Abstract
The pathologic phosphorylation and sub-cellular translocation of neuronal transactive response-DNA binding protein (TDP-43) was identified as the major disease protein in frontotemporal lobar degeneration (FTLD) with ubiquitinated inclusions, now termed FTLD-TDP, and amyotrophic lateral sclerosis (ALS). More recently, TDP-43 proteinopathy has been reported in dementia pugilistica or chronic traumatic encephalopathy caused by repetitive traumatic brain injury (TBI). While a single TBI has been linked to the development of Alzheimer’s disease and an increased frequency of neurofibrillary tangles, TDP-43 proteinopathy has not been examined with survival following a single TBI. Using immunohistochemistry specific for both pathological phosphorylated TDP-43 (p-TDP-43) and phosphorylation-independent TDP-43 (pi-TDP-43), we examined acute (n = 23: Survival < 2 weeks) and long-term (n = 39; 1–47 years survival) survivors of a single TBI versus age-matched controls (n = 47). Multiple regions were examined including the hippocampus, medial temporal lobe, cingulate gyrus, superior frontal gyrus and brainstem. No association was found between a history of single TBI and abnormally phosphorylated TDP-43 (p-TDP-43) inclusions. Specifically, just 3 of 62 TBI cases displayed p-TDP-43 pathology versus 2 of 47 control cases. However, while aggregates of p-TDP-43 were not increased acutely or long-term following TBI, immunoreactivity to phosphorylation-independent TDP-43 was commonly increased in the cytoplasm following TBI with both acute and long-term survival. Moreover, while single TBI can induce multiple long-term neurodegenerative changes, the absence of TDP-43 proteinopathy may indicate a fundamental difference in the processes induced following single TBI from those of repetitive TBI.
Keywords: TDP-43, 43 kDa transactive response (TAR) DNA binding protein, Traumatic brain injury, Head injury, Diffuse axonal injury, DAI, Neurodegeneration, Dementia, Alzheimer’s disease, Long-term survival, Single versus repetitive TBI
Introduction
Since its discovery as the major disease protein in frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U), now termed FTLD-TDP, and amyotrophic lateral sclerosis (ALS) [55], the 43 kDa transactive response (TAR) DNA binding protein (TDP-43) has emerged as important in the pathophysiology of neurodegenerative disease [14, 22, 23, 39, 41, 54, 75]. First identified for its capacity to bind the long-terminal repeat transactive response element of HIV-1 [59], TDP-43 was later revealed as the primary disease-associated protein in both ALS and FTLD-TDP [55]. It has since been demonstrated that TDP-43 pathology is a secondary feature in several other neurodegenerative diseases including Huntington’s disease, Parkinson’s disease and Alzheimer’s disease (AD) [4, 5, 29, 30, 51, 67, 73]. Further studies suggest TDP-43 pathology may also feature in syndromes of cognitive impairment associated with repetitive traumatic brain injury (TBI) including former boxers with dementia pugilistica [38] and retired American football players with chronic traumatic encephalopathy (CTE) [44].
While repetitive mild TBI has long been associated with progressive neurodegeneration, epidemiological evidence indicates that just a single traumatic brain injury may trigger or accelerate the onset of Alzheimer’s disease (AD) in later life [17, 21, 26, 27, 48–50, 52, 56, 60, 64, 66, 70]. Pathological analyses of individuals who died following a single TBI demonstrate a hallmark AD pathology, amyloid-β (Aβ) plaques, in up to 30% of individuals [12, 31, 33, 62, 63, 68, 72]. Moreover, long-term survivors (>1 year) following a single TBI display AD-like neurofibrillary tangles (NFTs) at a younger age and to a greater extent than age-matched controls [36]. These findings suggest a single TBI may trigger or accelerate the formation of pathologies, similar to those observed in AD and dementia pugilistica [15, 16, 19, 61, 71], as well as in CTE [43, 57, 58].
However, since it remains unknown whether a TDP-43 proteinopathy is also part of the acute or delayed pathological sequelae of a single TBI, we examined an established cohort of single moderate/severe TBI cases with survival times ranging from 10 h to 47 years post-injury for this pathology.
Materials and methods
Cohort: demographic and clinical data
All tissue was obtained from the TBI Archive of the Department of Neuropathology, Southern General Hospital, Glasgow, UK. Tissue was acquired at routine autopsy and approval for its use was granted by the South Glasgow and Clyde Research Ethics Committee.
Three groups were selected for examination (Table 1). Group 1 consisted of patients who died acutely (survival of less than 14 days) following moderate/severe TBI (n = 23). These cases were aged 9–75 years (mean 40 years), included 16 males and 7 females and had survival times from TBI ranging from 10 h to 14 days (mean 3.9 days).
Table 1.
