Abstract
Objective
Recently, our laboratory recapitulated a natural form of axon growth that occurs between late embryogenesis and early adulthood. In this article, we describe how this novel neural engineering approach may be used to produce a nervous tissue interface to integrate disconnected motor and sensory functions for external control.
Methods
For nervous system repair, we recently developed a unique method to engineer nervous tissue constructs in vitro consisting of bundles of axons spanning two populations of neuronal somata. To integrate electronics and nervous tissue to transform electrophysiological signals into electronic signals, we have designed a nervous tissue interface.
Results
Our nervous tissue interface consists of stretch-grown nervous tissue with one end interfaced with a multiple electrode array, enabling us to detect and record real-time efferent signals conducted down the nerve and stimulate afferent sensory signaling.
Conclusion
Our ultimate goal is to develop a neurally controlled prosthesis and a nervous system interface that could be linked to the patient's thoughts, providing two-way signaling for motor control and feedback from multiple external stimuli.
Keywords: Nervous system repair, Neurally controlled prosthesis, Peripheral nerve injury, Signaling
The history of surgery contains many instances of productive collaborations between surgeons and engineers. Certainly the partnership of Harvey Cushing and William T. Bovie is a classic example (4, 21, 35). Since 1928, the Bovie electrocautery device has been improved and has been widely used in all surgical specialties. In the neurosurgery field, the Spitz-Holter valve for cerebrospinal fluid shunting was developed through a close collaboration between John Holter, an engineer, and Dr. Eugene Spitz, a pediatric neurosurgeon in Philadelphia (2). At the University of Pennsylvania and University of Rochester, neurosurgeons continue to work closely with biomedical engineers and research scientists to search for therapeutic strategies for nervous system injuries.
Recently, our Neurosurgery Research Laboratory recapitulated a natural form of axon growth that occurs between late embryogenesis and early adulthood. For example, in contrast to axon extension via a growth cone, an estimate of the average rate of growth of a blue whale suggests that synapsed spinal axons must grow at a rate of approximately 4 cm/d to keep pace with the expansion of its body (Fig. 1) (15). Using an in vitro axon stretch growth bioreactor system, we have shown that axons interconnecting two populations of neurons can be induced to grow under mechanical stretching conditions (25, 32). Our primary focus has been to grow long axonal tracts to bridge areas of damage for nerve repair (12, 13, 24, 26). Here, we describe how this novel tissue engineering approach may be used to produce a nervous tissue interface to integrate disconnected motor and sensory functions for external control.
Figure 1.
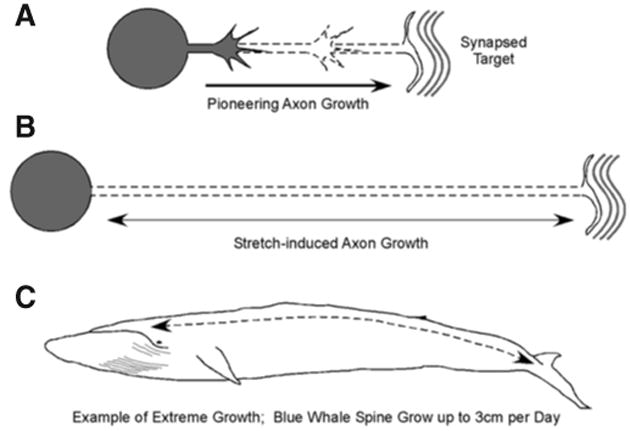
Two forms of axon growth. A, early in development, pioneering axons navigate to their targets under growth cone-mediated axon growth. B, after axons reach and establish synaptic connections with their targets, animals and their nervous systems continue to grow several orders of magnitude in size. Despite the absence of a growth cone, axons are forced to elongate several centimeters to meters in length, keeping pace with the growth of the animal. C, an example of extreme axon stretch growth occurs in the blue whale, which grows in length at a rate of 4 cm/d during peak growth.
Background
Prosthetic devices can be offered to replace a lost limb. In an effort to improve quality of life, engineers have advanced the performance of prosthetics; however, they remain passive devices with limited function. Even with the aid of a prosthesis, patients continue to face pervasive deficits throughout their life. Although the mechanical capability of prosthetic devices has become sophisticated, prosthetic motor tasks are currently driven by gross anatomic movements, making them cumbersome. Importantly, current devices do not replace lost sensory systems and cannot adapt movements based on sensory feedback, as a natural limb functions (6, 19).
