Abstract
Background
Hypoxia induces an inflammatory response in the lung manifested by alternative activation of macrophages with elevation of pro-inflammatory mediators that are critical for the later development of hypoxic pulmonary hypertension (HPH). Mesenchymal stromal cell (MSC) transplantation inhibits lung inflammation, vascular remodeling and right heart failure, and reverses HPH in experimental models of disease. In this study, we aimed to investigate the paracrine mechanisms by which MSCs are protective in HPH.
Methods and Results
We fractionated mouse MSC-conditioned media to identify the biologically-active component affecting in vivo hypoxic signaling and determined that exosomes, secreted membrane microvesicles, suppressed the hypoxic pulmonary influx of macrophages and the induction of pro-inflammatory and pro-proliferative mediators, including monocyte chemoattractant protein-1 and hypoxia-inducible mitogenic factor, in the murine model of HPH. Intravenous delivery of MSC-derived exosomes (MEX) inhibited vascular remodeling and HPH, whereas MEX-depleted media or fibroblast-derived exosomes had no effect. MEX suppressed the hypoxic activation of signal transducer and activator of transcription 3 (STAT3) and the upregulation of the miR-17 superfamily of microRNA clusters, whereas it increased lung levels of miR-204, a key microRNA whose expression is decreased in human PH. MEX produced by human umbilical cord MSCs inhibited STAT3 signaling in isolated human pulmonary artery endothelial cells demonstrating a direct effect of MEX on hypoxic vascular cells.
Conclusions
This study indicates that MEX exert a pleiotropic protective effect on the lung and inhibit PH through suppression of hyperproliferative pathways, including STAT-3 mediated signaling induced by hypoxia.
Keywords: hypertension, pulmonary, inflammation, hypoxia, signal transduction
The lung’s response to low levels of environmental oxygen is multifactorial. Diverse signaling pathways, activated or impaired by alveolar hypoxia, converge on endothelial and vascular smooth muscle cells to perturb pulmonary vascular homeostasis. Chronic hypoxia results in pulmonary vascular remodeling, a key pathological feature of pulmonary hypertension (PH). Inflammation plays a prominent detrimental role in most types of human PH and also in animal models of the disease, such as the monocrotaline- and hypoxia-induced PH (HPH) in rodents. The early component of hypoxia-induced lung inflammation, peaking during the first two to three days of hypoxic exposure1, is characterized by alternative activation of alveolar macrophages and appears to be causal to the subsequent vascular remodeling and the development of HPH2.
Despite the significant progress in our understanding of the pathophysiology of PH as well as the treatment of its symptoms, there is no cure for this disease and no single therapy has been proven effective. Given the complex pathways involved in the pathogenesis of PH, therapies aimed at more than one pathway and perhaps more than one cellular target may prove to be more efficacious. Stem cell-based therapeutic approaches hold such a promise as they may simultaneously target multiple signaling pathways and have long lasting effects. The therapeutic potential of mesenchymal stromal cells (MSCs; also referred to as mesenchymal stem cells or multipotent stromal cells) derived from the bone marrow, adipose, and other tissues, has been recognized in several animal models of lung disease3. In models of pro-inflammatory lung diseases, such as bleomycin or endotoxin-induced lung injuries4-6 as well as the neonatal murine model of bronchopulmonary dysplasia (BPD)7, 8, MSC delivery ameliorated lung injury, decreased lung inflammation and fibrosis, and increased survival. MSC delivery was reported to inhibit PH induced by monocrotaline in the rat9 and HPH in the mouse10. However, although a robust protection against lung injury upon MSC treatment was observed in most of the above animal models, only a small fraction of donor cells were retained in the recipient lung. This observation suggested that engraftment and direct tissue repair was not the sole mechanism of MSC therapeutic function and paracrine mechanisms were contemplated. In support of this, we observed that injections with culture media conditioned by MSCs can efficiently inhibit parenchymal injury, vascular remodeling and right ventricular hypertrophy (RVH), completely supplanting MSC treatment on the neonatal murine model of BPD7, 11. In vitro experiments demonstrated anti-proliferative properties of the MSC secretome on pulmonary vascular smooth muscle cells10, again suggesting that MSC paracrine factors can play a major role in preventing lung injury and vascular remodeling. Concordant with our observations, paracrine and immunomodulatory paradigms have been recently proposed to account for enhanced MSC therapeutic function in the context of a number of disease models12.
We have previously performed proteomic analysis of MSC-conditioned medium7, which in addition to immunomodulatory factors, revealed the presence of a number of proteins including CD63, CD81, moesin, lactadherin (MFGE8), heat-shock protein 90 (hsp90), and hsp70, reported to be associated with secreted vesicles known as exosomes13, 14. Secreted membrane microvesicles, especially the better-defined subclass represented by exosomes15, have been recognized as important mediators of cell-to-cell communication and as participants in immunomodulatory mechanisms16. Exosomes are small heterogeneous microvesicles, 30~100 nm in diameter, that are stored within multivesicular bodies (MVB) and released into the environment upon fusion of the MVB with the plasma membrane. Exosomes and microvesicles have been isolated and characterized from various cell types including dendritic cells17, macrophages18, tumor cells19, and embryonic stem cells20 and information is rapidly accumulating on their diverse biological function and their cell type-specific molecular composition. The physiologic relevance of MSC-derived exosomes (MEX) has not yet been evaluated in lung diseases, even though their cellular origin and the recently recognized paracrine function of MSCs may imply a promising therapeutic potential for secreted microvesicles in lung injury.
To address the above questions, we fractionated MSC-conditioned media (CM) through size-exclusion chromatography to identify the biologically-active component protecting against hypoxia-induced lung inflammation and HPH. Using the murine model of HPH, we demonstrate here that MEX are the critical vectors of MSC action: MEX delivery in vivo suppressed HPH and vascular remodeling. Moreover, pro-proliferative pathways were also blocked by MEX treatment, as evidenced by the suppression of signal transducer and activator of transcription (STAT3) phosphorylation resulting in increased lung levels of miR-204, a microRNA enriched in distal pulmonary arterioles that is down-regulated in both human PH and in experimental models of disease21. We found that hypoxia upregulates members of the miR-17 family of microRNA clusters in lung tissue, microRNAs shown to be under the regulatory control of STAT3, and show that MEX treatment efficiently suppresses this pro-proliferative signal. Combined, our findings point to MEX as the key effectors of MSC paracrine function with the potential to serve as vehicles of lung-targeted therapy.