Demographic and clinical data for traumatic brain injury cases and uninjured controls
| Group 1: acute TBI cases (n = 23) | Group 2: long-term TBI cases (n = 39) | Group 3: non-TBI control cases (n = 47) | |||
|---|---|---|---|---|---|
| Mean age (range) | 40 years (9–75 years) | 53 years (19–89 years) | 47 years (14–92 years) | ||
| Males | 16 (69.6%) | 35 (89.7%) | 30 (63.8%) | ||
| Mean PM delay (range) | 60 h (3–240 h) | 64 h (12–184 h)—3 unknown | 57 h (5–264 h) | ||
| Mean survival time (range) | 3.9 days (10 h–14 days) | 8.2 years (1–47 years) | Not applicable | ||
| Cause of TBI | |||||
| Assault | 4 (17.4%) | Assault | 8 (20.5%) | Not applicable: control cases had no known history of TBI | |
| Fall | 11 (47.8%) | Fall | 16 (41.0%) | ||
| MVC | 6 (26.1%) | MVC | 8 (20.5%) | ||
| Cycle accident | 1 (4.3%) | Unknown | 7 (17.9%) | ||
| Unknown | 1 (4.3%) | ||||
| Cause of death | TBI 23 (100%) | Pulmonary (incld. bronchopneumonia) | 9 (23.1%) | Pulmonary disease (incld. pulmonary edema) | 9 (19.1%) |
| Chronic heart failure | 6 (15.4%) | Chronic heart failure | 4 (8.5%) | ||
| Sudden unexpected death due to epilepsy | 6 (15.4%) | Sudden unexpected death in epilepsy | 14 (29.8%) | ||
| Acute cardiovascular death | 4 (10.3%) | Acute cardiovascular death | 7 (14.9%) | ||
| GI disease (incld. liver failure) | 3 (7.7%) | GI disease (incld. liver failure) | 1 (2.1%) | ||
| Hypothermia | 1 (2.6%) | Hypothermia | 1 (2.1%) | ||
| Malignancy | 2 (5.1%) | Malignancy | 2 (4.3%) | ||
| Acute intracerebral hemorrhage | 1 (2.6%) | Sepsis | 2 (4.3%) | ||
| ARDS water inhalation | 1 (2.6%) | Vasculitis | 1 (2.1%) | ||
| Renal disease (incld. pyelonephritis) | 3 (7.7%) | GSW—chest | 1 (2.1%) | ||
| Unknown | 3 (7.7%) | Drug overdose | 4 (8.5%) | ||
| Myasthenia gravis | 1 (2.1%) | ||||
PM post mortem, GI gastrointestinal, ARDS acute respiratory distress syndrome, GSW gunshot wound, MVC motor vehicle collision
Group 2 comprised an established cohort [36] of long-term survivors of TBI (n = 39). Specifically, all patients survived at least 1 year following injury (survival range: 1–47 years; mean 8.2 years). Cases were aged 19–89 years (mean 53 years) and included 35 males and 4 females. Detailed reports from the diagnostic post-mortem and/or forensic reports were available for all and indicated a history of single moderate–severe TBI, confirmed at diagnostic post-mortem. In all long-term survival cases, patients were discharged from hospital following recovery and, ultimately, died from causes of death unrelated to TBI or trauma—none were in a persistent vegetative state due to TBI prior to death (Table 1).
Finally, group 3 comprised uninjured, age-matched controls (n = 47). All controls had no documented history of head trauma, AD or Down’s syndrome and included 30 males and 17 females ranging in age from 14 to 92 years (mean 47 years). Causes of death are listed in Table 1.
All three groups are closely demographically matched by virtue of their acquisition at the same institution serving a distinct regional population. Any patients with a history of amateur or professional boxing or any other known history of repetitive head trauma were excluded from this study.
Based on initial immunohistochemical findings specific for the full-length TDP-43 protein, a subset of cases with positive findings from groups 1–3 (n = 5 per group) were further examined using antibodies specific for the extreme N-terminus and C-terminus of TDP-43. Two control cases with an absence of immunoreactivity for the full-length protein were included as negative controls. Positive control tissue was included as described below.
Brain tissue preparation and immunohistochemistry
For all examinations, the intact brain was immersed in 10% formol saline at autopsy and fixed for at least 3 weeks prior to dissection. Sampling using a standardized protocol and paraffin embedding was as described previously [25]. Analyses were performed using sections from: (1) the medial temporal lobe including the hippocampus at the level of the lateral geniculate nucleus extending out through the entorhinal cortex to include the inferior temporal gyrus; (2) the corpus callosum and cingulate gyrus extending through the superior frontal gyrus; (3) the brainstem, including the midbrain pons and medulla. For the TBI group no brainstem tissue was available in 6 cases (2 of which were short-term survivors). Similarly, brainstem tissue was unavailable in 2 control cases.
Immunohistochemistry (IHC) was performed on 8-µm sections. Following deparaffinization and rehydration, sections were immersed in aqueous hydrogen peroxide (10 min) to quench endogenous peroxidase activity. Antigen retrieval was performed in a microwave pressure cooker and subsequent blocking achieved using 1 drop of normal horse serum (Vector Labs, Burlingame, CA, USA) per 5 ml of Optimax buffer (BioGenex, San Ramon, CA, USA) for 30 min. Incubation with the primary antibodies was performed for 20 h at 4°C. Specifically, a rat monoclonal antibody specific to TDP-43 abnormally phosphorylated at residues 409/410 (p-TDP-43) [53] at a concentration of 1:500 was used. This antibody does not detect normal, non-phosphorylated TDP-43. In addition, serial sections were stained with a rabbit polyclonal antibody generated against the N-terminal of the full-length protein, which is phosphorylation independent (pi-TDP-43) and therefore stains both p-TDP-43 and normal non-phosphorylated TDP-43 (1:22 K, Proteintech, Chicago, IL). In addition, a subset of cases with positive findings was stained with antibodies generated against the extreme N-terminal (N-t) region (1065N; rabbit polyclonal; 1:60K) and extreme C-terminal (C-t) region (1039C; rabbit polyclonal; 1:30K) of TDP-43 as described previously [32].
A biotinylated universal secondary antibody was then applied for 1 h (Vectastain Elite ABC Kit, Vector Labs, Burlingame, CA, USA) followed by an avidin biotin complex as per the manufacturer’s instructions (Vectastain Elite ABC Kit, Vector Labs, Burlingame, CA, USA). Finally, visualization was achieved using the DAB peroxidase substrate kit (Vector Labs, Burlingame, CA, USA). Counterstaining with haematoxylin was performed and sections were examined using light microscopy on a Leica DMRB microscope (Leica Microsystems, Wetzlar, Germany).
Positive control tissue for all TDP-43 IHC included sections from cases with a history of FTLD-TDP (frontal lobe) and ALS (brainstem), and a case of clinicopathologically confirmed dementia pugilistica in a former professional boxer known to have extensive TDP-43 pathology. Omission of the primary antibody was performed on the same material to control for non-specific binding.
Observations were conducted blind to the demographic and clinical information for all cases by two independent observers (V.J. and W.S.). IHC was used to generate anatomical maps of observed TDP-43 pathology. For p-TDP- 43, the presence of any positive profile was recorded. For pi-TDP-43 IHC, a normal pattern of immunoreactivity was considered to be predominantly nuclear with, at most, a light cytoplasmic ‘blush’. Regions with unequivocal cytoplasmic staining were recorded for each case.
Results
Abnormal p-TDP-43 inclusions following TBI
There was no association between a history of TBI, either acutely or following long-term survival, and p-TDP-43 inclusions when compared to uninjured age-matched controls.