Recent advances in neuroscience have brought the concept of neurally controlled prostheses to reality. The tactic is to use regions of the undamaged nervous system to provide command signals to drive prosthetic functions. The most vigorously pursued approach attempts to harness external recordings obtained from the motor cortex (6, 20, 41). These noninvasive techniques obtain the user's intent from scalp-derived sensorimotor rhythms, typically those of the μ or fl frequency bands. Studies in rats, nonhuman primates, and quadriplegic patients have shown that after sufficient training, the user can learn to move a cursor in two-dimensional space by manipulating these frequencies (42). Although the risk of surgery is avoided, sophisticated systems requiring several degrees of freedom are still far from being realized (6, 7, 20).
To achieve complex signal integration, methods that are more invasive have been developed. These techniques chronically implant microelectrodes into the primary motor cortex or spinal cord to record local neuronal activity (1, 6, 18–20, 22, 31, 33). Neuronal population decoding algorithms are used to decipher the recorded signals in real time (6, 16). This method, however, involves complex computations and significant clinical risks arising from the chronic implantation of electrodes (6). Moreover, findings from functional magnetic resonance imaging studies indicate that there is extensive overlap of the cortical representations of different limb regions, adding additional difficulty to implant positioning and signal decoding (28).
The Challenge
For a prosthetic to perform like a natural limb, it would ideally be controlled by the patient's thoughts, converting nervous system signaling into movements and adaptation to sensory stimuli. The key to this approach is a nervous system interface, connecting the brain to the prosthetic device. Ideally, this interface would record efferent nervous signaling with a complexity capable of fine motor control. In addition, sensors would convey afferent sensory signals back into the nervous system. Although microelectrode implantation has had some success driving motor control, there is still not a clear approach to relay sensory signals or to use such signals for feedback control. Nevertheless, two-way signaling could tap into the processing power of the human brain, providing feedback control from multiple external stimuli, a necessity for any fine motor function and proprioception.
Rather than placing microelectrodes into delicate nervous tissue, one could consider interfacing through the peripheral nervous system and exploit its capacity to “remap” afferent and efferent signaling. For example, regenerating peripheral axons re-innervate tissue different from their original locations; however, with training, a patient can assimilate this new axon distribution and regain limb function (8–10, 14, 40). If the proximal nerve stump was used to drive a device, however, regeneration would not be necessary. Simply, it provides an existing biological connection with the central nervous system that may require minimal training to induce central nervous system plasticity to map motor and sensory function (3, 5, 17, 27, 29, 30, 39). Simple operant conditioning could allow a patient implanted with a nervous system interface to skillfully drive a sophisticated prosthetic device (5, 6). Accordingly, there would be little need to interpret complex neural processing computationally from a small region of the brain or spinal cord, a process better handled by the central nervous system.
To realize bi-directional signaling, an interface between the central nervous system and an electronic device must somehow be established. A more direct route would be to allow the nervous tissue to integrate with itself, which is the same goal for repairing the injured nervous system. Nervous tissue engineered in vitro could be coupled to an electronic interface at one end and allowed to integrate with the peripheral nervous system at the other end, providing a natural pathway of communication with the central nervous system.
Engineering Living and Functional Nervous Tissue in Vitro
For nervous system repair, we recently developed a unique method to engineer nervous tissue constructs in vitro consisting of bundles of axons spanning two populations of neuronal somata (Fig. 2) (26). These constructs may also be used to create a nervous tissue interface, serving as jumper cables between the electronics and nervous system. One population of neurons interfaces with an electronic device, and the other is allowed to integrate synaptically with the nervous system.
Figure 2.