Methods
Animal model and hypoxic exposure
The HPH mouse model has been well-established and used by our group extensively in previously-published work 1, 2, 22. The hypoxic exposure and treatment protocols used in this study are described in the Supplemental Material. All animal experiments were approved by the Boston Children’s Hospital Animal Care and Use Committee.
Preparation of exosomes
Isolation of mouse bone marrow-derived MSCs and MSCs from human umbilical cord Wharton’s Jelly (hUC-MSCs) followed by immunoselection (Supplemental Figure 1) and collection of conditioned media (CM) is outlined in the Supplemental Material.
Concentrated conditioned media were applied on a column of 16/60 Hiprep Sephacryl S-400 HR (GE Healthcare, Piscataway, NJ) that was pre-equilibrated with a buffer containing 20 mM sodium phosphate (pH 7.4) and 300 mM NaCl using an ÄKTA purifier liquid chromatography system (GE Healthcare, Piscataway, NJ). Fractions (1 ml) were collected at a flow rate of 0.5 ml/min. Polystyrene nanospheres of 50 nm diameter (Phosphorex, Fall River, MA) were used as a size reference and elution fractions corresponding to this standard’s retention volume were pooled and further analyzed.
For the isolation of exosomes from hUC-MSCs and human dermal fibroblasts, serum-free culture medium conditioned for 24 hours was filtered (0.2 μm) and concentrated by ultrafiltration device with 100 kDa cut-off (Millipore). Exosomes in CM were precipitated with 1/3 volume of polyethylene glycol (PEG) buffer (33.4% PEG 4000, 50 mM HEPES (pH 7.4), 1 M NaCl) overnight at 4°C followed by centrifugation at 12,000 xg for 5 min and resuspension in PBS (pH7.4). Exosomes in PEG-precipitated fraction were further purified by S200 size-exclusion chromatography. Seventy-five μl sample was applied on a S200 column (Clontech, Mountain View, CA) preequilibrated with PBS by spinning at 700 xg for 5 min and the exosomal fraction was subsequently eluted in the flow-through by centrifugation at 700 xg for 5 min.
In some experiments, exosomes were isolated by ultracentrifugation at 100,000 xg for 2 hours and the pellet was subsequently washed with PBS followed by repeat ultracentrifugation for 2 hours at the same speed. Exosome pellet resuspended in PBS was measured for protein concentration by Bradford assay (Bio-Rad, Hercules, CA). Expression of exosomal markers between the two preparations was similar, as shown in Supplemental Figure 2.
Statistical Analysis
All values are expressed as mean ± standard deviation (SD). All comparisons between experimental and control groups were performed by One-way ANOVA (analysis of variance) with Tukey-Kramer post-test using PRISM 5 statistical software (GraphPad software, San Diego, CA) unless otherwise indicated. A value of p < 0.05 was considered to indicate statistically significant differences. Student’s t-test was used to compare two groups.
See Supplemental Material for a detailed description of further experimental methods.
Results
Factors secreted by MSCs can prevent hypoxia-induced pulmonary inflammation
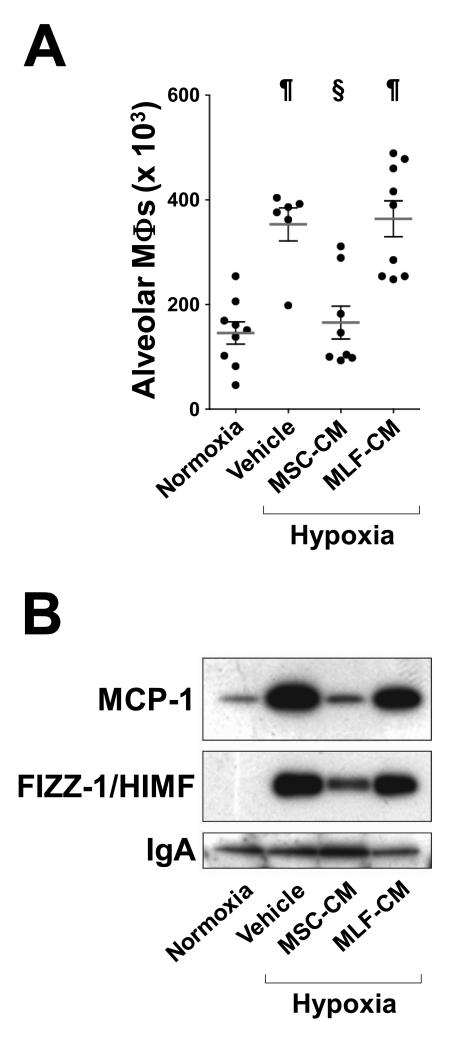
To determine if hypoxic lung inflammation responds to MSC paracrine signals, we injected mice with concentrated serum-free culture media conditioned by either mouse MSCs (MSC-CM) or by mouse lung fibroblasts (MLF-CM) and exposed the animals to normobaric hypoxia (8.5% O2) for 48 hours. In the control group injected with vehicle (serum-free culture media), hypoxia resulted in pulmonary influx of macrophages, as assessed in bronchoalveolar lavage fluid (BALF) and this response was blocked in animals treated with MSC-CM but not in the group treated with MLF-CM (Figure 1A). We also assessed, in cell-free BALF, levels of monocyte chemoattractant protein-1 (MCP-1), a cytokine transiently upregulated in the lung by early hypoxia2, 23 and levels of hypoxia-induced mitogenic factor (HIMF), a pleiotropic factor with pro-inflammatory, mitogenic and chemokine-like properties24. In animals injected with either vehicle or MLF-CM, BALF levels of both MCP-1 and HIMF were highly increased by hypoxia and this increase was effectively suppressed by MSC-CM treatment (Figure 1B). These results indicate that, as we have previously reported on the model of hyperoxia-induced BPD7, 11, the protective effects of MSC treatment in HPH10 involve mainly paracrine mechanisms.
Figure 1.