Acute TBI
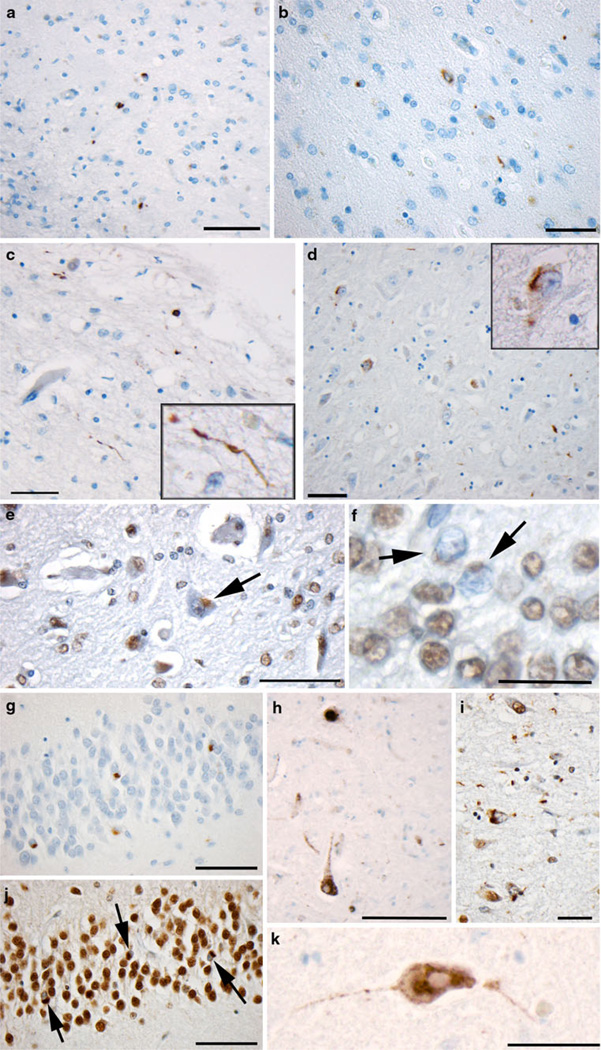
Of the 23 cases that died acutely (survival of less than 14 days) following TBI, only 1 (4.3%) showed immunoreactivity for p-TDP-43. This was an 18-year-old male who died 10 h after sustaining blunt-force trauma to the head (Fig. 1d). Cytoplasmic p-TDP-43 positive inclusions were identified within the hippocampal formation, predominantly within the CA1 region. No intranuclear pathology was identified and nuclear clearing was evident using the pi-TDP-43 antibody. This case has previously been identified as having NFTs and Aβ plaques, also within the medial temporal lobe [36].
Fig. 1.
TDP-43 proteinopathy in select positive cases and positive controls. a–b Immunoreactivity to p-TDP-43 in the medial temporal lobe of an 89-year-old female who died 7 years following a single moderate/severe TBI (scale bar 100 µm). c Immunoreactivity to p-TDP-43 in the medial temporal lobe of a 76-year-old female control who died acutely following a myocardial infarction and had no previous history of dementia or other neurological disease (scale bar 100 µm). d p-TDP-43 immunoreactivity in the CA1 region of hippocampus of an 18-year-old male who died 10 h following severe TBI (scale bar 100 µm). e, f Nuclear clearing of TDP-43 in cells following TBI as evidenced using the pi-TDP-43 antibody (same case as a, b) scale bar e100 µm, f 30 µm. g, j Granule cells of the dentate gyrus showing TDP-43 proteinopathy in a case of dementia pugilistica demonstrated using antibodies reactive to p-TDP-43 (g) and pi-TDP-43 (j) (scale bar 70 µm). i Extensive TDP-43 pathology observed in the collateral sulcus of the same case of dementia pugilistica (scale bar 100 µm). h A brainstem neuron reactive for the pi-TDP-43 antibody in a case of ALS. Note the clearing of the nucleus (scale bar 50 µm). k Similar cytoplasmic accumulation with nuclear clearing is observed in a case of FTLD (scale bar 50 µm)
Long-term survival from TBI
Of the 39 cases examined with a history of at least 1 year’s survival from TBI, only 2 (5.1%) displayed p-TDP-43 immunoreactivity. One was an 89-year-old female who died 7 years following a motor vehicle collision, which resulted in both intracerebral hemorrhage and brain contusion. Having survived this TBI, the patient was discharged to the community and died 7 years later from bronchopneumonia complicating colonic adenocarcinoma. Immunoreactivity to p-TDP-43 was found within the transentorhinal cortex, fusiform gyrus and inferior temporal lobe affecting both superficial and deep layers of cortex (Fig. 1a, b, e, f). Immunoreactive profiles were predominantly cytoplasmic with very occasional neuritic profiles observed. No intranuclear pathology was identified and nuclear clearing was evident using the pi-TDP-43 antibody. This case had previously been identified to have widespread thioflavine-S positive NFTs and Aβ-plaques [36].
The second positive long-term survival case was a 53-year-old male who died 8 years following a TBI from a fall which resulted in subdural hematoma, intracerebral contusions and diffuse axonal injury. This individual went on to recover and died 8 years later from acute pyelonephritis. Only a small isolated cluster of 5–6 cells displayed cytoplasmic p-TDP-43 immunoreactive inclusions within the medulla of this individual, with nuclear clearing evident following staining using the pi-TDP-43 antibody. This case was previously identified to have minimal NFTs in the transentorhinal cortex, yet had no Aβ plaques [36].
Uninjured control cases
Of the 47 controls examined, just 2 cases demonstrated abnormal p-TDP-43 inclusions (4.3%). The first was a 76-year-old female with no documented history of neurological disease or dementia who died acutely from a myocardial infarction. Immunoreactivity to p-TDP-43 was minimal and confined to the superficial layers of the transentorhinal cortex (Fig. 1c). Again, immunoreactive profiles were predominantly cytoplasmic, with occasional neuritic profiles observed. No intranuclear pathology was identified and nuclear clearing evident using the pi-TDP-43 antibody. This case was also previously identified to have extensive Aβ-plaques in the medial temporal lobe, though they were typically diffuse in nature. Moderate NFTs were also observed in the same region [36].
The second control displaying pathology was a 57-year-old male who died as a result of non-Hodgkin’s lymphoma. A small isolated cluster of cells within the medulla displayed cytoplasmic p-TDP-43 immunoreactive inclusions. No plaque or tangle pathologies were present in this case on previous examination [36].
