTI may provide a means with which to tease out pathological differences between AD and PD dementias.
Keywords: Neurodegenerative disorders, Dementia, Corticospinal tract, Corpus callosum, Cingulum
Introduction
Aging is accompanied by alterations in physiological, functional, and structural biological changes in the brain associated with neurodegeneration [1]. In older adults the most frequent neurodegenerative disorder is Alzheimer's disease (AD) followed by Parkinson's disease (PD) [2-4]. AD is characterized by impaired memory and cognitive decline, roughly affecting 4.5 million people in the US [5] while PD is a movement disorder with primary motor features such as rest tremor, bradykinesia and rigidity [6]. The risk of developing dementia in PD (PDD) is reported to be as high as 70% with a 6-fold chance to develop dementia compared to age matched controls [7,8]. The loss of dopaminergic neurons in the substantia nigra is considered the hallmark neuropathological finding of PD [9,10]. Yet it is evident that the white matter pathological changes go beyond the basal ganglia, as shown by significant white matter deterioration in the cingulum [11,12] corticospinal tract, corpus callosum [13], and projection fibers along the thalamus, putamen, temporal, and frontal cortices [10,14].
In the last few decades, in vivo non-invasive techniques such as diffusion tensor imaging (DTI) have been developed to investigate white matter alterations in neurodegenerative diseases. DTI measures the orientation and direction of water molecules in neural tissue and primarily characterizes the integrity of white matter fibers [15,16]. Diffusion studies on individuals with PD have been inconsistent. Two studies reported a lack of white matter deficit when comparing PD to healthy elderly subjects [3,17], while others found reduced white matter integrity by means of fractional anisotropy (FA), mainly in the substantia nigra [3,18,19], cingulum [20], thalamus, putamen, frontal, and temporal cortices [10]. A study using a more global approach (tract based spatial statistics (TBSS)) showed reduced FA in PD compared to CON mainly in superior and inferior pre/post central gyrus, posterior striatum and frontal white matter regions [10].
Alterations in PDD white matter as evidenced by decreased FA have also been reported in a number of brain regions such as the corpus callosum [20], frontal, temporal, and occipital lobes [11,17]. However negative comparisons results between PD, PDD, and CON have also been reported in regions such as the cingulum and corpus callosum [12]. Additional DTI studies examined individuals with Lewy body dementia (LBD), which presents with early cognitive changes as well as parkinsonism and has a pathology similar to PDD [21]. Hattori et al. found that individuals with PDD and LBD both had decreased FA within posterior and anterior regions compared to controls [17]. Watson et al. compared LBD and AD using DTI and found reduced FA in LBD subjects compared to CON predominantly in the parieto-occipital tracts with relative sparing in the frontal areas, while the AD cohort showed a more widespread reduced FA in frontal regions when compared to CON [22]. Kantarci et al. found decreased diffusivity mainly in the amygdala and the inferior longitudinal fasciculus (associated with visual hallucinations) when patients with DLB were compared to controls while AD were characterized with higher diffusivity in temporo-parietal regions [23].
Though recent studies have investigated white matter changes associated with various neurodegenerative diseases, to our knowledge limited studies have compared PD, and AD dementias at the level of white matter microstructure with inconclusive results. It is important to understand these microstructural differences, which will help us have a better understanding of each disease progression. Thus, in this study we aimed to characterize group differences in white matter neural fibers using two anisotropic directionality markers: fractional anisotropy (FA) and mean diffusivity (MD). Whole brain voxelwise analyses were performed using tract based-spatial statistics (TBSS), among three neurodegenerative disease groups: PDD, AD, and non-demented PD.Secondly, we aimed to measure DTI markers of dopaminergic neuronal loss using aregion of interest (ROI) analysis in the substantia nigra (SN). We hypothesized that the cohort with combined cognitive decline and impaired motor control (PDD) would show the most expansive global white matter degeneration compared to AD, PD and healthy aging individuals (CON).