MSC-CM but not MLF-CM suppress hypoxia-induced pulmonary influx of macrophages. (A) Macrophage numbers in the BALF of hypoxic mice and littermate normoxic controls. Animals received either serum-free culture media (vehicle), MSC-CM (5 μg protein) or MLF-CM (5 μg protein) prior to hypoxic exposure for 48 hours. Each dot represents the total number of Kimura stain-positive cells in BALF from individual animals. Horizontal lines represent the group mean and vertical brackets the standard deviation. (B) BALF levels of MCP-1 and HIMF. Cell-free BALF specimens from all animals in each treated group were pooled and an amount corresponding to one animal equivalent was subjected to western blot analysis. IgA levels were assessed as a control for equal loading. n > 6 for all groups. ¶, p<0.001 vs. untreated normoxic controls; §, p<0.001 vs. PBS-treated hypoxic controls (One-way ANOVA with Tukey-Kramer post-test).
The anti-inflammatory activity in MSC-CM is associated with exosomes
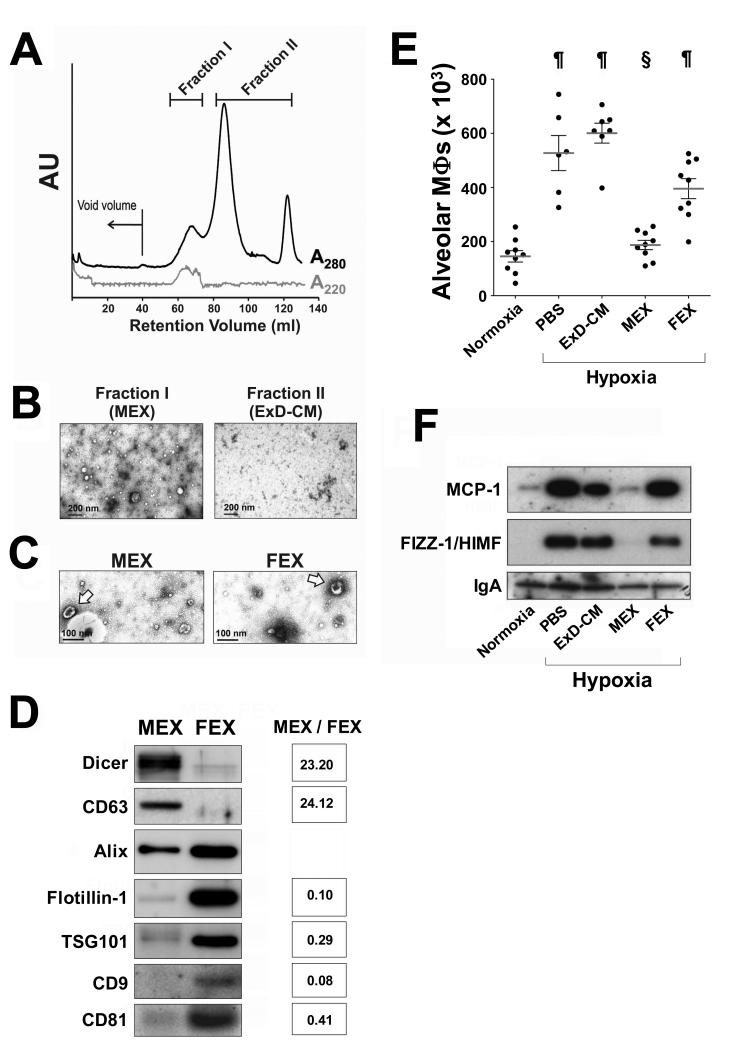
In order to identify the biologically-active component of MSC-CM, we fractionated concentrated conditioned media through size-exclusion chromatography. Polystyrene nanospheres of 50 nm diameter served as a hydrodynamic radius standard to identify the exosomal fraction, and fractions in a protein peak eluting with a retention volume corresponding to that of the standard were pooled (Figure 2A, Fraction I). Negative staining electron microscopic analysis revealed Fraction I to contain heterogeneous microvesicles that were absent in fractions corresponding to the retention volume of moieties of smaller size (Figure 2B, Fraction II). Fraction I was highly enriched in microvesicles 30-100 nm in diameter exhibiting biconcave morphology, a distinct morphological feature of exosomes (Fig 2C, arrows). Exosomes were present in both MSC- and MLF-CM and, in this report, MSC exosome preparations are termed MEX, whereas MLF exosome preparations are termed FEX. Both MEX and FEX contain diverse mature microRNAs (see below) and also Dicer (Figure 2D), a component of the cytoplasmic microRNA maturation complex. However, relative abundance of each exosomal marker differs depending on the cellular origin of the microvesicles.
Figure 2.
(A) Size fractionation of concentrated conditioned media by Sephacryl-400 chromatography. The black trace represents elution of protein (A280) and the gray trace represents the elution profile of 50 nm diameter polystyrene nanospheres (A220). The fractions of conditioned media in the retention volume corresponding to the 50 nm diameter range were pooled and termed Fraction I, and fractions representing moieties of smaller size were pooled as Fraction II. (B) Negative staining electron microscopy at 30,000X magnification revealed heterogeneous microvesicles specifically in Fraction I. (C) Fraction I preparations from either MEX or FEX were enriched in 30-100 nm diameter microvesicles exhibiting typical exosome morphology (arrows). (D) Preparations of exosomes produced by either MSCs or MLFs are associated with typical exosomal markers as well as Dicer. The relative levels of these markers depends on the cellular origin of the microvesicles. Density for each band relative to Alix was measured and relative abundance in MEX compared to FEX for each marker is shown on right. (E) Macrophage numbers in the BALF of animals exposed to hypoxia for 48 hours and treated with either vehicle (PBS), MEX, FEX, or the exosome-depleted fraction of MSC-CM (ExD-CM). Normoxic animals were used as a control. ¶, p<0.001 vs. normoxic control animals; §, p<0.001 vs. PBS-treated hypoxic animals (One-way ANOVA with Tukey-Kramer post-test). (F) Western blot analysis of MCP-1 and HIMF levels in BALF of hypoxic animals treated with isolated exosomes and controls.
MEX preparations were efficacious in suppressing hypoxic inflammation when injected into animals, whereas the MSC-CM fraction depleted of exosomes (ExD-CM) had no significant effect and FEX had a partial inhibiting effect (Figure 2E). Concordantly, levels of pro-inflammatory mediators in cell-free BALF of hypoxic animals were suppressed only by MEX but not by FEX or ExD-CM treatment (Figure 2F), indicating that the ability to suppress early hypoxia-induced pulmonary inflammation is associated specifically with exosomes of MSC origin.