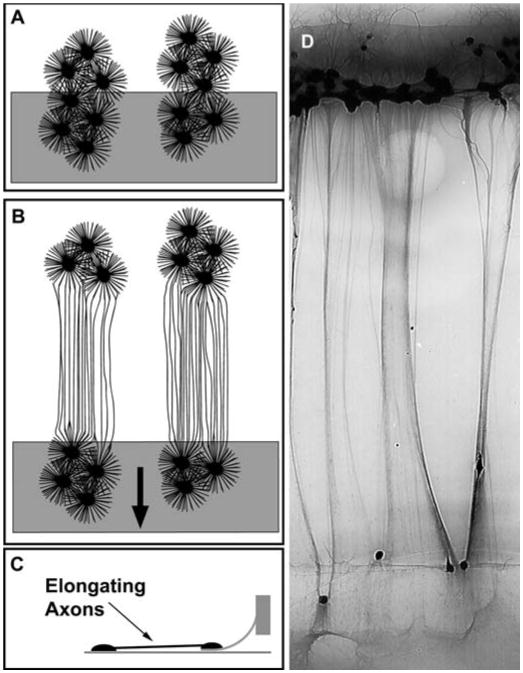
Extreme axon stretch growth. A, rat dorsal root ganglia are plated on two adjoining substrates. Axons are then allowed to grow across the interface of the two substrates and into the population of neurons on either side. B, axons bridging the two populations of neurons are stretch-grown by displacing the two adjoining substrates apart. A gradually increasing growth rate was strategically applied (arrow) to achieve stretch growth rates up to 1 cm/d. C, side view illustration of stretch-growing axons. D, axon bundles stretch-grown in our axon stretch growth bioreactor system.
The key to creating living nervous tissue in culture is through continuous mechanical tension on axons spanning two populations of neurons, inducing axon “stretch growth” (25, 32). This technique has produced nerve tracts consisting of up to 106 axons grown at rates of at least 10 mm/d and reaching a remarkable 10 cm in length while maintaining normal structure and function (32). Importantly, they retain the ability to generate normal action potentials that propagate across stretch-grown axons (23).
We recently demonstrated that nervous tissue engineered in vitro can survive long term and maintain its geometry after transplantation in spinal cord (13) and peripheral nerve lesions (12). Survival of transplanted axon tracts seems to have greatly facilitated outgrowth and guidance of host axons through the grafted region. In the peripheral nerve, the detail of the final destinations of the axonal growth is unclear because appropriate afferent and efferent connection with the appropriate neurotransmitters will be necessary for functioning grafts. Our histological evaluation revealed that regenerated axons were mostly large myelinated axons and conducted compound action potentials in the range of A-β fibers. Because most nociceptors are conducted by unmyelinated C-fibers or thinly myelinated A-δ fibers, we think that the termination of our axons will not contribute to any increased nociception (11, 38). In our preliminary behavioral evaluation, the animals showed significantly improved motor function. We also think that the regenerated axons contributed to improved sensory function with action potential conducted by low threshold mechanoreceptors (mostly A-β fibers). However, we are still in the process of conducting further studies to define this process.
The Nervous Tissue Interface
To connect to the nervous system biologically, we must integrate electronics and nervous tissue to transform electrophysiological signals into electronic signals. However, current approaches to coupling an electronic interface to nervous tissue simply places electrodes in the proximity of neurons in the brain or spinal cord and has not provided an environment that encourages neurons to assimilate (6, 20). The central feature of our nervous tissue interface is the ability to create transplantable living nervous tissue already coupled to an electronic interface. Stretch-grown nervous tissue interfaces could be used to synapse with the nervous system biologically at one end, thereby providing a jumper cable in which the opposing end interfaces with the electronic device.
Common in vitro techniques have been used extensively to couple cultured neurons to commercially available multiple electrode arrays for electrophysiological analysis (36, 37). Exploiting this technology, we modified our axon stretch growth bioreactor to create long lengths of nervous tissue in vitro, interfaced with a multiple electrode array (Fig. 3). Because stretch-grown axons can transmit active electrical signals (23), we propose that this nervous tissue interface, through the multiple electrode array, could detect and record real-time efferent signals conducted down the nerve and stimulate afferent sensory signaling. Our ability to grow axons of unparalleled length will allow us to position the attached electrode array at any convenient location, allowing easy exteriorization.
Figure 3.
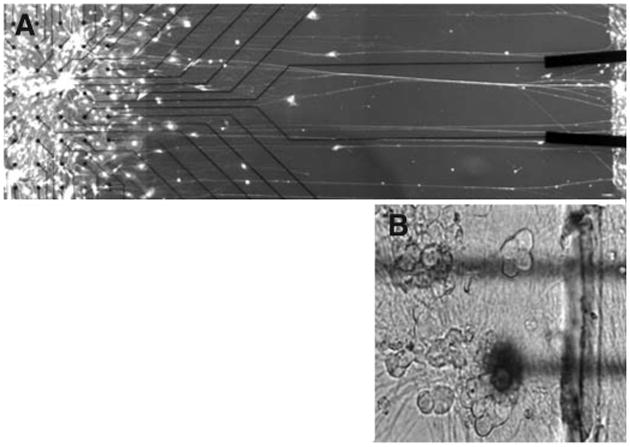
A, nervous tissue can be stretch grown in vitro already interfaced to a commercially available multiple electrode array. On the left, a group of dorsal root ganglion somata are fixed on top of an electrode array. On the right, a group of somata are mechanically displaced, stretch growing the interconnecting axons. B, magnified view of dorsal root ganglion cells located in the proximity of electrodes. After transplantation and integration of this nervous tissue interface with host nerves, efferent activity could be used as commands to drive a prosthetic limb. Likewise, sensory stimuli detected by the prosthetic limb could be transmitted back into the nervous system by stimulating these same neurons with the electrodes.