As expected, the positive controls for TDP-43 immunocytochemistry displayed a typical pattern of immunoreactivity to both the p-TDP-43 and pi-TDP-43 antibodies with multiple p-TDP-43 cytoplasmic inclusions and associated nuclear clearing evident with pi-TDP-43 staining (Fig. 1g–k).
Increased cytoplasmic immunoreactivity to pi-TDP-43
While TBI cases did not exhibit p-TDP-43 immunoreactive inclusions to a greater extent than the uninjured controls, increased immunoreactivity to pi-TDP-43, was commonly observed in the cytoplasm following TBI when compared to uninjured controls (Fig. 2). However, positive nuclear staining remained in all examples, with no nuclear clearing characteristic of the TDP-43 proteinopathies.
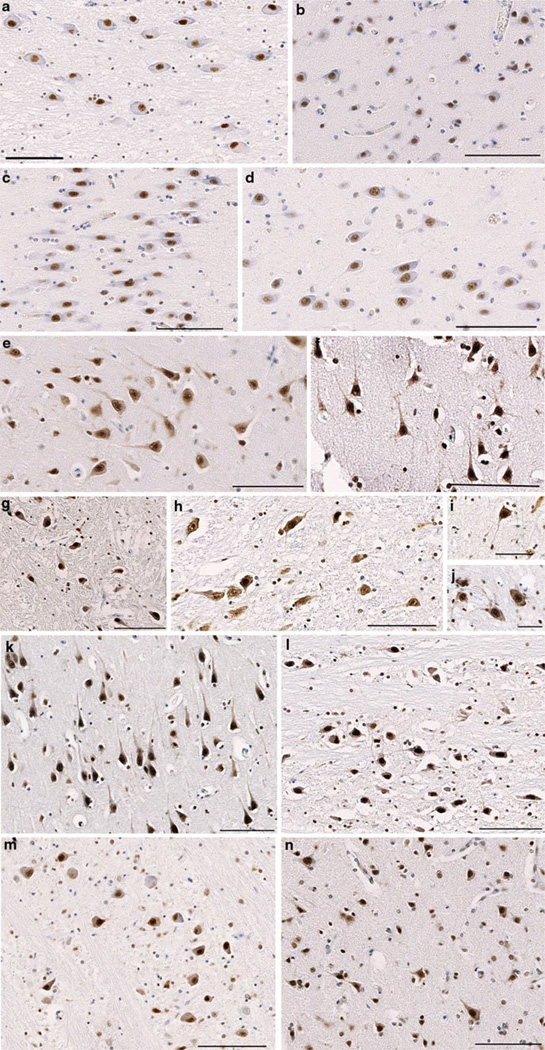
Fig. 2.
Pi-TDP-43 Immunoreactivity. a–d Controls with immunoreactivity to the antibody specific for pi-TDP-43. Sections display neuronal somata with a well-circumscribed nucleus and minimal or absent staining within the cytoplasmic compartment. a A region of the pons in a 16-year-old male who died following inhalation of gastric contents. b–c Regions of the cingulate gyrus and hippocampus, respectively in a 39-year-old female who died following a sudden unexplained death due to epilepsy. d The CA4 region of the hippocampus in a 22-year-old male who died acutely following a gun shot wound to the chest. All scale bars 100 µm. e–j Immunoreactivity specific for pi-TDP-43 acutely following TBI. In contrast to typical staining in TDP-43 proteinopathies, the nuclei remain positively stained. However, increased immunoreactivity within the cytoplasm is apparent and often extends into processes. e–f Regions of the cingulate gyrus and hippocampus, respectively in a 41-year-old male who died 20 h following TBI caused by a fall (scale bars 100 µm). g The medulla in a 16-year-old male 84 h following TBI caused by violent assault including blunt-force trauma to the head (scale bar 100 µm). h The midbrain of a 58-year-old male who died 6 h following TBI caused by a fall (scale bar 100 µm). i–j Individual neurons in the midbrain and medulla respectively of a 56-year-old female who died 72 h also following TBI caused by a fall (scale bars 50 µm). k–n Immunoreactivity specific for pi-TDP-43 in patients who survived at least 1 year following TBI. k, m The CA3 region of the hippocampus and a region of the medulla, respectively in a 50-year-old male who died 1 year following a TBI caused by violent assault. Cause of death was bronchopneumonia l. A region of the pons in a 42-year-old male who died 7 years following a TBI caused by a fall. The patient died as a result of an acute cardiac event. n The cingulate gyrus in a 37-year-old male who died 4 years following a fall. The cause of death was attributed to bronchopneumonia. All scale bars 100 µm
The vast majority of controls displayed no cytoplasmic pi-TDP-43 immunoreactivity (Fig. 2a–d), with only 15 of 47 (31.9%) positive and with staining almost exclusively observed in the brainstem. Only 2 (4.3%) controls displayed cytoplasmic staining in the cortex.
Increased cytoplasmic immunoreactivity for pi-TDP-43 was commonly and most extensively observed following acute TBI, with almost all cases (20 of 23; 86.9%) displaying cytoplasmic pi-TDP-43 immunoreactivity to some degree (Fig. 2e–j). While no distinct regional pattern was observed, increased pi-TDP-43 immunoreactivity was frequently extensive and observed in all regions examined including the cortex and brain stem. Indeed, cortical pi- TDP-43 staining was common, being present in 19 of 23 cases (82.6%) in this group.
In the long-term survival group, where there was a minimum 1 year survival from TBI, there was also increased cytoplasmic pi-TDP-43 immunoreactivity compared to controls, although this was generally less extensive than observed acutely following TBI (Fig. 2k–n). Specifically, 28 of 39 (71.8%) had increased cytoplasmic immunoreactivity and with 21 of 39 (53.8%) demonstrating increased cytoplasmic pi-TDP-43 immunoreactivity in the cortex.
Cytoplasmic staining was diffuse in nature and frequently extended into processes. Notably, neurons that demonstrated cytoplasmic immunoreactivity for pi-TDP-43 frequently displayed altered morphology, including shrunken cell bodies and an angular appearance.
A relative absence of N-t truncated and C-t fragments of TDP-43
In order to determine the presence of N-truncated or C-terminal fragments of TDP-43, a subset of 5 cases per group displaying increased cytoplasmic immunoreactivity for the full-length pi-TDP-43, were examined using antibodies raised against the extremes of the N-t and C-t [32].