Methodologies
Demographics
A total of 48 participants were included in this study. Individuals with PD and PDD were recruited from the Parkinson's Disease and Movement Disorder Center at the University of Kansas Medical Center. All patients were diagnosed with idiopathic PD by a neurologist specializing in movement disorders according to the United Kingdom Parkinson's Disease Society Brain Bank Criteria for diagnosis [24]. Diagnostic criteria for PDD were based on recommendations from the Movement Disorder Society Task Force for level 1 testing [25] and included tests from the Uniform Data Set (UDS) used by the national network of Alzheimer's Disease Centers [26]. Extra pyramidal signs were assessed using the motor subscale of the Unified Parkinson's Disease Rating Scale (UPDRS). Healthy elderly non-demented subjects (Clinical Dementia Rating [CDR], 0; n=13) and individuals with AD (CDR, 0.5; n=12, CDR, 1; n=2) were included from ongoing studies at the University of Kansas Alzheimer's Disease Research Center [27]. Diagnostic criteria for AD require the gradual onset and progression of impairment in memory and in at least one other cognitive and functional domain [28]. The presence or absence of AD dementia, and its severity if present, was determined using the CDR scale [29].
Diffusion imaging acquisition
Diffusion weighted images were acquired in a 3.0 Tesla Allegra MRI scanner using single-shot echo-planar imaging sequences with a repetition time [TR]=1000ms and echo time [TE]=81ms. Diffusion gradients were applied in 36 directions with 2 b-values (b = 0 s/mm2 and b= 800 s/mm2). Sixty-eight 2-mm sections were acquired in at in-plane resolution of 128×128 with a 300 mm field of view (FOV). The total image acquisition time was 12 min.
Tract based spatial statistics
We performed voxelwise analysis of FA and MD using tract based spatial statistics (TBSS) [30], part of the Functional Software Laboratory (FSL 4.1.9) [31]. In the first step, 1×1×1 mm FA and MD images were created by fitting a tensor model to the raw eddy corrected diffusion data using FMRIB's Diffusion Toolbox(FDT) and then brain-extracted using the Brain Extraction Tool (BET) [32]. All subjects’ FA/MD images were aligned into a common space target (FMRIB58_FA) using the nonlinear registration tool FNIRT [33,34] which uses a b-spline representation of the registration warp field [35]. After registration, the mean FA image was created and thinned (at 0.2 threshold) to create a mean FA skeleton, which represents the centers of all tracts common to all the groups. Each subject's aligned FA/MD data were then projected perpendicular onto this skeleton and the data fed into voxelwise cross-subject statistics.
We performed group differences among AD, PD, PDD and CON for FA and MD using randomise, a TBSS statistical tool that computes non-parametric permutations using the generalized linear model. We used threshold-free cluster enhancement (TFCE), which identifies “clusters” of significant results in the dataset [36-38]. The number of permutations was set to 5,000 using age and gender as the confound regressors. We compared each group to the other. Significance values were reported at p<0.05 family-wise error (FWE) corrected. The principal white matter tracts derived from the John Hopkins University (JHU) white-matter probabilistic tractography atlas [39,40] were used. Fiber tract threshold criteria included any tract greater than 0.7 with mention of no secondary fibers in that skeleton location.
Region of interest (ROI) analysis
Substantia nigra ROI were drawn on each image using FSLView 3.1.8, part of the Oxford Center for Functional MRI of the Brain (FMRIB) software library (FSL) [31]. After identifying the slice where the substantia nigra (SN), red nucleus and subthalamic nucleus were noticeable, we moved one slice ventral where the SN was visible. At this specific slice location, two raters (R.P. and R.R.) drew ROIs in the red-green-blue (RGB) principal directionally invariant eigenvector map, using the green color as a reference for fibers in the SN traveling in the ventral-dorsal direction. Values were extracted from the FA and MD map (Figure 1). Inter-class correlation was 0.834 while intra-class correlations were 0.913 and 0.949 respectively. Additionally, a Bland-Altman analysis [41,42] was used to compare values giving a mean difference of 0.03 percentage points with a 95% confidence interval between −0.13 and 0.064. For comparisons between groups, one-way analysis of variance (ANOVA) was performed using age as a covariate. All statistical analyses were performed using SPSS 20.0 (SPSS Inc, Chicago, IL) and significant values were set to p<0.05.
Figure 1.
Region of Interest (ROI) image representations in the substantia nigra (SN). (A) Shows the b0 slice image with brighter SN contrast. (B) Shows the FA image. (C) Shows the principal eigenvectors in RGB color. (D) is a zoomed image of the region of interest mask (in red).