Dose response effects of MEX on lung inflammation
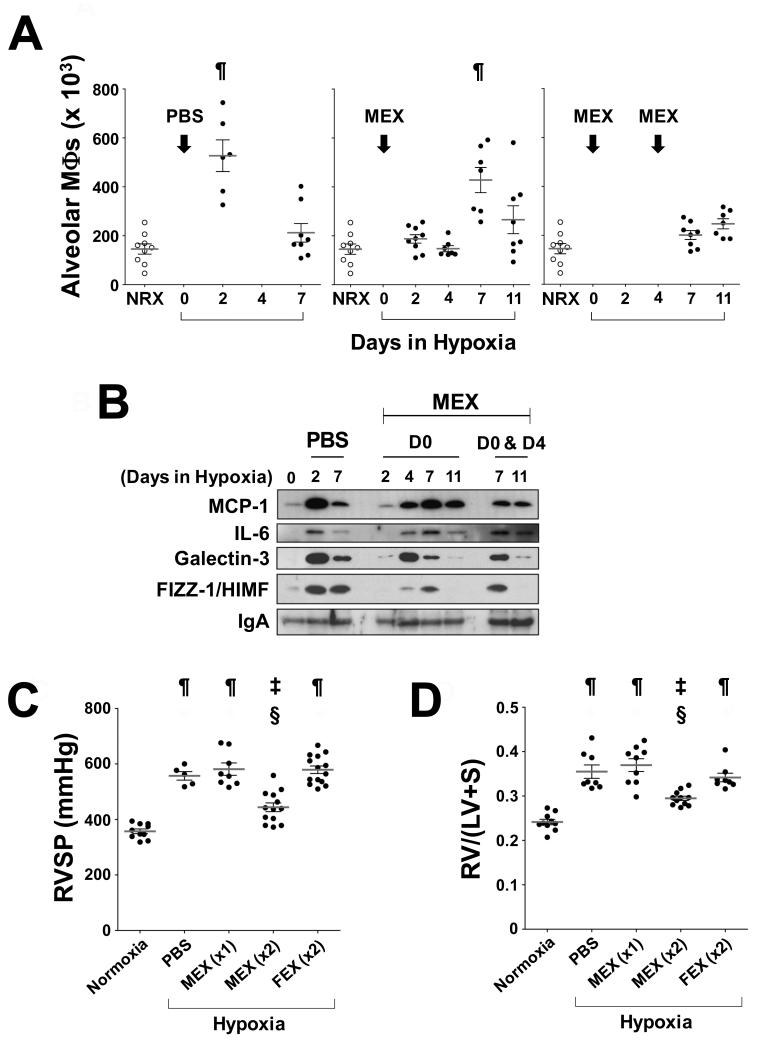
We have previously reported that suppression of the entire period of the inflammatory response to early hypoxia is required to protect animals from later development of PH 2. This inflammatory response is transient in the murine model, peaking within 2-3 days of hypoxic exposure and subsiding by day 7 (Figure 3A, left panel). We therefore assessed the effect of MEX treatment on the temporal profile of hypoxic lung inflammation, and we found that a low dose of MEX (0.1 μg/animal, via jugular vein) was able to delay but not to completely suppress the pulmonary influx of macrophages, resulting in a shift of the inflammatory peak towards later times (Figure 3A, middle panel). The observed temporal profile of pulmonary macrophage influx was paralleled by the temporal profile of induction of the pro-inflammatory markers, MCP-1, interleukin-6 (IL-6), Galectin-3, and HIMF, in cell-free BALF, which also shifted to a later time (4 to 7 days, Figure 3B). In contrast, a treatment consisting of two sequential injections of MEX, one prior to exposure to hypoxia and a second injection at day 4, just prior to the delayed inflammatory peak (Figure 3A, middle panel), efficiently suppressed pulmonary influx of macrophages over the entire period of inflammatory responses to early hypoxia (Figure 3A, right panel). However the peak of pro-inflammatory markers in BALF, although delayed by the first dose, was not affected by the second injection of MEX (Figure 3B).
Figure 3.
Repeated administration of a low MEX dose (x2) can suppress pulmonary influx of macrophages and ameliorate HPH. (A) Animals were injected with one dose of PBS (left panel, n=14) or 0.1 μg MEX (Middle panel, n=31) through the jugular vein prior to hypoxic exposure and alveolar macrophages were measured in BALF at sacrifice on the days indicated on the X axis. Animals (Right panel, n=15) received 0.1 μg MEX prior to exposure to hypoxia and 0.1 μg MEX again at day 4 of hypoxia and alveolar macrophages were measured in BALF at sacrifice on the days indicated on the X axis. Each dot represents an individual animal. Macrophage numbers in BALF from normoxic control animals (open circles) are replicated in all three panels for direct comparison. ¶, p<0.001 vs. normoxic control group (One-way ANOVA with Tukey-Kramer post-test). (B) Representative Western blot analysis of cytokine levels in BALF of animals treated with a single low dose MEX. BALF from 6 animals in each group was pooled for immunoblot analysis and repeated at least 3 times. (C) RVSP and (D) Fulton’s Index determined after 3 weeks of hypoxic exposure. The Fulton’s Index (RV/[LV+S]) is a measurement of right ventricular hypertrophy expressed as a weight ratio. RV, right ventricle; LV, left ventricle; S, septum. MEX (x1): Animals were injected once with MEX. MEX(x2), FEX(x2): Animals were injected twice with equivalent amounts of MEX or FEX. Black dots represent values for individual animals, horizontal lines represent the group mean and vertical brackets the standard deviation. ¶, p<0.001 vs. normoxic control group; ‡, p<0.01 vs. normoxic control group; §, p<0.001 vs. PBS-injected hypoxic control group (One-way ANOVA with Tukey-Kramer post-test).