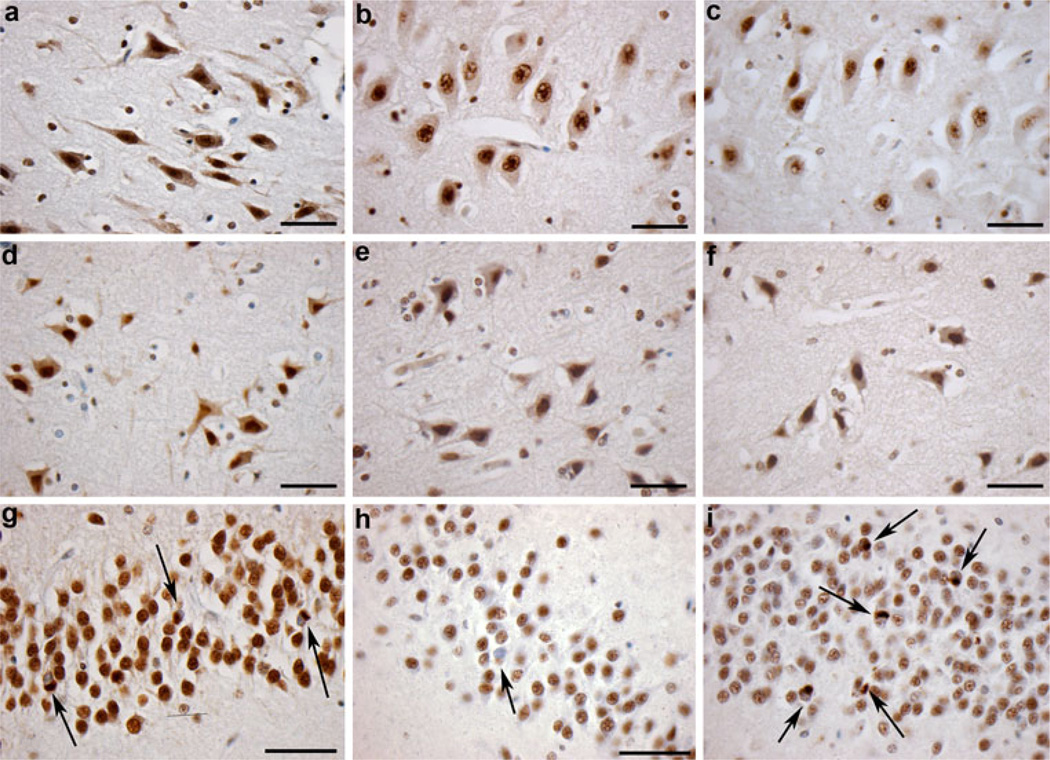
In cases displaying positive cytoplasmic staining for full-length pi-TDP-43, comparatively weak cytoplasmic immunoreactivity was observed when using antibodies raised against the N-t and C-t in serial sections (Fig. 3a–f). Notably, both N-t and C-t antibodies revealed immunoreactivity indicative of normal nuclear TDP-43 consistent with previous observations [32] (Fig. 3a–i). Given that both the N-t and C-t antibodies detect endogenous nuclear TDP-43, the comparatively diminished immunoreactivity in the cytoplasm to these antibodies, when compared to the robust immunoreactivity observed to the full-length pi-TDP-43 antibody, indicates that the observed cytoplasmic TDP-43 immunoreactivity most likely is full-length rather than truncated TDP-43, while the affinities of the N-t and C-t antibodies to TDP-43 may be less than pi-TDP-43 to account for their diminished immunoreactivity relative to pi-TDP-43 antibody. This, compared to positive controls (including cases of dementia pugilistica, FTLD and ALS), where pathological inclusions displayed greatly increased immunoreactivity to the C-terminus, is consistent with previous observations [32].
Fig. 3.
Immunoreactivity to full-length pi-TDP-43, and the N-terminus and C-terminus of TDP-43. Cytoplasmic and nuclear immunoreactivity specific for a pi-TDP-43, b the extreme N-terminus of TDP-43 and c the extreme C-terminus of TDP-43 in a 41-year-old male who died 20 h following TBI caused by a fall. Scale bars a–c 50 µm. d–f Cytoplasmic and nuclear immunoreactivity specific for d pi-TDP-43, e the extreme N-terminus of TDP-43 and f the extreme C-terminus of TDP-43 following TBI in a 48-year-old male who died 3 years following injury caused by a fall. Scale bars d–f 50 µm). g Cytoplasmic TDP-43 inclusions with associated nuclear clearing identified using pi-TDP-43 specific antibody in the granule cells of the dentate gyrus in a case of dementia pugilistica (pathology indicated by arrows). h Cytoplasmic TDP-43 inclusions with associated nuclear clearing identified using an antibody specific for the extreme N-terminus of TDP-43 in the same case and region as g. Pathology indicated by arrows. i Cytoplasmic TDP-43 inclusions with associated nuclear clearing identified using an antibody specific for the extreme C-terminus of TDP-43 in the same case and region as g, h. Pathology indicated by arrows. Scale bars g, h 70 µm)
Discussion
Based on these results, it does not appear that a classical TDP-43 proteinopathy is a feature of either acute or long-term survival from a single TBI. This relative absence of pathological, p-TDP-43 positive inclusions differs from AD (a secondary TDP-43 proteinopathy [4, 5, 29, 30, 51, 73]) an d the pathologies described in material from patients with a history of repetitive head injury, such as the limited case descriptions of CTE in former American football players and dementia pugilistica in former boxers [38, 44]. These data suggest there is perhaps a different spectrum of long-term pathological processes induced by a single, compared with repetitive TBI and AD. As such, TDP-43 IHC may be important in differentiating the chronic neuropathology of a single TBI from that of repetitive injury. However, while pathologic inclusions were absent following single TBI, increased cytoplasmic immunoreactivity to TDP-43, independent of its phosphorylation status, was frequently observed by pi-TDP-43 IHC. This may indicate increased cytoplasmic TDP-43 is part of a normal injury response.