Results
Demographics and clinical features
Table 1 shows the demographics data for the PD, PDD, AD, and CON groups. Education and MMSE were found to be significantly different among groups. Post-hoc analyses showed that PDD had a significantly lower mean education compared to CON (p=0.002), and the AD group (p =0.003). The PDD group also had a significantly lower MMSE than the AD, CON, and PD groups (p<0.001) while AD was significantly lower than the CON (p =0.006) and the PD (p=0.016). UPDRS and disease duration was significantly higher in PDD group compared to the PD group (p <0.001).
Table 1.
Demographics data for the PD, PDD, AD, and CON groups.
| Control | AD | PD | PDD | |||
|---|---|---|---|---|---|---|
| (n=13) | (n=14) | (n=12) | (n=9) | P-Value | Post-hoc significance (LSD) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age (yrs) | 71.54 (7.45) | 71.07 (7.32) | 67.5 (4.01) | 74.78 (5.12) | 0.086 | |
| Sex (M:F) | 6:7 | 8:6 | 5:7 | 8:1 | 0.14 | |
| Education (yrs) | 16.77 (2.09) | 16.64 (2.27) | 15.33 (2.23) | 13.67 (2.18) | 0.007 | CON, AD >PDD* |
| MMSE (max 30) | 28.92 (1.32) | 26.79 (2.45) | 28.67 (1.44) | 22.22 (2.17) | <0.001 | CON, AD, PD>PDD** and CON >AD** |
| Total UPDRS | NA | NA | 19.58 (7.10) | 32.89 (5.86) | <0.001 | PDD>PD** |
| Disease Duration (years) | NA | NA | 6.38 (4.19) | 16.33 (6.92) | <0.001 | PDD>PD** |
NA= not applicable; n= number; SD = standard deviation
p<0.01
p<0.001.
Region of interest (ROI) analysis
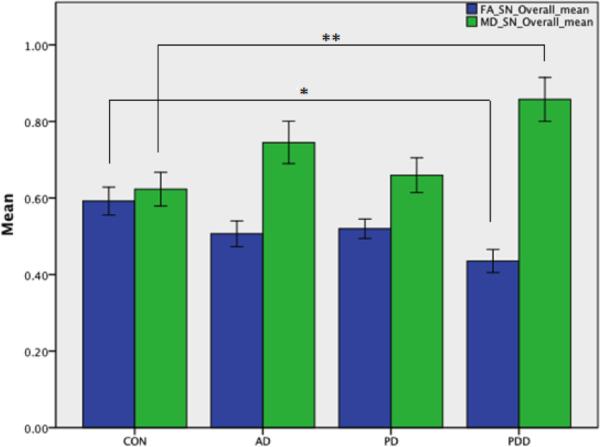
We found a significant decrease in FA in the substantia nigra in the PDD group compared to controls (p=0.004). We found that individuals with PDD also had increased mean diffusivity when compared to controls (p=0.011). There was no significant FA or MD differences between CON and AD, PD and AD, PDD and AD, PD and CON, or PD and PDD. Mean values and standard errors are shown in Figure 2.
Figure 2.
Substantia nigra ROIs. Overall means across different groups: fractional anisotropy (blue) and mean diffusivity (green). Error bars denote ± 1 standard error of the mean (SEM). One-way ANOVA analyses with age and sex as covariates were performed among each group. Mean values for each group are CON (FA mean= 0.59, MD mean=0.62), AD (FA mean= 0.51 MD mean= 0.75), PD (FA mean= 0.52 MD mean= 0.67), and PDD (FA mean=0.44 MD mean=0.86). Stars denote significant values for FA CON>PDD (at p=0.004*), and MD at PDD>CON (at p=0.01**).
Tract Based Spatial Statistics
Non-parametric voxel wise TBSS analysis was performed to compare group differences between PD, PDD, AD, and control groups on FA and MD projected skeleton maps. Significant results were divided in ten different probabilistic tracts [43]. Table 2 shows the significant group differences for fractional anisotropy while Table 3 shows the significant group comparisons for the mean diffusivity. Fractional anisotropy and mean diffusivity brain maps differences are shown in Figure 3.
Table 2.