Multiple administrations of low doses of MEX, but not FEX, ameliorate pulmonary hypertension, right ventricular hypertrophy, and lung vascular remodeling
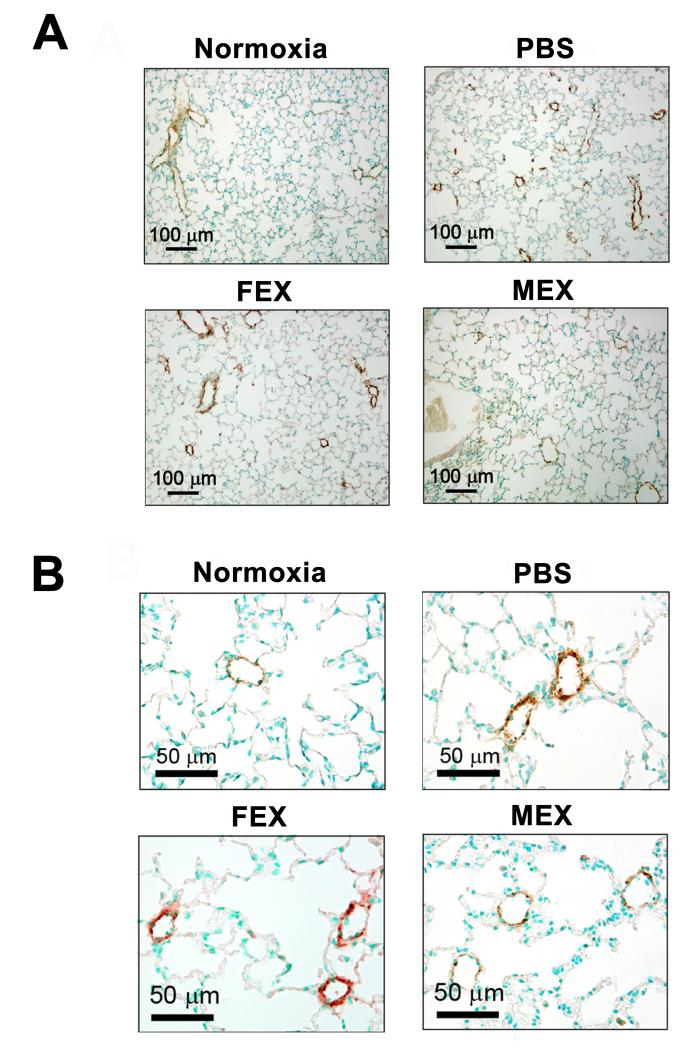
The physiologic consequences of partial or complete abrogation of the early inflammatory response to hypoxia are seen in Figure 3C and 3D. A single low dose of MEX did not protect against the elevation of RVSP or the development of RVH after three weeks of hypoxic exposure, whereas the double injection regimen significantly improved both variables. These results mirror the physiologic response we had observed using pulses of heme oxygenase-1 (HO-1) overexpression to completely or partially suppress the early hypoxic lung inflammation 2 and suggest a dose- and time-sensitive window for anti-inflammatory treatments to confer protection from HPH. Importantly, FEX treatment using the double injection protocol did not have any physiologic effect, buttressing the assertion that the function(s) protecting against HPH reside specifically with exosomes produced by MSCs. Furthermore, animals treated with two doses of MEX and exposed to three weeks of hypoxia did not develop vascular remodeling as determined by α-smooth muscle actin (α–SMA) staining, whereas the same treatment protocol with FEX resulted in medial wall hypertrophy similar to vehicle-treated controls (Figure 4).
Figure 4.
Repeated administration of low MEX dose (x2) inhibits lung vascular remodeling. Representative lung sections are shown from animals exposed to the same conditions as described in the legend of Figure 3 and stained for α-SMA to evaluate medial vessel wall thickness. (A) 100X magnification and (B) 400X.
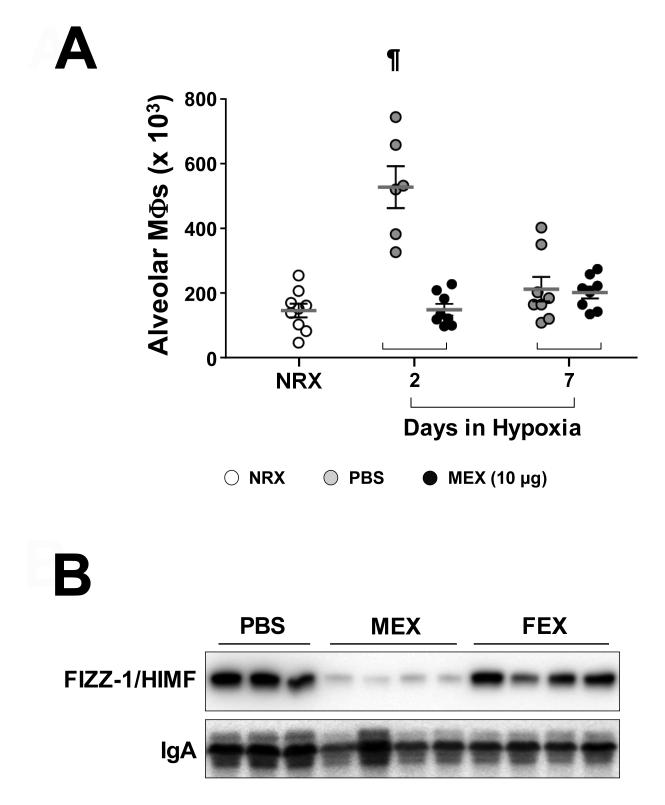
A single high dose MEX treatment inhibits hypoxic inflammation, vascular remodeling and HPH
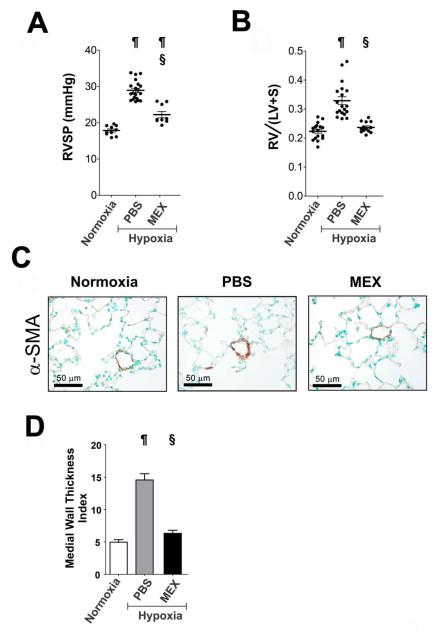
The incomplete protection from HPH by two sequential low doses of MEX could be related to the failure in completely suppressing early hypoxic inflammation. To test the efficacy of higher MEX dosages on early hypoxic inflammation and HPH, 10 μg MEX were injected through the tail vein and mice were exposed to hypoxia for 2 and 7 days. A higher dose of MEX prevented pulmonary influx of macrophages similarly with two sequential injections of MEX (Figure 5A) and importantly, also completely abrogated the elevation of pro-inflammatory marker FIZZ-1/HIMF in the lung for the entire period of early hypoxic responses. An equivalent dose of FEX had no effect (Figure 5B). Next, to examine the efficacy of higher dose of MEX on vascular remodeling and HPH, 10 μg of MEX were injected and mice were exposed to chronic hypoxia for three weeks. Compared to vehicle-injected (PBS) controls, the MEX-treated group had significantly (p < 0.001, One-way ANOVA) decreased RVSP (Figure 6A) and did not develop RVH in response to chronic hypoxia (Figure 6B). MEX treatment prevented pulmonary vascular remodeling, as assessed by α-SMA staining (Figure 6C). Morphometric analyses on small arterioles revealed a significant effect on the medial wall thickness index, with values in the MEX-treated group approximating those of the minimally-muscularized normoxic vessels (Figure 6D). Taken together, the above results strongly suggest that the protective mechanism of MEX action is through blocking inflammatory lung responses to early hypoxia, which when left unchecked, activate pro-proliferative pathways in the vascular wall thereby increasing medial wall thickness and altering vascular cell phenotype.