While the normal functions of TDP-43 have not been comprehensively characterized, it has been identified as a regulator of both gene expression [1, 2] and exon splicing [6, 10, 45, 74], most likely via its DNA and RNA binding capabilities [8, 9, 11, 14]. Accordingly, though synthesized in the cytoplasm, TDP-43 predominantly resides within the nuclear compartment of both neurons and glia under physiological conditions [55]. By comparison, in neurodegenerative disease, TDP-43 accumulates within the cytoplasm as relatively insoluble, truncated, ubiquitinated and hyperphosphorylated inclusions [28, 34, 53, 55]. The mechanisms leading to these changes are not well understood; however, damage to axons has been implicated as a potential trigger for the cytoplasmic redistribution of TDP-43 and is of particular relevance to TDP-43 proteinopathies observed where there is a history of repetitive mild TBI.
Damage to axons or diffuse axonal injury (DAI) is one of the most common pathologies of TBI [3, 18, 20] and has been documented in all severities of TBI, including mild TBI/concussion [7]. In addition, axonal pathology has been indicated as a potential mechanism by which AD-like pathologies are initiated post-TBI [35]. Specifically, the extensive accumulation of amyloid precursor protein (APP) within damaged axons post-TBI has been revealed as a potential source of Aβ plaques both acutely and with long-term survival following a single TBI [12, 13, 35, 69, 72]. Moreover, due to tau protein’s primary physiological function as a microtubule stabilizer in axons, it is suspected that traumatic injury to axons may contribute to the dysregulation of tau and NFT formation [36].
Recent reports have implicated axonal injury as a modulator of TDP-43 homeostasis [46, 47, 65]. Specifically, axotomy of mouse spinal motor neurons resulted in upregulation of TDP-43 with associated relocalization to the cytosolic compartment in vivo [46, 47]. Similar results were observed following ligation of mouse hypoglossal neurons [65]. Moreover, as axons recovered over time, TDP-43 was restored to the nucleus, suggesting the transient redistribution of TDP-43 may be potentially beneficial [47, 65]. Of note, cytoplasmic redistribution of TDP-43 in the absence of abnormal phosphorylation has also been observed following acute cerebral ischemia in rats [37]. Similarly, our results show that, following TBI, immunoreactivity to phosphorylation-independent TDP-43 using pi-TDP-43 IHC can be observed within the cytoplasmic compartment acutely following injury and in some cases may persist long term. This may represent an increase in TDP-43 post-TBI, but it might also reflect a change in epitope exposure or other factors, thereby underlining the need for direct quantification of TDP-43 in traumatically injured brain using biochemical analyses. Notably, strong cytoplasmic immunoreactivity was observed to the antibody recognizing full-length pi-TDP-43, compared to relatively weaker immunoreactivity observed with N-terminus and C-terminus specific antibodies. Moreover, immunoreactivity for truncated species of TDP-43 was considerably weaker than positive control cases, which, as expected, displayed robust immunoreactivity for the C-terminus in pathological inclusions. Whilst this strongly suggests that it is the full-length, non-truncated species of TDP-43 accumulating within the cytoplasm, again, direct biochemical analyses are indicated for confirmation. Further, the affinities of the N-t and C-t antibodies to TDP-43 may be less than pi-TDP-43 to account for their diminished immunoreactivity relative to pi-TDP-43.
Notably, neurons with increased cytoplasmic TDP-43 immunoreactivity frequently displayed an abnormal morphology. This may suggest TDP-43 simply accumulates within the cytoplasm as a consequence of nuclear membrane breakdown in degenerating neurons. Future studies to characterize neurons immunoreactive for TDP-43 using pi-TDP-43 IHC will be important. Similarly, it has been reported that acutely injured tissue has an increased propensity for non-specific antibody binding. However, the morphological appearance of positive cells, regional patterns and distribution of staining, and immunoreactivity with survival of many years suggests that non-specific binding is unlikely occurring in the cases described.
Alternatively, an increased presence of TDP-43 within the cytoplasm supports the hypothesis that it may be an important modulator of the injury response, perhaps via trafficking of mRNA species to the cytosol [47]. Interestingly, TDP-43 was observed to co-localize with markers of transport and stress granules in both the somata and the axonal compartment following axotomy in vivo [47] and following exposure to various stressors including oxidative stress, heat shock and thapsigargin in vitro [42]. Moreover, TDP-43 was identified as important in both the assembly and maintenance of stress granules in vitro under conditions of oxidative stress, suggesting a potential role in regulating stress-induced gene expression [42]. Examination of the interplay between stress granule formation and TDP-43 dynamics following TBI will be an important future consideration.
While the potential mechanisms driving cytosolic accumulation of pi-TDP-43 post-injury require further examination, it is notable that following human TBI and in rodent models of axotomy/ischemia, aggregated, ubiquitinated TDP-43 inclusions characteristic of neurodegenerative disease were absent [37, 46, 47, 65]. Further, TDP-43 pathology does not appear to be a reactive response to diverse injuries [40]. While abundant inclusions have been identified at post-mortem in a limited number of cases in which there is a history of repetitive mild TBI [38, 44], these are not a feature of single TBI. It is possible that only multiple insults, persistently reinforcing the acute upregulation of TDP-43, are sufficient to cause aggregation and associated proteinopathy.
Of the small number of cases where TDP-43 inclusions were identified in this cohort, the two cases with the most significant pathology were aged 89 (long-term TBI group) and 76 (control), respectively. Increasing evidence suggests TDP-43 proteinopathy may be a feature of so-called ‘normal aging’, similar to that observed for Aβ plaques and NFTs. Indeed, the largest examination of TDP-43 proteinopathy in ‘normal’ controls to date demonstrated that approximately 29% of individuals over the age of 65 displayed some degree of TDP-43 pathology [24]. By comparison, individuals aged less than 65 had no significant pathology. Moreover, the burden of pathology was observed to increase with advancing age suggesting an age-related mechanism [24]. Notably, we also observed very minimal TDP-43 inclusions in the medulla of two cases aged 53 (long-term TBI group) and 57 (control). While there is not currently a precedent to indicate that pathology in these cases is age-associated, the evidence presented indicates that it is unlikely attributable to TBI. In addition, TDP-43 pathology was observed in the medial temporal lobe of one 18-year-old individual who died acutely following TBI. TDP-43 proteinopathy in such a young individual renders this case an outlier, both within the context of the data presented here and indeed the known literature. With no known history of neurological disease or of repetitive TBI in this case, there is no clear explanation for the observed findings. However, as the only acute TBI case within the cohort displaying TDP-43 positive inclusions, there is insufficient evidence to suggest this finding is associated with acute brain injury.