The significant group differences for fractional anisotropy.
| Fractional Anisotropy (FA) | CON > PDD | PD > PDD | AD > PDD | PD > AD | ||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Anterior Thalamic Radiation | - | - | ◆ | ◆ | ■ | - | - | - |
| Corticospinal Tract | - | - | – | – | ■ | - | - | - |
| Forceps Major | ◆ | ◆ | – | – | ■ | ■ | ■ | - |
| Forceps Minor | ■ | ■ | ◆ | ◆ | – | ■ | - | ■ |
| Inferior fronto-occipital fasciculus | ■ | ■ | ◆ | ◆ | ■ | ■ | ■ | - |
| Inferior Longitudinal fasciculus | ◆ | ◆ | ◆ | ◆ | ■ | ■ | ■ | - |
| Superior longitudinal fasciculus | – | ■ | ◆ | - | ■ | - | - | - |
| Uncinate fasciculus | ■ | - | ◆ | - | ■ | - | - | ■ |
| Cingulum (hippocampus) | - | - | – | - | – | - | - | - |
| Cingulum (cingulate gyrus) | - | - | ◆ | - | - | - | - | - |
■ denotes p<0.05. ◆ denotes p<0.01
TBSS fractional anisotropy significant results for each group comparison on each probabilistic tract. Results were divided between left and right hemisphere. CON vs. AD and CON vs. PD did not show any significant differences and thus they were omitted from the table.
Table 3.
TBSS mean diffusivity significant results for each group comparison on each probabilistic tract.
| Mean Diffusivity (MD) | CON < PDD | PD < PDD | AD < PDD | PD < AD | ||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Anterior Thalamic Radiation | ■ | ■ | ■ | ■ | ◆ | ◆ | – | – |
| Corticospinal Tract | ■ | ◆ | ■ | ■ | ◆ | ◆ | – | – |
| Forceps Major | ■ | ■ | – | – | ◆ | ◆ | ■ | – |
| Forceps Minor | ◆ | ◆ | ◆ | ◆ | ■ | ■ | ■ | ■ |
| Inferior fronto-occipital fasciculus | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | – | – |
| Inferior longitudinal fasciculus | – | – | ◆ | ■ | ◆ | ◆ | – | – |
| Superior longitudinal fasciculus | ◆ | ◆ | ◆ | ◆ | ◆ | ◆ | – | – |
| Uncinate fasciculus | ◆ | ◆ | ◆ | ■ | ◆ | ■ | ■ | – |
| Cingulum (hippocampus) | – | – | – | ■ | – | ■ | – | – |
| Cingulum (cingulate gyrus) | ■ | – | – | – | – | – | – | – |
■ denotes p<0.05, and ◆ denotes p<0.01
Results were divided between left and right hemisphere. CON vs. AD and CON vs. PD did not show any significant differences and thus they were omitted from the table.
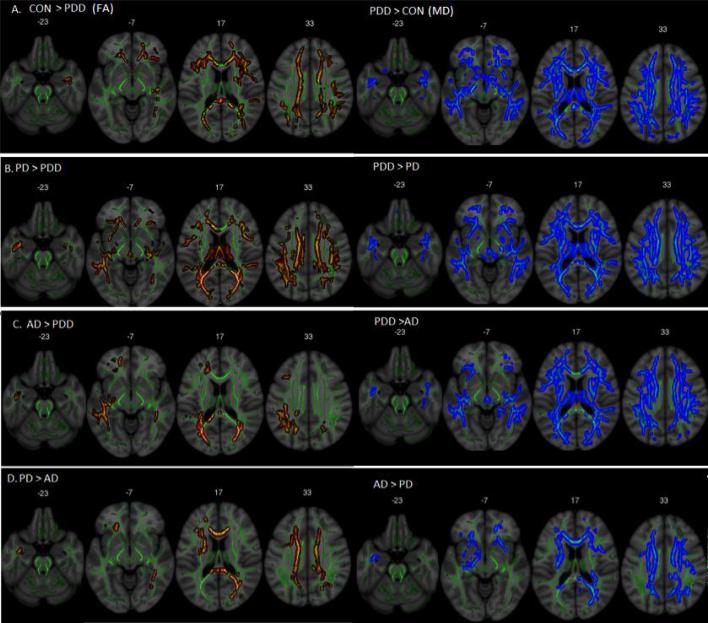
Figure 3.