After transplantation and integration of this nervous tissue interface with host nerves, willful stimulation of motor fibers would be recorded by the multiple electrode array, which in turn, drives the prosthesis (Fig. 4). By tapping into the peripheral nervous system, there would be little need to decipher the “neural code.” Through rehabilitation, the patient will be able to learn to control particular motor functions directly. Moreover, this approach can address an important aspect of prosthetic control that is generally overlooked—sensory function. Signals from sensor transducers on a prosthetic would be transferred to the multiple electrode array to stimulate sensory fibers for afferent conduction and central processing.
Figure 4.
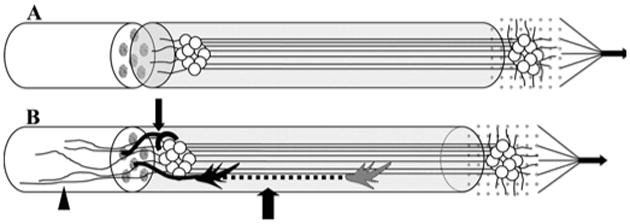
Illustration of the transplantable nervous tissue interface. A, theinterface, engineered in vitro (Fig. 3), is surgically transplanted to a proximalperipheral nerve stump in the effected limb. On the left end, the construct isallowed to integrate with the nerve stump. On the right end, the multipleelectrode array can be placed in any convenient location for exteriorizationof signal processing and prosthetic connection. B, after transplantation,three modes of axon integration may occur: 1) sprouting axons from the hostproximal nerve stump grow into the transplanted construct and synapse onneurons (smallarrow), 2) axons grow through the construct (largearrow), 3) axons from transplant grow out proximally into the host nerve and spinalcord (arrowhead).
Future Perspective
Traumatic injury and disease of the brain, spinal cord, and peripheral nerves can lead to loss of body functions. A limb could also be completely lost because of amputation. Although prosthetic devices can be offered to replace a lost limb, for patients who simply lose neurological control of an existing limb, there are currently no options to consider.
With success, our nervous tissue interface could offer an important alternative to more conventional brain machines for nonfunctional limbs as well as prosthetics. For instance, functional electrical stimulation has been used to stimulate motor fibers in nerves or the muscle itself to control disabled body functions (34). This approach could eventually be controlled through a nervous system interface, returning function to people who have been paralyzed by a spinal cord injury.
For clinical application, a reliable and expedient supply of neurons for the nervous tissue construct is needed. Dorsal root ganglia could be obtained from cervical and thoracic gan-glionectomies from both live patients and organ donors. We have recently shown the feasibility of using adult human dorsal root ganglion cells for this purpose (24). Not only can these adult neurons survive for at least 3 months, they also remain electrophysiologically active.
A neurally controlled prosthesis is quickly becoming a reality. However, for a prosthesis to operate like a natural limb, the most straightforward approach is to use the processing power of the human brain. We describe a nervous system interface that could be linked to the patient's thoughts, providing two-way signaling for motor control and feedback from multiple external stimuli. Whether it is a prosthetic device or a disabled body function, the mind could regain control.
Acknowledgments
Jason H. Huang, M.D. was supported by a National Research Service Award from the National Institutes of Health (Grant NS46170), a David Kline Award from the American Association of Neurological Surgeons, and a Charles Elsberg Award from the New York Academy of Medicine.