The lack of association between single TBI and TDP-43 proteinopathy brings to light a fundamental difference in the pathology observed in cases where there is a history of repetitive versus single TBI. Notably, both tau pathologies and Aβ plaques have been identified in a proportion of cases following chronic survival from single TBI [36]. To date, there are only limited short series showing an association between a history of repetitive TBI and TDP-43 proteinopathy. Here, using a large series across a temporal survival range, we find no association between a single moderate/severe TBI and TDP-43 inclusions. Further elucidation of the physiological role of TDP-43 in the injury state will undoubtedly be important in comprehending its evolution into aggregated inclusions.
Acknowledgments
This work was supported by National Institutes of Health grants NS038104, AG10124, AG17546 and NS056202. We would also like to thank Ms. Janice E. Stewart for her technical assistance with immunohistochemical staining procedures.
Footnotes
Conflict of interest None of the authors have any conflict of Interest to declare.
Contributor Information
Victoria E. Johnson, Department of Neurosurgery, Penn Center for Brain Injury and Repair, University of Pennsylvania, 105 Hayden Hall, 3320 Smith Walk, Philadelphia, PA 19104, USA Division of Clinical Neurosciences, University of Glasgow, Glasgow, UK.
William Stewart, Division of Clinical Neurosciences, University of Glasgow, Glasgow, UK; Department of Neuropathology, Institute of Neurological Sciences, Southern General Hospital, Glasgow, UK.
John Q. Trojanowski, Department Pathology and Laboratory Medicine, Center for Neurodegenerative Disease Research, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA Alzheimer’s Disease Core Center, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA; Institute on Aging, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
Douglas H. Smith, Email: smithdou@mail.med.upenn.edu, Department of Neurosurgery, Penn Center for Brain Injury and Repair, University of Pennsylvania, 105 Hayden Hall, 3320 Smith Walk, Philadelphia, PA 19104, USA.
References
- 1.Abhyankar MM, Urekar C, Reddi PP. A novel CpG-free vertebrate insulator silences the testis-specific SP-10 gene in somatic tissues: role for TDP-43 in insulator function. J Biol Chem. 2007;282:36143–36154. doi: 10.1074/jbc.M705811200. [DOI] [PubMed] [Google Scholar]
- 2.Acharya KK, Govind CK, Shore AN, Stoler MH, Reddi PP. cis-Requirement for the maintenance of round spermatid-specific transcription. Dev Biol. 2006;295:781–790. doi: 10.1016/j.ydbio.2006.04.443. [DOI] [PubMed] [Google Scholar]
- 3.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 4.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai T, Mackenzie IR, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 6.Ayala YM, Pagani F, Baralle FE. TDP43 depletion rescues aberrant CFTR exon 9 skipping. FEBS Lett. 2006;580:1339–1344. doi: 10.1016/j.febslet.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 7.Blumbergs PC, Scott G, Manavis J, et al. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 8.Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- 9.Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci. 2008;13:867–878. doi: 10.2741/2727. [DOI] [PubMed] [Google Scholar]
- 10.Buratti E, Brindisi A, Pagani F, Baralle FE. Nuclear factor TDP-43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: a functional link with disease penetrance. Am J Hum Genet. 2004;74:1322–1325. doi: 10.1086/420978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buratti E, Dork T, Zuccato E, et al. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen XH, Siman R, Iwata A, et al. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 2004;165:357–371. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 2010;6:211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 16.Dale GE, Leigh PN, Luthert P, Anderton BH, Roberts GW. Neurofibrillary tangles in dementia pugilistica are ubiquitinated. J Neurol Neurosurg Psychiatry. 1991;54:116–118. doi: 10.1136/jnnp.54.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geddes JF, Vowles GH, Beer TW, Ellison DW. The diagnosis of diffuse axonal injury: implications for forensic practice. Neuropathol Appl Neurobiol. 1997;23:339–347. [PubMed] [Google Scholar]
- 19.Geddes JF, Vowles GH, Nicoll JA, Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- 20.Geddes JF, Whitwell HL, Graham DI. Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol. 2000;26:105–116. doi: 10.1046/j.1365-2990.2000.026002105.x. [DOI] [PubMed] [Google Scholar]
- 21.Gedye A, Beattie BL, Tuokko H, Horton A, Korsarek E. Severe head injury hastens age of onset of Alzheimer’s disease. J Am Geriatr Soc. 1989;37:970–973. doi: 10.1111/j.1532-5415.1989.tb07283.x. [DOI] [PubMed] [Google Scholar]
- 22.Geser F, Martinez-Lage M, Kwong LK, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis, frontotemporal dementia and beyond: the TDP-43 diseases. J Neurol. 2009;256:1205–1214. doi: 10.1007/s00415-009-5069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geser F, Martinez-Lage M, Robinson J, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66:180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geser F, Robinson JL, Malunda JA, et al. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol. 2010;67:1238–1250. doi: 10.1001/archneurol.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham DI, Gentleman SM, Lynch A, Roberts GW. Distribution of beta-amyloid protein in the brain following severe head injury. Neuropathol Appl Neurobiol. 1995;21:27–34. doi: 10.1111/j.1365-2990.1995.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 26.Graves AB, White E, Koepsell TD, et al. The association between head trauma and Alzheimer’s disease. Am J Epidemiol. 1990;131:491–501. doi: 10.1093/oxfordjournals.aje.a115523. [DOI] [PubMed] [Google Scholar]
- 27.Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa M, Arai T, Nonaka T, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 30.Hu WT, Josephs KA, Knopman DS, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber AGK, Kelemen J, Cervod-Navarro J. Density of amyloid plaques in brains after head trauma. J Neurotrauma. 1993;10(Suppl):S180. [Google Scholar]
- 32.Igaz LM, Kwong LK, Xu Y, et al. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikonomovic MD, Uryu K, Abrahamson EE, et al. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Inukai Y, Nonaka T, Arai T, et al. Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett. 2008;582:2899–2904. doi: 10.1016/j.febslet.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson VE, Stewart W, Smith DH. Amyloid and tau pathologies many years following single traumatic brain injury in humans. Brain Pathol. 2011 doi: 10.1111/j.1750-3639.2011.00513.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanazawa M, Kakita A, Igarashi H, et al. Biochemical and histopathological alterations in TAR DNA-binding protein-43 after acute ischemic stroke in rats. J Neurochem. 2011;116:957–965. doi: 10.1111/j.1471-4159.2010.06860.x. [DOI] [PubMed] [Google Scholar]