Figure 5.
A single high dose MEX efficiently suppresses the entire period of inflammatory responses to early hypoxia. (A) Macrophage numbers in the BALF of animals injected with a high dose (10 μg) MEX prior to hypoxic exposure for 2 and 7 days. Dots represent values for individual animals. Black dots represent values for MEX-treated group. White and gray dots represent values for normoxic group (NRX) and PBS-treated groups, respectively, which are adopted from Fig 3A. Horizontal lines represent the group mean and vertical brackets the standard deviation. ¶, p<0.001 vs. normoxic control group (One-way ANOVA with Tukey-Kramer post-test). (B) Representative Western blot analysis of FIZZ-1/HIMF levels in BALF of animals treated with a single high dose MEX and FEX (10 μg) prior to hypoxic exposure for 7 days.
Figure 6.
A single high dose MEX treatment prevents HPH. (A) RVSP measurements and (B) Fulton’s Index of normoxic animals, and animals injected with either PBS or 10 μg MEX prior to a three week hypoxic exposure. Black dots represent values for individual animals, horizontal lines represent the group mean and vertical brackets the standard deviation. ¶, p<0.001 vs. normoxic control group; §, p<0.001 vs. PBS-treated hypoxic control group (One-way ANOVA with Tukey-Kramer post-test). (C) Histology of paraffin-embedded lung sections stained for αSMA at 400X magnification, depicting representative pulmonary arterioles. (D) Assessment of medial wall thickness of small pulmonary arterioles (20-40 μm diameter) in the three experimental groups (sections from four animals per group; n>40 vessels measured per animal). ¶, p<0.001 vs. normoxic control group; §, p<0.001 vs. PBS-treated hypoxic group (One-way ANOVA with Tukey-Kramer post-test).
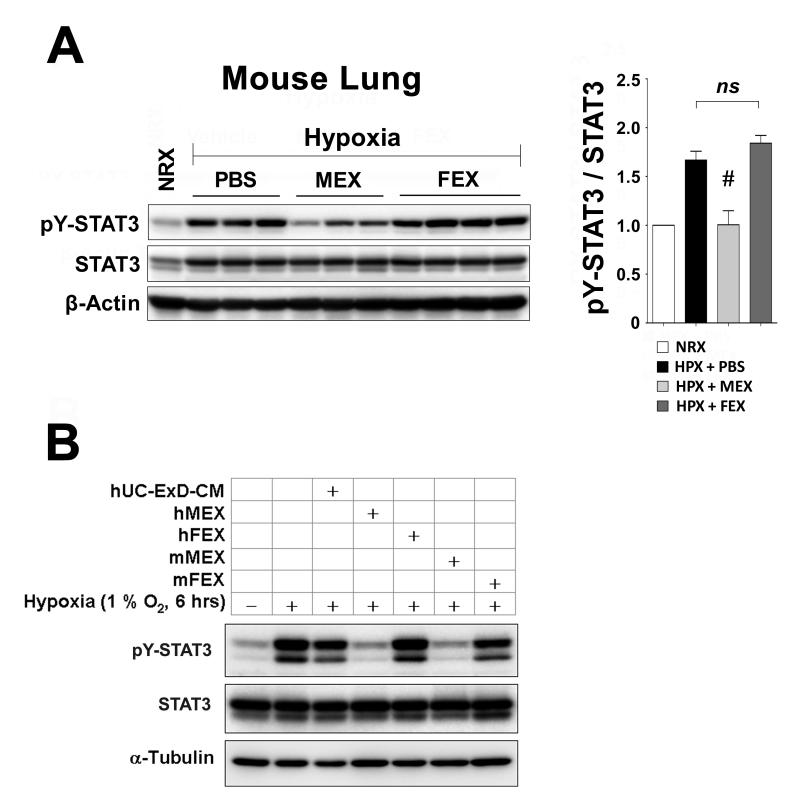
MEX inhibit STAT3 activation by hypoxia
Early hypoxia resulted in activation of STAT3 in the mouse lung through phosphorylation at Tyr-705 and without any effect on the total levels of STAT3 protein. This activation was efficiently suppressed by MEX treatment, but not FEX (Figure 7A). STAT3 is a transcription factor integral to signaling pathways of many cytokines and growth factors and its activation plays a critical role in respiratory epithelial inflammatory responses25, 26. Importantly, persistent ex vivo STAT3 activation, has been linked to the hyperproliferative and apoptosis-resistant phenotype observed in pulmonary artery endothelial cells (PAECs) 27 and pulmonary artery smooth muscle cells (PASMCs)28 from patients with idiopathic pulmonary arterial hypertension (IPAH). Therefore, to determine whether MEX regulate STAT3 activation on lung vascular cells, we exposed primary human PAECs (hPAECs) to hypoxia and assessed pY-STAT3 levels. As depicted in Figure 7B, exposure of hPAECs to hypoxia results in robust activation of STAT3 by Tyr-705 phosphorylation. Treatment with mouse MEX or MEX derived from MSCs isolated from human umbilical cord stroma29 completely abrogated this response. In contrast, neither mouse FEX, human FEX, nor the fraction of human umbilical cord MSC-CM depleted of microvesicles (hUC-ExD-CM) had any effect. Besides demonstrating that suppression of STAT3 activation is a property shared by MEX of both human and mouse origin, these results strongly suggest that direct suppression of hypoxic signaling in pulmonary vascular cells is a primary function underlying the protection conferred by MEX treatment. In concordance, we found that MEX dose-dependently inhibited PASMC proliferation rate in response to serum-derived mitogens (Supplemental Figure 3), confirming that MEX have direct effects on lung vascular cells.
