Abstract
Factors other than age and genetics may increase the risk of developing Alzheimer disease (AD). Accumulation of the amyloid-β (Aβ) peptide in the brain seems to initiate a cascade of key events in the pathogenesis of AD. Moreover, evidence is emerging that the sleep–wake cycle directly influences levels of Aβ in the brain. In experimental models, sleep deprivation increases the concentration of soluble Aβ and results in chronic accumulation of Aβ, whereas sleep extension has the opposite effect. Furthermore, once Aβ accumulates, increased wakefulness and altered sleep patterns develop. Individuals with early Aβ deposition who still have normal cognitive function report sleep abnormalities, as do individuals with very mild dementia due to AD. Thus, sleep and neurodegenerative disease may influence each other in many ways that have important implications for the diagnosis and treatment of AD.
Introduction
Disturbances of sleep and circadian rhythm frequently impair the quality of life and safety of individuals with alzheimer disease (AD). insomnia at night, agitated behaviour at sunset and excessive sleeping during the daytime affect 25-40% of patients with mild to moderate dementia due to AD in the community setting, and the intensity of these changes correlates with the severity of dementia.1 Circadian rhythms decrease in amplitude and show a phase delay, particularly in patients with advanced stages of dementia due to AD.2 Sleep problems occur very early on in the course of AD, consistent with the finding that brain regions involved in sleep and circadian control are affected early in the pathogenesis of the condition.3 Individuals with amnestic mild cognitive impairment, many of whom have very early AD,4,5 show EEG abnormalities during sleep, including fewer sleep spindles and reduced amounts of slow-wave sleep (SWS).6
The pathology of AD emerges prior to any symptoms, with the first identifiable changes occurring ~10–15 years before cognitive symptoms. In the earliest stage of preclinical AD, soluble amyloid-β (Aβ) becomes insoluble and aggregates into amyloid plaques, initially manifesting as a reduction in soluble Aβ42 levels in the cerebrospinal fluid (CSF).7 Our research group has focused on individuals in this first stage of preclinical AD who are cognitively normal, but have biomarker evidence of amyloid plaques. Compared with their peers who do not have evidence of amyloid plaques, these individuals have worse quality of sleep, as assessed by actigraphy-measured sleep efficiency and wake time after sleep onset.8 These differences are significant even after adjustment for age, sex, and the presence of the APOE ε4 allele (an important risk factor for late-onset, sporadic AD). Tau tangles, the other pathological hallmark of AD, might also adversely affect sleep or circadian rhythms, but have not been investigated.
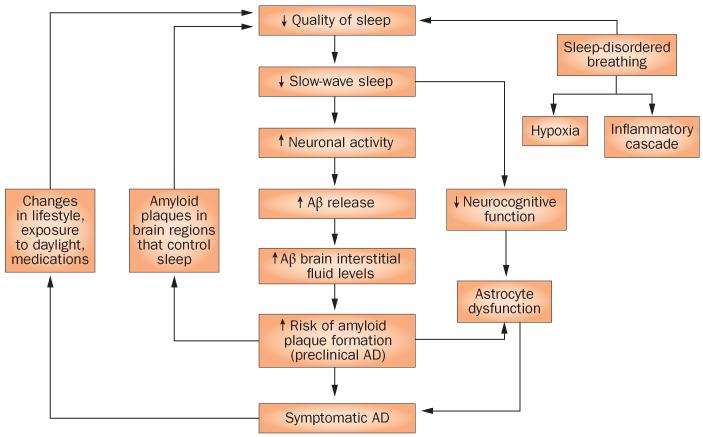
Changes in sleep seem to precede the onset of cognitive symptoms in patients with AD, and sleep quality and/or circadian function declines further in parallel with both cognitive dysfunction and the progression of AD pathology. However, the time course of changes in sleep, from preclinical AD to the clinical stages of dementia due to AD, is yet to be defined. In this Perspectives article, we discuss the evidence that an association exists between AD and disrupted sleep; that amyloid accumulation disrupts sleep; and that disrupted sleep increases the risk of Aβ accumulation in mice, as well as dementia due to AD in humans. On the basis of this information, we propose a bidirectional relationship between AD and sleep quality, and provide a hypothesis for the mechanisms underlying this relationship (Figure 1).