Fractional anisotropy (in red) and mean diffusivity values (in blue). A) PDD showed a higher diffusion compared to controls in the corpus callosum, bilateral posterior inferior fronto-occipital fasciculus, and anterior projections regions. B) Lower diffusion in PDD compared to PD in posterior, anterior, and CC regions. C) AD shows higher diffusion when compared to PDD in the splenium of the CC and bilateral posterior fibers. D) AD showed lower diffusion values when compared to PD mainly in the corpus callosum, posterior right and anterior IFOF. All images are displayed in a Montreal Neurological Institute (MNI) background (in grey) with the mean FA skeleton superimposed (green).
Discussion
We characterized white matter micro structural differences in AD, PD, PDD, and healthy non-demented controls using tract based spatial statistics and ROI analysis in the substantia nigra. We found that the PDD group showed extensive global white matter degeneration when compared to PD, AD and healthy controls. In addition, we found that the AD group had smaller regional deficits in projection tracts of the corpus callosum (forceps minor and forceps major) when compared to PD. Our ROI analysis of the substantia nigra showed white matter deterioration in the PDD group when compared to controls by means of decreased FA and increased MD. No significant white matter abnormalities in the substantia nigra were found in the PD, AD, or non-demented control groups. Our findings suggest that individuals with PDD have a whole brain deterioration of white matter using TBSS and in the substantia nigra by means of FA and MD when compared to AD, PD, and non-demented controls.
We found degeneration of the corticospinal tracts (CST) in the PDD group when compared to CON and AD. Injuries in this tract are directly related to motor weakness and rigidity leading to an impaired human gait [44]. There was no deterioration difference in the CST when the PD group was compared to controls. A possible explanation could be that these cohort was in the early stages of PD (UPDRSMean= 19.58), which may account for a non-evident white matter deterioration. Diffusion of the inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), and superior longitudinal fasciculus (SLF) were also significantly increased in the PDD when compared to PD, AD, and controls. Even though the IFOF is poorly understood, it interconnects the frontal, temporal, and occipital lobes and its known to affect auditory and visual processing and neuro-motor functioning. The ILF provides a direct connection from occipital cortex to temporal lobe [45,46]. Anteriorly the ILF joins the uncinate fasciculus to relay information to the orbito-frontal brain [47]. Lesions in these ILF and SLF fibers have been related to thought disorders, visual emotion, and cognitive impairments [48,49]. A recent investigation [23], also found decreased diffusion in the ILF and amygdala, which was associated with visual hallucinations. In AD, the SLF has shown to be significantly reduced bilaterally [50]. The SLF has also been reported to control movement integrity, gait related functions, and early gait disturbances [51,52]. Finally, we found that the uncinate fasciculus showed significant FA decrease in PDD and AD when compared to PD, and controls. The uncinate fasciculus interconnects the amygdala, and hippocampus with the orbitofrontal cortex [53]. In AD, degeneration of the uncinate fasciculus has been associated as a secondary effect after grey matter atrophy [54] and along with the SLF, corpus callosum and cingulum are the fibers most affected [51-55].
Our results showed greatest white matter deterioration in the PDD group followed by a slight deterioration in the AD group. WM structural differences were greatest in the SN for PDD patients (ROI analyses) and observed in other motor tracts, suggesting a disease-specific effect. In global TBSS analyses, PDD was associated with more widespread WM structural differences even when compared to the early-stage AD group. Although this is surprising, the PDD group was more cognitively impaired than the early-stage AD group, suggesting that these more widespread changes may be due to the differing levels of dementia severity. Further studies controlling for dementia severity would be necessary to definitively assess this possibility. We did not find global white matter deterioration in PD compared to healthy aging individuals, which is in agreement with the recent literature [12,17,20]. A possible explanation could be the age differences between PD, AD and healthy elderly individuals, with PD being relatively younger. The decrease in anisotropy could be related to increased age and not driven by a specific pathology.
We found abnormalities in the substantia nigra only in individuals with PDD. This finding is consistent with PD dopaminergic nigrostriatal system deterioration where a greater number of neurons are lost [56]. However, no difference was found when PD was compared to the healthy individuals. Previous ROIs investigations in the basal ganglia also failed to distinguish differences in FA and MD between PD and controls [3,4,18]. Yet, a recent study found reduced FA in the substantia nigra in early PD when compared to controls, with greater differences in the caudal region [19]. A possible explanation for the lack of a difference between the PD group and controls in our study is that microstructural white matter changes of dopaminergic neurons at early stages of PD may not be detectable with our diffusion tensor acquisition methodology. High resolution and specific imaging sequencing for regions of interest across the cortex could help identifying microstructural changes at these early stages of the disease that were not apparent with our acquisition methodology.