Figure 7.
MEX of either mouse or human origin suppress the hypoxic phosphorylation (activation) of STAT3. (A) Total protein extracts from lungs of individual animals treated with 10 μg MEX. Hypoxia exposure for 2 days resulted in activation of STAT3 through phosphorylation at Tyr-705 (pY-STAT3) and this was prevented by treatment with MEX of mouse origin. Right panel: Quantitation of STAT3 phosphorylation. #, p<0.01 vs. PBS-treated hypoxic group; ns, statistically non-significant (One-way ANOVA with Tukey-Kramer post-test). (B) Primary cultures of hPAECs exposed to hypoxia (1% O2, 6 hours) exhibit robust activation of STAT3 that is efficiently suppressed by MEX secreted by MSCs from mouse (mMEX) and human umbilical cord stroma (hMEX). Note that neither the microvesicle-depleted fraction of media conditioned by human umbilical cord MSCs (hUC-ExD-CM), nor hFEX or mFEX, have any effect on STAT3 phosphorylation. Data are representative of at least 3 independent experiments.
Differential miRNA content in MEX vs. FEX
A number of recent studies report the successful horizontal transfer of functional mRNA and miRNA species from exosomes into recipient cells30, 31. To evaluate potential differential signals released by MEX vs FEX that could mediate their therapeutic effects in vivo, we quantified relative levels of a number of candidate miRNAs in MEX and FEX preparations. We found that relative to FEX, MEX contain significantly increased levels of miRNA-16 and miRNA-21. Interestingly, although let7b miRNA levels were comparable within these two types of exosomes, let7b pre-miRNA was significantly enriched in MEX vs FEX (>10 fold) (Supplemental Figure 4). These findings point to distinct and potentially important microRNA-mediated regulatory signals delivered to the lung by MSC-derived exosomes.
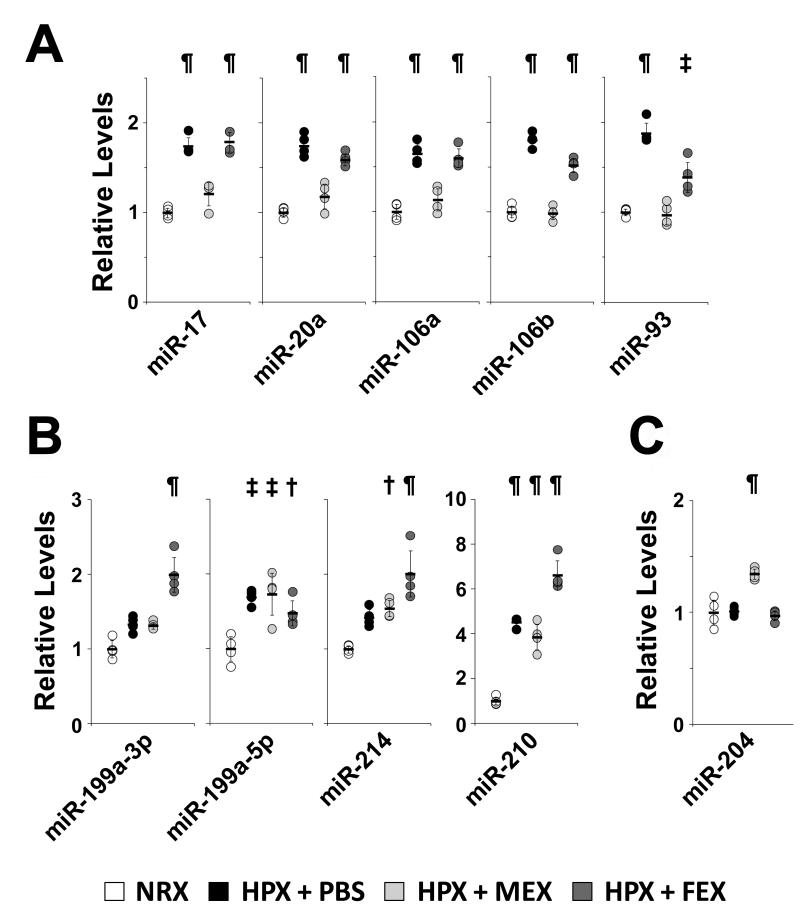
MEX treatment suppresses the hypoxic induction of the miR-17 microRNA superfamily and increases levels of anti-proliferative miR-204 in the lung
STAT3 (activated by either vascular endothelial growth factor or IL-6) has been reported to directly regulate the transcription of the miR-17~92 cluster of microRNAs in PAECs, resulting in decreased levels of bone morphogenetic protein receptor-2 (BMPR2), a target of miR-1732. Therefore, we assessed the effect of hypoxia and MEX treatment on the miR-17~92 cluster of microRNAs and its conserved paralog clusters, miR-106b~25 and miR-106a~363. These microRNA clusters have been postulated to be pro-proliferative and to target an array of genes involved in the G1/S phase transition33. We found that select microRNAs representing all three clusters of the miR-17 superfamily were upregulated by hypoxia in the lung, and this transcriptional activation was efficiently suppressed by MEX treatment (Figure 8A). Interestingly, levels of microRNAs involved in hypoxic signaling networks, such as miR-199a-5p, a microRNA reported to stabilize HIF1α in cardiac myocytes34, miR-214, which shares the same host gene with miR-199 35, or miR-210, a hypoxamir under direct hypoxia-inducible factor-1 (HIF1α regulation36, were not affected by MEX treatment (Figure 8B), pointing to targeted effects of MEX on specific hypoxia-regulated signaling pathways. Treatment with an equivalent dose of FEX had no inhibitory effect on the hypoxamirs examined with only members of the miR106b/25/93 cluster being moderately affected by FEX (Fig 8A).
Figure 8.