Figure 1.
The bidirectional relationship between sleep and AD. Potential positive-feedback mechanisms exist between the accumulation of Aβ, impaired sleep quality and effects on cognitive function. Abbreviations: Aβ, amyloid-β; AD, Alzheimer disease.
Sleep and AD pathology
Although Aβ accumulation in the brain is one of the first key pathological findings in AD and may serve as the instigator of disrupted sleep, other factors probably contribute to the severity of sleep problems in patients with AD. Elderly individuals, especially if they have other medical conditions, may not have regular physical activity or mealtimes and, therefore, lack strong zeitgebers to entrain their circadian rhythms. Inadequate daylight exposure for patients in institutional care2 might result in deficient input to the suprachiasmatic nucleus via the retinohypothalamic tract, further diminishing circadian amplitude. Medications for common comorbidities, such as depression, hypertension or cardiac disease, can also disrupt sleep–wake functions. Obstructive sleep apnoea is also very common in patients with AD, and might further impair sleep quality.
Studies in mouse models of AD confirm the association between disrupted sleep and AD pathology, and indicate a possible causal relationship. In one transgenic mouse model (the amyloid precursor protein/presenilin 1 or APP/PS1 model), which develops Aβ deposition in the brain, increased wakefulness and decreased sleep starts around the time that amyloid plaques begin to accumulate in the hippocampus and cortex (6 months of age), and significantly disrupted sleep patterns are evident by the time that plaques become widespread (9 months of age).9 The APP/PS1 knockin model of AD demonstrates a circadian rhythm delay,10 whereas the PLB1 triple knock-in model (in which mice carrying a single copy each of mutant human APP and tau transgenes are crossed with mice overexpressing PS1) show reduced sleep duration, slowed EEG traces during wake-fulness, shorter sleep bouts and reduced circadian amplitude by 12 months of age.11
Studies in the APP/PS1 model demonstrate diurnal variation in the level of soluble Aβ in the interstitial fluid, which increases during wakefulness and decreases during sleep. This diurnal variation disappears with the onset of accumulation of amyloid plaques, which occurs at 6 months in the hippocampus, but not until 9 months in the striatum. Importantly, active immunization with Aβ prior to amyloid deposition prevented the formation of amyloid plaques, maintained diurnal variation in the level of soluble Aβ, and normalized sleep–wake patterns in this model.9 This observation strongly suggests that a form of Aβ that accumulates leads to the sleep–wake disruptions in this model, and possibly those observed in preclinical AD. Further research is required to tease apart the contributions of these and other factors to sleep problems in AD. However, data from preclinical AD in humans and mouse models support a direct negative effect of Aβ accumulation on sleep function.
Disrupted sleep and the risk of AD
In multiple cross-sectional studies, sleep durations ≤5 h12 and ≥11 h per night13 have been associated with an increased risk of cognitive impairment. Additional markers of poor quality sleep, such as low sleep efficiency, prolonged latency of sleep onset, increased wakefulness after sleep onset, and increased daytime napping, have all been associated with impaired cognitive function, in both cross-sectional14 and prospective studies over 1 year.15 A large prospective study of sleep, measured by actigraphy, demonstrated that increased sleep fragmentation also increased the risk of developing AD.16 Evidence that disrupted sleep might result in cognitive impairment has come from studies of individuals with sleep-disordered breathing. In a prospective study of 298 women without dementia, the 105 individuals with sleep-disordered breathing had an adjusted odds ratio of 1.85 of developing mild cognitive impairment or dementia.17 This increased risk was associated with frequent oxygen desaturations (>15 per hour), but not with measurement of sleep fragmentation or sleep duration.
Studies of incident dementia suggest that sleep problems increase the risk of dementia.15-17 However, an important consideration is that diagnosis of AD typically occurs years after the onset of pathological changes of AD in the brain. These incident dementia studies were conducted over 1–6 years, which is considerably shorter than the 10–15 years during which preclinical AD is present prior to onset of symptomatic AD.7 These studies should probably be considered to represent cross-sectional trials in which some individuals already had preclinical AD, rather than true prospective studies of incident AD. Until human studies with substantially longer follow-up times have been completed, animal models of AD are likely to provide the best available data with which to discern the directionality and underlying mechanisms of the relationship between AD and sleep abnormalities.