- 38.King A, Sweeney F, Bodi I, et al. Abnormal TDP-43 expression is identified in the neocortex in cases of dementia pugilistica, but is mainly confined to the limbic system when identified in high and moderate stages of Alzheimer’s disease. Neuropathology. 2010;30:408–419. doi: 10.1111/j.1440-1789.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 39.Kwong LK, Uryu K, Trojanowski JQ, Lee VM. TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis. Neurosignals. 2008;16:41–51. doi: 10.1159/000109758. [DOI] [PubMed] [Google Scholar]
- 40.Lee EB, Lee VM, Trojanowski JQ, Neumann M. TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system. Acta Neuropathol. 2008;115:305–311. doi: 10.1007/s00401-007-0331-5. [DOI] [PubMed] [Google Scholar]
- 41.Lee EB, Lee VM-Y, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP-43 mediated neurodegeneration. Nat Rev Neurosci. 2011 doi: 10.1038/nrn3121. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald KK, Aulas A, Destroismaisons L, et al. TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet. 2011;20:1400–1410. doi: 10.1093/hmg/ddr021. [DOI] [PubMed] [Google Scholar]
- 43.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKee AC, Gavett BE, Stern RA, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercado PA, Ayala YM, Romano M, Buratti E, Baralle FE. Depletion of TDP 43 overrides the need for exonic and intronic splicing enhancers in the human apoA-II gene. Nucleic Acids Res. 2005;33:6000–6010. doi: 10.1093/nar/gki897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moisse K, Mepham J, Volkening K, et al. Cytosolic TDP-43 expression following axotomy is associated with caspase 3 activation in NFL−/− mice: support for a role for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009;1296:176–186. doi: 10.1016/j.brainres.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Moisse K, Volkening K, Leystra-Lantz C, et al. Divergent patterns of cytosolic TDP-43 and neuronal progranulin expression following axotomy: implications for TDP-43 in the physiological response to neuronal injury. Brain Res. 2009;1249:202–211. doi: 10.1016/j.brainres.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 48.Molgaard CA, Stanford EP, Morton DJ, et al. Epidemiology of head trauma and neurocognitive impairment in a multiethnic population. Neuroepidemiology. 1990;9:233–242. doi: 10.1159/000110778. [DOI] [PubMed] [Google Scholar]
- 49.Mortimer JA, French LR, Hutton JT, Schuman LM. Head injury as a risk factor for Alzheimer’s disease. Neurology. 1985;35:264–267. doi: 10.1212/wnl.35.2.264. [DOI] [PubMed] [Google Scholar]
- 50.Mortimer JA, van Duijn CM, Chandra V, et al. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case–control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 51.Nakashima-Yasuda H, Uryu K, Robinson J, et al. Comorbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 52.Nemetz PN, Leibson C, Naessens JM, et al. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- 53.Neumann M, Kwong LK, Lee EB, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neumann M, Kwong LK, Sampathu DM, Trojanowski JQ, Lee VM. TDP-43 proteinopathy in frontotemporal lobar degeneration and amyotrophic lateral sclerosis: protein misfolding diseases without amyloidosis. Arch Neurol. 2007;64:1388–1394. doi: 10.1001/archneur.64.10.1388. [DOI] [PubMed] [Google Scholar]
- 55.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 56.O’Meara ES, Kukull WA, Sheppard L, et al. Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am J Epidemiol. 1997;146:373–384. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- 57.Omalu BI, DeKosky ST, Hamilton RL, et al. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59:1086–1092. doi: 10.1227/01.NEU.0000245601.69451.27. (discussion 1092–1083) [DOI] [PubMed] [Google Scholar]
- 58.Omalu BI, DeKosky ST, Minster RL, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–134. doi: 10.1227/01.neu.0000163407.92769.ed. (discussion 128–134) [DOI] [PubMed] [Google Scholar]
- 59.Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol. 1995;69:3584–3596. doi: 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 61.Roberts GW, Allsop D, Bruton C. The occult aftermath of boxing. J Neurol Neurosurg Psychiatry. 1990;53:373–378. doi: 10.1136/jnnp.53.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts GW, Gentleman SM, Lynch A, Graham DI. beta A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- 63.Roberts GW, Gentleman SM, Lynch A, et al. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1994;57:419–425. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salib E, Hillier V. Head injury and the risk of Alzheimer’s disease: a case control study. Int J Geriatr Psychiatry. 1997:363–368. doi: 10.1002/(sici)1099-1166(199703)12:3<363::aid-gps515>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 65.Sato T, Takeuchi S, Saito A, et al. Axonal ligation induces transient redistribution of TDP-43 in brainstem motor neurons. Neuroscience. 2009:1565–1578. doi: 10.1016/j.neuroscience.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 66.Schofield PW, Tang M, Marder K, et al. Alzheimer’s disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry. 1997;62:119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008;67:1159–1165. doi: 10.1097/NEN.0b013e31818e8951. [DOI] [PubMed] [Google Scholar]
- 68.Smith DH, Chen XH, Iwata A, Graham DI. Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg. 2003;98:1072–1077. doi: 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- 69.Smith DH, Chen XH, Nonaka M, et al. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999;58:982–992. doi: 10.1097/00005072-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan P, Petitti D, Barbaccia J. Head trauma and age of onset of dementia of the Alzheimer type. JAMA. 1987:2289–2290. doi: 10.1001/jama.1987.03390170045014. [DOI] [PubMed] [Google Scholar]
- 71.Tokuda T, Ikeda S, Yanagisawa N, Ihara Y, Glenner GG. Re-examination of ex-boxers’ brains using immunohistochemistry with antibodies to amyloid beta-protein and tau protein. Acta Neuropathol. 1991;82:280–285. doi: 10.1007/BF00308813. [DOI] [PubMed] [Google Scholar]
- 72.Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang HY, Wang IF, Bose J, Shen CK. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004:130–139. doi: 10.1016/s0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- 75.Wang IF, Wu LS, Shen CK. TDP-43: an emerging new player in neurodegenerative diseases. Trends Mol Med. 2008:479–485. doi: 10.1016/j.molmed.2008.09.001. [DOI] [PubMed] [Google Scholar]