Contributor Information
Bryan J. Pfister, Department of Biomedical Engineering, New Jersey Institute of Technology, Newark, New Jersey
Jason H. Huang, Department of Neurosurgery, University of Rochester Medical Center, Rochester, New York
Niranjan Kameswaran, Department of Neurosurgery, University of Pennsylvania, Philadelphia, Pennsylvania
Eric L. Zager, Department of Neurosurgery, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania
Douglas H. Smith, Department of Neurosurgery, University of Pennsylvania, Philadelphia, Pennsylvania
References
- 1.Bai Q, Wise KD. Single-unit neural recording with active microelectrode arrays. IEEE Trans Biomed Eng. 2001;48:911–920. doi: 10.1109/10.936367. [DOI] [PubMed] [Google Scholar]
- 2.Boockvar JA, Loudon W, Sutton LN. Development of the Spitz-Holter valve in Philadelphia. J Neurosurg. 2001;95:145–147. doi: 10.3171/jns.2001.95.1.0145. [DOI] [PubMed] [Google Scholar]
- 3.Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 4.Cushing H. Electro-surgery as an aid to the removal of intracranial tumors: With a preliminary note on a new surgical-current generator by W.T. Bovie, Ph.D, Chicago. Surg Gynecol Obstet. 1928;47:751–784. [Google Scholar]
- 5.Dhillon GS, Lawrence SM, Hutchinson DT, Horch KW. Residual function in peripheral nerve stumps of amputees: Implications for neural control of artificial limbs. J Hand Surg [Am] 2004;29:605–615. doi: 10.1016/j.jhsa.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Donoghue JP. Connecting cortex to machines: Recent advances in brain interfaces. Nat Neurosci. 2002;5(Suppl):1085–1088. doi: 10.1038/nn947. [DOI] [PubMed] [Google Scholar]
- 7.Donoghue JP, Sanes JN, Hatsopoulos NG, Gaal G. Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. J Neurophysiol. 1998;79:159–173. doi: 10.1152/jn.1998.79.1.159. [DOI] [PubMed] [Google Scholar]
- 8.Elbert T, Rockstroh B. Reorganization of human cerebral cortex: The range of changes following use and injury. Neuroscientist. 2004;10:129–141. doi: 10.1177/1073858403262111. [DOI] [PubMed] [Google Scholar]
- 9.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 10.Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236–250. doi: 10.1111/j.1085-9489.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang JH, Thalhammer JG, Raymond SA, Strichartz GR. Susceptibility to lidocaine of impulses in different somatosensory afferent fibers of rat sciatic nerve. J Pharmacol Exp Ther. 1997;282:802–811. [PubMed] [Google Scholar]
- 12.Huang JH, Zhang JZ, Groff RF, Pfister BJ, Zager EL, Smith DH. Living nerve contructs bridging peripheral nerve lesions demonstrate long-term survival and restoration of function. J Neurotrauma. 2004;21:1297. [Google Scholar]
- 13.Iwata A, Browne KD, Pfister BJ, Gruner JA, Smith DH. Long-term survival and outgrowth of mechanically engineered nervous tissue constructs implanted into spinal cord lesions. Tissue Eng. 2006;12:101–110. doi: 10.1089/ten.2006.12.101. [DOI] [PubMed] [Google Scholar]
- 14.Kanamaru A, Homma I, Hara T. Movement related cortical source for elbow flexion in patients with branchial plexus injury after intercostal-musculocutaneous nerve crossing. Neurosci Lett. 1999;274:203–206. doi: 10.1016/s0304-3940(99)00705-3. [DOI] [PubMed] [Google Scholar]
- 15.Kato H. [Accessed June 12, 2006];Biology of Blue Whales. Available at http://luna.pos.to/whale/jwa_v10_kato.html.