In both mice18 and humans,19 levels of soluble Aβ fluctuate with the sleep–wake cycle, in a diurnal pattern. This observation suggests a potential mechanism through which sleep problems may increase the risk of developing AD. The acute effect of sleep deprivation is an increase in Aβ concentrations; moreover, chronic sleep deprivation accelerates Aβ deposition into insoluble amyloid plaques in two different transgenic mouse models of amyloidosis (the APPSWE and APPSWE/PS1DE9 models).18 Conversely, enhanced sleep through treatment with an orexin receptor antagonist decreased Aβ plaque deposition in these models.18
Comparable sleep-deprivation studies in humans have not been published. However, the similarities in Aβ dynamics between humans and mouse models suggest that the same mechanisms might be involved. The same diurnal Aβ pattern has been observed in CSF in humans, albeit with a 6-h delay attributed to the Aβ transit time between the interstitial fluid in the brain and the lumbar CSF.9,19 In mice, diurnal variation in the levels of Aβ in brain interstitial fluid disappears when amyloid plaques are present in the corresponding brain region.9 Individuals with amyloid plaques (as determined by Pet imaging with Pittsburgh compound B) also lacked diurnal variation in the level of soluble Aβ, particularly Aβ42.19 In humans with presenilin mutations that cause familial AD, those whose imaging scans were negative for amyloid deposition had normal diurnal variation in the levels of Aβ in CSF, whereas those with amyloid deposition had attenuation of the diurnal pattern of soluble Aβ in CSF.9
In summary, humans and mice have remarkably similar Aβ dynamics, which change in a similar way when amyloid plaques are present in the brain. Robust data from mouse models support a causal role for disrupted sleep patterns in alterations of soluble Aβ dynamics and, subsequently, in amyloid accumulation. Prospective studies in humans also support the hypothesis that disrupted sleep contributes to the risk of incident dementia. Long-term studies in humans are required, however, to confirm the mouse model data showing that disrupted sleep accelerates AD at a pathophysiological level.
Sleep patterns and Aβ
Non-rapid eye movement (non-REM) sleep is a relatively quiescent state at the neuronal level. 18F-fluorodeoxyglucose Pet studies of individuals in the awake state and in non-REM and REM sleep have shown that the cerebral metabolic rate, as measured by glucose utilization, is similar during REM sleep and wakefulness, but declines by 43.8% on average during SWS, which is the deepest stage of non-REM sleep.20,21 The physiological differences in this parameter between SWS and awake states are—to some extent at least—manifestations of changes in neuronal activity. For instance, cortical neurons continuously fire irregularly in both the awake and REM states, resulting in low-amplitude, high-frequency waves on EEG. During SWS, cortical neurons oscillate between silent periods of hyperpolarization and firing during depolarization. this oscillation manifests on EEG as high-amplitude, low-frequency waves.22
Neuronal firing, or synaptic activity, releases Aβ into the brain interstitial fluid, and regional increases in neuronal activity are associated with regional increases in the concentration of Aβ in the interstitial fluid.23 During SWS, neurons spend most of the time in the hyperpolarized, silent state and, therefore, are predicted to have less overall neuronal activity and to release less Aβ than they do during other stages of sleep or wakefulness. The diurnal variations in Aβ concentration observed in interstitial fluid in mice18 and in CSF in humans,19 which are characterized in both cases by decreased levels of Aβ during sleep, supports this hypothesis.
If the quality of sleep is poor and an individual is awake or lightly sleeping, or cannot reach and sustain SWS, then the amount of time during the sleep period that cortical neurons will depolarize and fire is likely to be increased relative to that during a good-quality sleep period. This increase in neuronal firing during poor-quality sleep will result in greater release of Aβ, and higher Aβ levels in the interstitial fluid, compared with that occurring during a good-quality sleep period. Indeed, acute sleep deprivation in mice led to an increase in the concentration of soluble Aβ independent of the stress-response pathway, as demonstrated by blocking corticotropin-releasing factor.18 Experiments to selectively restrict specific stages of sleep are pending but, on the basis of the above data, we hypothesize that SWS has the strongest relationship with reductions in Aβ release from synapses and concentration in the interstitial fluid.