We did not find any significant abnormalities in white matter microstructure of the AD group when compared to healthy non-demented controls perhaps because the AD patients were in the early stages of the disease (mean MMSE = 26.79). The accumulation of amyloid beta and tau neurofibrillary tangles in AD may lead to synaptic loss and degeneration [4,57,58]. However, at early stages of the disease the accumulation of these aggregates primarily damage the neuronal body, which is a major component of gray matter, and thus axonal body deterioration, which includes white matter tissue, may occur later in the disease. Previous studies that compared early stages of AD (also defined in some cases as mild cognitive impairment (MCI)), later stages of AD, and healthy normal controls showed reduced white matter diffusion in AD, and to a lesser extent in MCI, when compared to healthy controls [59,60] while others did not find any white matter changes in MCI or AD [61,62].
Our primary limitation was the relatively small number of subjects in each disease group. Age differences between the younger PD and older PDD groups were also prominent and may have impacted the results, however, we controlled for age in our analyses. Moreover, even though TBSS aims to improve the sensitivity of subject registration and smoothing, it performs qualitative statistics in a normalized number of fibers, thus some pathological changes in each fiber may be disregarded due to the thinning out of the skeleton map. In addition, FA and MD results did not overlap in every tract due to its different diffusivity definition. FA is a marker of diffusivity along the main diffusion of direction while MD is used to measure diffusion integrity in every direction in a specific voxel. Future investigation with other diffusion markers such as radial diffusivity and/or axial diffusivity should be taken into account. Our results show that there is a higher decrease in white matter integrity rather than diffusion decrease along the main direction. Finally, this study is a cross-sectional study, and further investigation with longitudinal data could give us more insights on the progressive nature of each of these pathologies.
Conclusion
We found that individuals with Parkinson's disease dementia showed significant regional (substantia nigra) and global white matter deterioration when compared to non-demented individuals by means of fractional anisotropy and mean diffusivity. Individuals at early stages of Alzheimer's dementia and Parkinson's disease did not appear to have significant white matter degeneration compared to non-demented elderly subjects. Additional longitudinal studies within the same cohorts will be useful to characterize whether further changes appear in individuals with AD and PD as the diseases progress.
Acknowledgements
This study was supported by grants and R01AG033673 and R01AG034614 from the National Institutes of Aging, and a TEVA award from the Kansas City Area Life Sciences Initiative. The University of Kansas General Clinical Research Center (M01RR023940), which is now and is now the National Center for Advancing Translational Sciences (UL1RR033179) provided essential space, expertise, and nursing support. This research was also supported by the KU Alzheimer's Disease Center (NIH grant P30AG010129 through the National Institute on Aging). E.D.V is supported by KL2 TR000119, R.A.H and R.D.P are supported by K01AG030514, and J.K.M. is supported in part by the KU Medical Center Biomedical Research Training Program.
Footnotes
Citation: Perea RD, Rada RC, Wilson J, Vidoni ED, Morris JK, et al. (2013) A Comparative White Matter Study with Parkinson's disease, Parkinson's Disease with Dementia and Alzheimer's Disease. J Alzheimers Dis Parkinsonism 3: 123. doi: 10.4172/2161-0460.1000123
Disclosure Statement
Rajesh Pahwa-Dr. Pahwa has received grants to his institution from NIH, NPF, Teva Neuroscience, Novartis, Xenoport, Impax, EMD Serono, Allon, Acadia, Schering, Biotie, Phytopharm, Adamas. He received personal compensation from Novartis, Teva Neuroscience, Medtronic, St Jude Medical, GE Healthcare, EMD Serono, Adamas, Impax, Medscape. He served on a DSM committee for Ceregene. He received royalties from Oxford Press and Informa Healthcare. He is coeditor in chief for the International Journal of Neuroscience.