- 16.Kennedy PR, Kirby MT, Moore MM, King B, Mallory A. Computer control using human intracortical local field potentials. IEEE Trans Neural Syst Rehabil Eng. 2004;12:339–344. doi: 10.1109/TNSRE.2004.834629. [DOI] [PubMed] [Google Scholar]
- 17.Lee RG, van Donkelaar P. Mechanisms underlying functional recovery following stroke. Can J Neurol Sci. 1995;22:257–263. doi: 10.1017/s0317167100039445. [DOI] [PubMed] [Google Scholar]
- 18.Maynard EM, Hatsopoulos NG, Ojakangas CL, Acuna BD, Sanes JN, Normann RA, Donoghue JP. Neuronal interactions improve cortical population coding of movement direction. J Neurosci. 1999;19:8083–8093. doi: 10.1523/JNEUROSCI.19-18-08083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mussa-Ivaldi FA, Miller LE. Brain-machine interfaces: Computational demands and clinical needs meet basic neuroscience. Trends Neurosci. 2003;26:329–334. doi: 10.1016/S0166-2236(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 20.Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229–258. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor JL, Bloom DA. William T. Bovie and electrosurgery. Surgery. 1996;119:390–396. doi: 10.1016/s0039-6060(96)80137-1. [DOI] [PubMed] [Google Scholar]
- 22.Palmer C. A microwire technique for recording single neurons in unrestrained animals. Brain Res Bull. 1978;3:285–289. doi: 10.1016/0361-9230(78)90129-6. [DOI] [PubMed] [Google Scholar]
- 23.Pfister BJ, Bonislawski DP, Smith DH, Cohen AS. Stretch-grown axons retain the ability to transmit active electrical signals. FEBS Lett. 2006;580:3525–3531. doi: 10.1016/j.febslet.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfister BJ, Huang JH, Zager EL, Iwata A, Meaney DF, Cohen AS, Smith DH. Engineering Nerve Constructs for Clinical Application. J Neurotrauma. 2004;21:1288. [Google Scholar]
- 25.Pfister BJ, Iwata A, Meaney DF, Smith DH. Extreme stretch growth of integrated axons. J Neurosci. 2004;24:7978–7983. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfister BJ, Iwata A, Taylor AG, Wolf JA, Meaney DF, Smith DH. Development of transplantable nervous tissue constructs comprised of stretch-grown axons. J Neurosci Methods. 2005;153:95–103. doi: 10.1016/j.jneumeth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science. 1992;258:1159–1160. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]
- 28.Rao SM, Binder JR, Hammeke TA, Bandettini PA, Bobholz JA, Frost JA, Myklebust BM, Jacobson RD, Hyde JS. Somatotopic mapping of the human primary motor cortex with functional magnetic resonance imaging. Neurology. 1995;45:919–924. doi: 10.1212/wnl.45.5.919. [DOI] [PubMed] [Google Scholar]
- 29.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 30.Sanes JN, Wang J, Donoghue JP. Immediate and delayed changes of rat motor cortical output representation with new forelimb configurations. Cereb Cortex. 1992;2:141–152. doi: 10.1093/cercor/2.2.141. [DOI] [PubMed] [Google Scholar]
- 31.Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 32.Smith DH, Wolf JA, Meaney DF. A new strategy to produce sustained growth of central nervous system axons: Continuous mechanical tension. Tissue Eng. 2001;7:131–139. doi: 10.1089/107632701300062714. [DOI] [PubMed] [Google Scholar]
- 33.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 34.Vitenzon AS, Mironov EM, Petrushanskaya KA. Functional electrostimulation of muscles as a method for restoring motor functions. Neurosci Behav Physiol. 2005;35:709–714. doi: 10.1007/s11055-005-0114-1. [DOI] [PubMed] [Google Scholar]
- 35.Voorhees JR, Cohen-Gadol AA, Laws ER, Spencer DD. Battling blood loss in neurosurgery: Harvey Cushing's embrace of electrosurgery. J Neurosurg. 2005;102:745–752. doi: 10.3171/jns.2005.102.4.0745. [DOI] [PubMed] [Google Scholar]
- 36.Wagenaar DA, Pine J, Potter SM. An extremely rich repertoire of bursting patterns during the development of cortical cultures. BMC Neurosci. 2006;7:11. doi: 10.1186/1471-2202-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagenaar DA, Potter SM. A versatile all-channel stimulator for electrode arrays, with real-time control. J Neural Eng. 2004;1:39–45. doi: 10.1088/1741-2560/1/1/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waikar SS, Thalhammer JG, Raymond SA, Huang JH, Chang DS, Strichartz GR. Mechanoreceptive afferents exhibit functionally-specific activity dependent changes in conduction velocity. Brain Res. 1996;721:91–100. doi: 10.1016/0006-8993(96)00165-5. [DOI] [PubMed] [Google Scholar]
- 39.Wall JT, Felleman DJ, Kaas JH. Recovery of normal topography in the somatosensory cortex of monkeys after nerve crush and regeneration. Science. 1983;221:771–773. doi: 10.1126/science.6879175. [DOI] [PubMed] [Google Scholar]
- 40.Wiech K, Preissl H, Lutzenberger W, Kiefer RT, Topfner S, Haerle M, Schaller HE, Birbaumer N. Cortical reorganization after digit-to-hand replantation. J Neurosurg. 2000;93:876–883. doi: 10.3171/jns.2000.93.5.0876. [DOI] [PubMed] [Google Scholar]
- 41.Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002;113:767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 42.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci U S A. 2004;101:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]