The amplitude of diurnal variation in Aβ concentration in healthy young adults—30% peak-to-peak19—is quite high, which suggests that sleep patterns could considerably affect levels of soluble, extracellular Aβ (that is, in the interstitial fluid). In states of chronic sleep disruption, such as obstructive sleep apnoea or behaviourally restricted sleep in individuals, we would predict decreased SWS, increased neuronal activity, increased Aβ release and, therefore, an increased Aβ concentration in the extracellular space.
From soluble Aβ to amyloid plaque formation
Aβ usually exists in a soluble, monomeric and nontoxic form. However, if this protein changes conformation, it becomes insoluble and aggregates into oligomers and amyloid plaques in the extracellular space. Studies in mouse models of AD have shown that a high extracellular concentration of Aβ is associated with early amyloid plaque formation.24 In APP transgenic mice that express a mutant form of APP (APPswe [also called TG2576]), the brain regions that are most vulnerable to amyloid plaque formation have increased concentrations of Aβ in the interstitial fluid when the mice are young and have not yet developed any amyloid plaques. Furthermore, a physiological increase in the neuronal activity of a specific cortical region also increases the level of Aβ and, subsequently, the amyloid plaque burden in that region.
Sleep in mice differs from that in humans in that mice are nocturnal and, therefore, sleep predominantly during the daytime. Sleep bouts are brief and occur throughout the 24-h period. However, the neurochemical mechanisms that drive wakefulness, non-REM sleep and REM sleep are highly conserved between mammalian species. In the hβAPP transgenic mouse model (also known as PDAPP), which develops specific aspects of AD pathology, including Aβ deposition, Aβ-associated neuritic degeneration and neuroinflammation, circadian sleep patterns are initially normal and degrade with age, similarly to their trajectory in human AD.25
Despite these differences between species, we hypothesize that through mechanisms that are at least partially similar in humans and mice, chronically elevated Aβ levels would increase the chance of Aβ aggregation and formation of amyloid plaques. Data that support this notion have come from functional connectivity MRI, which can be used to identify networks of separate brain regions with temporally correlated activity. The default mode network (DMN), which includes the precuneus, lateral parietal and medial prefrontal brain regions, was identified as such because these regions are most active when an individual is not attending to a specific task. This network, therefore, represents the areas of the brain that over time are likely to have the highest levels of neuronal activity. These areas correspond extraordinarily well with the brain regions most susceptible to amyloid plaque formation in AD.26
Evidence from functional MRI studies indicates that connectivity between DMN components such as the frontal cortex is decreased during sleep.27 The contribution of multiple brain regions, including the posterior cingulate cortex, parahippocampal gyrus and medial prefrontal cortex, to the DMN decreases with progression from wakefulness to SWS.28 Decreased functional connectivity between components of the DMN during sleep suggests that overall neuronal activity may be decreased in these regions. We further suggest the possibility that poor-quality sleep results in an increase in DMN connectivity during the sleep period, relative to that during high-quality sleep, leading to increased neuronal activity and, therefore, Aβ release. The correlation between high DMN connectivity and regionally selective formation of amyloid plaques in AD supports the hypothesis that increases in neuronal activity can lead to increases in soluble Aβ levels, which over time can lead to an increased risk of amyloid plaque formation.
Once amyloid plaques form, additional positive-feedback mechanisms might increase the likelihood that soluble Aβ will form insoluble oligomers and amyloid plaques. Astrocytes assist in the clearance of Aβ and also support neurons via metabolic coupling. When amyloid plaques are present, astrocytes cluster around them, and are consequently less capable of participating in metabolic coupling or Aβ clearance.29 Amyloid plaques act as a ‘sink’ for soluble Aβ, particularly the more toxic oligomeric and fibrillar Aβ42 form, such that an abnormally low level of Aβ42 in the CSF is highly correlated with amyloid plaques on Pet.30 Together with inefficient metabolic coupling and decreased Aβ clearance by astrocytes, the amount of Aβ available to be sequestered by amyloid plaques would be raised, increasing the rate of plaque growth and plaque toxicity.