References
- 1.Beal MF, Lang AE, Ludolph AC. Neurodegenerative Diseases: Neurobiology, Pathogenesis and Therapeutics. Cambridge University Press; 2005. [Google Scholar]
- 2.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa K, Nakata Y, Yamada K, Nakagawa M. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J Neurol Neurosurg Psychiatry. 2004;75:481–484. doi: 10.1136/jnnp.2003.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schocke MF, Seppi K, Esterhammer R, Kremser C, Jaschke W, et al. Diffusion-weighted MRI differentiates the Parkinson variant of multiple system atrophy from PD. Neurology. 2002;58:575–580. doi: 10.1212/wnl.58.4.575. [DOI] [PubMed] [Google Scholar]
- 5.Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2011;32:2322. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Factor SA, Weiner WJ. Parkinson's disease: diagnosis and clinical management. Demos Medical Publishing; 2007. [Google Scholar]
- 7.Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 8.Aarsland D, Laake K, Larsen JP, Janvin C. Donepezil for cognitive impairment in Parkinson's disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2002;72:708–712. doi: 10.1136/jnnp.72.6.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudow G, O'Brien R, Savonenko AV, Resnick SM, Zonderman AB, et al. Morphometry of the human substantia nigra in ageing and Parkinson's disease. Acta Neuropathol. 2008;115:461–470. doi: 10.1007/s00401-008-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan W, Kang GA, Glass GA, Zhang Y, Shirley C, et al. Regional alterations of brain microstructure in Parkinson's disease using diffusion tensor imaging. Mov Disord. 2012;27:90–97. doi: 10.1002/mds.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui H, Nishinaka K, Oda M, Niikawa H, Kubori T, et al. Dementia in Parkinson's disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116:177–181. doi: 10.1111/j.1600-0404.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- 12.Wiltshire K, Concha L, Gee M, Bouchard T, Beaulieu C, et al. Corpus callosum and cingulum tractography in Parkinson's disease. Can J Neurol Sci. 2010;37:595–600. doi: 10.1017/s0317167100010751. [DOI] [PubMed] [Google Scholar]
- 13.Gattellaro G, Minati L, Grisoli M, Mariani C, Carella F, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, et al. DTI correlates of distinct cognitive impairments in Parkinson's disease. Hum Brain Mapp . 2013 doi: 10.1002/hbm.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 16.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 17.Hattori T, Orimo S, Aoki S, Ito K, Abe O, et al. Cognitive status correlates with white matter alteration in Parkinson's disease. Hum Brain Mapp. 2012;33:727–739. doi: 10.1002/hbm.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan LL, Rumpel H, Yap K, Lee E, Loo HV, et al. Case control study of diffusion tensor imaging in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:1383–1386. doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaillancourt DE, Spraker MB, Prodoehl J, Abraham I, Corcos DM, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72:1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamagata K, Motoi Y, Abe O, Shimoji K, Hori M, et al. White matter alteration of the cingulum in Parkinson disease with and without dementia: evaluation by diffusion tensor tract-specific analysis. AJNR Am J of neuroradiology. 2012;33:890–895. doi: 10.3174/ajnr.A2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodel R, Csoti I, Ebersbach G, Fuchs G, Hahne M, et al. Lewy body dementia and Parkinson's disease with dementia. J Neurol. 2008;255(Suppl 5):39–47. doi: 10.1007/s00415-008-5007-0. [DOI] [PubMed] [Google Scholar]
- 22.Watson R, Blamire AM, Colloby SJ, Wood JS, Barber R, et al. Characterizing dementia with Lewy bodies by means of diffusion tensor imaging. Neurology. 2012;79:906–914. doi: 10.1212/WNL.0b013e318266fc51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantarci K, Avula R, Senjem ML, Samikoglu AR, Zhang B, et al. Dementia with Lewy bodies and Alzheimer disease: neurodegenerative patterns characterized by DTI. Neurology. 2010;74:1814–1821. doi: 10.1212/WNL.0b013e3181e0f7cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 25.Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 26.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, et al. The Alzheimer's Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidoni ED, Van Sciver A, Johnson DK, He J, Honea R, et al. A community-based approach to trials of aerobic exercise in aging and Alzheimer's disease. Contemp Clin Trials. 2012;33:1105–1116. doi: 10.1016/j.cct.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersson J, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2. 2007 [Google Scholar]