Circadian factors other than sleep might also have a role in the generation of plaques. Circadian rhythms become weaker and less synchronized at a regional level in the brain with ageing,31 and this lack of synchrony might also promote amyloid aggregation through effects on molecular transcription, autophagy, and formation of reactive oxygen species.32
From amyloid plaques to dementia
Despite the fact that amyloid plaques represent the first identifiable pathological change in patients with AD, dementia does not occur until 10–15 years after their initial formation. Any genetic, physiological, or environmental factor that hastens progression from the preclinical to the symptomatic stages of AD increases the burden of AD. Sleep serves a restorative function in the brain, and has a critical role in cognitive functions. Chronic partial sleep deprivation has been associated with accumulation of neurocognitive deficits in executive function and working memory.33 In another study, fragmented sleep–wake patterns, as measured by actigraphy, were associated with poor cognitive function, even after controlling for demographic factors such as age, sex and education, as well as daily hours of rest and total daily activity.34 Chronically disrupted sleep probably results in decreased cognitive function, the extent of which is influenced by the level of brain injury; thus, an individual with AD pathology is more likely to become symptomatic if they are also under the influence of poor sleep. In addition, hypoxia related to sleep-disordered breathing, the sympathetic nervous system response to sleep loss, and inflammatory immune cascades related to sleep problems, may either contribute directly to the pathological process of AD or hasten the progression from preclinical to symptomatic AD.
Effects of AD on sleep
As mentioned in the introduction, once amyloid plaques have formed, sleep–wake functions and circadian rhythms are disrupted in both mice9-11 and humans.8 This disruption may result in a positive-feedback loop, whereby poor sleep contributes to amyloid deposition, and amyloid plaque formation disrupts sleep through effects on sleep-promoting brain regions. Although ageing and the associated retirement from work tends to improve the day-to-day stability of sleep timing and duration,35 circadian rhythms become more fragmented, with more-frequent periods of wakefulness at night and inactivity during the day.36 Moreover, once AD progresses to the symptomatic stage, additional factors contribute to increasingly poor quality sleep. Concerns for the safety of an individual may prompt their care in an institution, which is associated with poor daylight exposure and decreased daytime activity levels.2 Medications used to treat agitation may further disrupt sleep patterns, or lead to reduced synchrony of circadian rhythms and sleep periods. These factors all contribute to disrupted sleep in individuals with dementia, which can then lead to further declines in their cognitive functioning, in addition to potentially feeding back into, and hastening, the pathophysiological cascade of AD.
Potential clinical applications
One estimate suggests that a hypothetical treatment that could delay the onset of symptomatic AD by 5 years would reduce the burden of AD by 43% by 2050.37 Understanding the bidirectional relationship between sleep and AD could, therefore, open up valuable opportunities for research that might have clinical application.
One obvious approach is to investigate whether improving the quality of sleep in humans can either reduce the risk of AD or delay the progression of preclinical to symptomatic AD. A study focusing on a high-risk population, such as individuals with preclinical AD, or those who have a known genetic mutation associated with dominantly inherited AD, would be needed to test this hypothesis in a reasonable length of time to enable the onset of symptoms. Interventions to improve sleep quality also have the potential to dramatically reduce the prevalence of symptomatic AD. Smallscale studies of melatonin therapy (which promotes sleep) in patients with mild cognitive impairment have shown modest effects on psychometric test performance.38 However, long-term outcome studies to assess the effects of such treatment on progression to AD are lacking. The most successful approaches will probably target the specific stages of sleep (namely SWS) and disrupted circadian rhythms that drive the pathological progression of AD.
The best time window for a therapeutic agent to be effective in slowing or stopping the progression of AD is probably the preclinical stage. By definition, however, preclinical AD is a stage without cognitive abnormalities. As a result, the number of therapies that can be tested is limited by the long duration and high cost of clinical trials to assess the effects of a treatment on progression to symptomatic AD. Sleep is a readily quantifiable brain function that is abnormal in preclinical AD. Thus, sleep quality could potentially be used as a biomarker of disease burden in patients with preclinical AD. A metric of sleep that correlates with disease burden in the preclinical stage could also enable rapid evaluation of novel therapeutic agents at a stage when they are most likely to be successful in slowing progression to symptomatic AD.
Acknowledgements
The authors’ work is supported by NIH grant p01NS074969 and the Ellison Medical Foundation Senior Scholar Award (both to D. M. Holtzman). The research described in this article was made possible by a grant to Y.-E. S. Ju from the National Center for research resources (UL1 rr024992), which is a component of the NIH, and the NIH roadmap for Medical research.
Footnotes
Author contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Competing interests
D. M. Holtzman declares associations with the following companies: AstraZeneca, C2N Diagnostics, Eli Lilly, Genentech. See the article online for full details of the relationships. The other authors declare no competing interests.
References
- 1.Moran M, et al. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6:347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, et al. variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997;20:18–23. [PubMed] [Google Scholar]
- 3.Swaab DF, Fliers E, partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 4.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 6.Westerberg CE, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 2012;18:490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ju YS, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh JH, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci. Transl. Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan MJ, et al. Effects of aging and genotype on circadian rhythms, sleep, and clock gene expression in APPxPS1 knock-in mice, a model for Alzheimer’s disease. Exp. Neurol. 2012;236:249–258. doi: 10.1016/j.expneurol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Platt B, et al. Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, pLB1. PLoS ONE. 2011;6:e27068. doi: 10.1371/journal.pone.0027068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis. Assoc. Disord. 2006;20:41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 13.Faubel R, et al. Usual sleep duration and cognitive function in older adults in Spain. J. Sleep Res. 2009;18:427–435. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell T, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–1356. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potvin O, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–499. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim AS, et al. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaffe K, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang J-E, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, et al. Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Arch. Neurol. 2012;69:51–58. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maquet P, et al. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and 18F2-fluoro-2-deoxy-d-glucose method. Brain Res. 1990;513:136–143. doi: 10.1016/0006-8993(90)91099-3. [DOI] [PubMed] [Google Scholar]
- 21.Dangvu TT, et al. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33:1589–1603. doi: 10.1093/sleep/33.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huitrón-reséndiz S, et al. Age-independent and age-related deficits in visuospatial learning, sleep-wake states, thermoregulation and motor activity in PDAPP mice. Brain Res. 2002;22:126–137. doi: 10.1016/s0006-8993(01)03373-x. [DOI] [PubMed] [Google Scholar]
- 26.Jagust WJ, Mormino EC. Lifespan brain activity, β-amyloid, and Alzheimer’s disease. Trends Cogn. Sci. 2011;15:520–526. doi: 10.1016/j.tics.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horovitz SG, et al. Decoupling of the brain’s default mode network during deep sleep. Proc. Natl Acad. Sci. USA. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sämann PG, et al. Development of the brain’s default mode network from wakefulness to slow wave sleep. Cereb. Cortex. 2011;21:2082–2093. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- 29.Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J. Neurosci. 2012;32:10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagan AM, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann. Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 31.Farajnia S, et al. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J. Neurosci. 2012;32:5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hastings MH, Goedert M. Circadian clocks and neurodegenerative diseases: time to aggregate? Curr. Opin. Neurobiol. 2013;23:880–887. doi: 10.1016/j.conb.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 34.Lim ASP, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35:633–640B. doi: 10.5665/sleep.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vahtera J, et al. Effect of retirement on sleep disturbances: the GAZEL prospective cohort study. Sleep. 2009;32:1459–1466. doi: 10.1093/sleep/32.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luik AI, et al. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol. Int. doi: 10.3109/07420528.2013.813528. http://dx.doi.org/10.3109/07420528.2013.813528. [DOI] [PubMed]
- 37.Alzheimer’s Association Changing the trajectory of Alzheimer’s disease: a national imperative. 2010 www.alz.org [online], http://www.alz.org/documents_custom/trajectory.pdf.
- 38.Cardinali DP, et al. Therapeutic application of melatonin in mild cognitive impairment. Am. J. Neurodegener. Dis. 2012;1:280–291. [PMC free article] [PubMed] [Google Scholar]