- 34.Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB Analysis Group Technical Reports: TR07JA02. 2007 [Google Scholar]
- 35.Schnabel J, Rueckert D, Quist M, Blackall JM, Castellano-Smith AD, et al. A generic framework for non-rigid registration based on non-uniform multi-level free-form deformations. Springer; 2001. pp. 573–581. [Google Scholar]
- 36.Anderson MJ, Robinson J. Permutation tests for linear models. Australian & New Zealand Journal of Statistics. 2001;43:75–88. [Google Scholar]
- 37.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 39.Hua K, Zhang J, Wakana S, Jiang H, Li X, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 42.Hanneman SK. Design, analysis, and interpretation of method-comparison studies. AACN Adv Crit Care. 2008;19:223–234. doi: 10.1097/01.AACN.0000318125.41512.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 44.Barthélemy D, Grey MJ, Nielsen JB, Bouyer L. Involvement of the corticospinal tract in the control of human gait. Prog Brain Res. 2011;192:181–197. doi: 10.1016/B978-0-444-53355-5.00012-9. [DOI] [PubMed] [Google Scholar]
- 45.Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer's disease: preliminary findings and future directions. Biochim Biophys Acta. 2012;1822:416–422. doi: 10.1016/j.bbadis.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kvickström P, Eriksson B, van Westen D, Lätt J, Elfgren C, et al. Selective frontal neurodegeneration of the inferior fronto-occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC Neurol. 2011;11:13. doi: 10.1186/1471-2377-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashtari M. Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Dev Med Child Neurol. 2012;54:6–7. doi: 10.1111/j.1469-8749.2011.04122.x. [DOI] [PubMed] [Google Scholar]
- 48.Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20:209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 50.Parente DB, Gasparetto EL, da Cruz LC, Jr, Domingues RC, Baptista AC, et al. Potential role of diffusion tensor MRI in the differential diagnosis of mild cognitive impairment and Alzheimer's disease. AJR Am J Roentgenol. 2008;190:1369–1374. doi: 10.2214/AJR.07.2617. [DOI] [PubMed] [Google Scholar]
- 51.Scherder E, Eggermont L, Visscher C, Scheltens P, Swaab D. Understanding higher level gait disturbances in mild dementia in order to improve rehabilitation: ‘last in-first out’. Neurosci Biobehav Rev. 2011;35:699–714. doi: 10.1016/j.neubiorev.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 53.Papagno C, Miracapillo C, Casarotti A, Romero Lauro LJ, Castellano A, et al. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011;134:405–414. doi: 10.1093/brain/awq283. [DOI] [PubMed] [Google Scholar]
- 54.Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, et al. White matter tract integrity in aging and Alzheimer's disease. Hum Brain Mapp. 2009;30:1051–1059. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. Neuroimage. 2011;55:880–890. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodaie M, Neimat JS, Lozano AM. The dopaminergic nigrostriatal system and Parkinson's disease: molecular events in development, disease, and cell death, and new therapeutic strategies. Neurosurgery. 2007;60:17–28. doi: 10.1227/01.NEU.0000249209.11967.CB. [DOI] [PubMed] [Google Scholar]
- 57.Simon DK, Chu CT, Swerdlow RH. Mitochondria and Parkinson's disease. Parkinsons Dis. 2011;2011:261791. doi: 10.4061/2011/261791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swerdlow RH. Brain aging, Alzheimer's disease, and mitochondria. Biochim Biophys Acta. 2011;1812:1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuang L, Wen W, Zhu W, Trollor J, Kochan N, et al. White matter integrity in mild cognitive impairment: a tract-based spatial statistics study. Neuroimage. 2010;53:16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]
- 60.Shu N, Wang Z, Qi Z, Li K, He Y. Multiple diffusion indices reveals white matter degeneration in Alzheimer's disease and mild cognitive impairment: a tract-based spatial statistics study. J Alzheimers Dis. 2011;26:275–285. doi: 10.3233/JAD-2011-0024. [DOI] [PubMed] [Google Scholar]
- 61.Scola E, Bozzali M, Agosta F, Magnani G, Franceschi M, et al. A diffusion tensor MRI study of patients with MCI and AD with a 2-year clinical follow-up. J Neurol Neurosurg Psychiatry. 2010;81:798–805. doi: 10.1136/jnnp.2009.189639. [DOI] [PubMed] [Google Scholar]
- 62.Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, et al. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis. 2010;22:507–522. doi: 10.3233/JAD-2010-100234. [DOI] [PubMed] [Google Scholar]