MEX treatment suppresses the hypoxic induction of the miR-17 microRNA superfamily and increases levels of anti-proliferative miR-204 in the lung. Mice were treated with either PBS, MEX (10 μg) or FEX (10 μg) and then exposed to hypoxia for 7 days. Another untreated group remained in normoxia as control. MicroRNA levels in total mouse lung were assessed by qPCR and fold changes in the hypoxic groups are presented relative to the mean of the normoxic group. (A) Select miRs representing the miR-17~92, miR-106b~25 and miR-106a~363 clusters. (B) Select miRs reported to be involved in hypoxic signaling. (C) Upregulation of basal levels of the pulmonary arteriole-specific miR-204 upon MEX treatment. Dots represent expression levels in individual animals. NRX : Normoxia; HPX : Hypoxia. ¶, p<0.001 vs. normoxia; ‡, p<0.01 vs. normoxia; †, p
Importantly, we observed that MEX treatment, but not FEX, resulted in the increase of lung levels of miR-204 (Figure 8C), a microRNA enriched in distal pulmonary arterioles that is transcriptionally suppressed by STAT3 but also inhibits the activation of STAT3 in a feed-forward regulatory loop21. The proliferative and anti-apoptotic phenotype of PASMCs isolated from patients with IPAH is inversely related to the level of miR-204 and delivery of exogenous miR-204 to the lungs of animals with PH ameliorated established disease21. Therefore, we interpret these results as an indication that MEX treatment, by suppressing STAT3 activation at the early stages of hypoxic exposure, prevents the hypoxic induction of the pro-proliferative miR-17 superfamily in the lung vasculature and blocks the STAT3-miR-204-STAT3 feed-forward loop in distal pulmonary vessels.
Discussion
This report, demonstrates that the protective functions of MSCs are mediated by secreted microvesicles. Thus, our work provides an explanation for the paradox of the consistently observed significant physiologic effect of MSC treatment despite very low retention of donor cells in the lung. Secreted membrane microvesicles, of which exosomes represent the better characterized subclass, have been recognized as important mediators of intercellular communication, especially in the immune system. It has been proposed that such microvesicles, called exosomes, can act as a vector for the transfer of genetic information (mRNA and microRNAs) or the shuttling of effector proteins to recipient cells15, 16. Heat-shock protein 72 from tumor-derived exosomes mediates immunosuppressive function to myeloid-derived suppressive cells through activation of STAT319. Through putative transfer of microRNAs and mRNAs, microvesicles secreted from tumor-initiating cells positive for the mesenchymal marker CD105 have been reported to confer angiogenic phenotype to normal endothelial cells37. Supporting our observations here, microvesicles released from MSCs have been recently reported to improve recovery in animal models of experimentally-induced renal failure38 and myocardial ischemia/reperfusion injury39, however, the underlying mechanisms mediating these protective effects were not characterized.
Our results show that treatment with MSC-derived exosomes prevents the activation of hypoxic signaling that underlies pulmonary inflammation and the development of PH in the murine model. MCP-1 and HIMF/FIZZ1 are highly upregulated by hypoxia in the lung and both factors are potent proinflammatory mediators. Moreover, both MCP-1 and HIMF/FIZZ1 have been linked to the development of PH in murine models of disease40, 41 as well as in human PH42. Egashira et. al. demonstrated that administration of exogenous recombinant MCP-1 resulted in prominent medial wall thickening of pulmonary arterioles and MCP-1 receptor blockade prevented monocyte recruitment as well as the subsequent vascular remodeling 41. HIMF/FIZZ1, a marker of alternative activated macrophages43, is also induced by hypoxia in the respiratory epithelium and plays a critical role in the development of HPH in a murine model and in scleroderma-associated PH. 24, 44, 45. The knockdown of HIMF/FIZZ1 partially blocked increases in pulmonary artery pressure, RVH, and vascular remodeling caused by chronic hypoxia. The important role of HIMF in the development of PH was further confirmed through intrapulmonary gene transfer of HIMF/FIZZ1 which recapitulated the findings of HPH40. We demonstrate here that MEX administration inhibited the hypoxic induction of both MCP-1 and HIMF/FIZZ1 in the lung and this was associated with prevention of HPH.
Directly related to of the anti-inflammatory action of MEX treatment is the observed prevention of hypoxia-activated pro-proliferative pathways. Examining total lung tissue we found that, in addition to MCP-1, hypoxic levels of IL-6 were also suppressed by MEX administration. IL-6 is a proinflammatory cytokine known to activate STAT346 and, in a number of studies, was associated with PH32, 47. It is thus possible that STAT3 may be a key mediator of hypoxic, pro-inflammatory signaling leading to PH in the in vivo lung. Indeed, phosphorylation of STAT3 at the 705 tyrosine residue is required for STAT3 dimerization and subsequent nuclear translocation48, 49, and this was markedly increased in both lung and PAECs in response to hypoxia but significantly suppressed by MEX treatment. Importantly, persistent ex vivo STAT3 activation, has been linked to the hyperproliferative and apoptosis-resistant phenotype observed in PAECs27 and PASMCs28 from patients with IPAH. Mathew et. al. reported a marked upregulation of STAT3 phosphorylation in the lungs of rats with monocrotaline-induced PH50. STAT3 directly regulates the transcription of the miR-17~92 cluster of microRNAs in hPAECs, resulting in decreased levels of BMPR2, a target of miR-1732 whose downregulation is recognized as a hallmark of PH. We found that hypoxia induced select microRNAs of the miR-17 superfamily in the lung and MEX effectively suppressed this induction whereas MEX did not suppress other microRNAs involved in hypoxic signaling networks, including the hypoxamir miR-210 which is induced by HIF36, pointing to selective, STAT3 targeted effects of MEX action in the lung rather than global suppression of all hypoxamirs.
In addition to STAT3 being a central determinant of the hyperproliferative vascular cell phenotype in patients with IPAH, the suppression of miR-204 (a distal pulmonary artery specific microRNA) correlates with PH severity in human disease and rodent models of PH21. In this study, miR-204 was suppressed by STAT3 and miR-204 was shown to inhibit the activation of STAT3 in a self-regulatory loop. Although during the acute hypoxic phase we did not observe the decrease in miR-204 levels observed under chronic hypoxia in the mouse21 we consider the fact that MEX treatment increases the basal level of miR-204 as a strong indication that MEX treatment is shifting the balance of the STAT3-miR-204 loop to an anti-proliferative state.
A schema of a hypothesis synthesizing the above results with previous work from our group and the work of others is shown in Supplemental Figure 5. We have previously shown that hypoxia shifts the Th1/Th2 balance of immunomodulators in the lung, resulting in alternative activated alveolar macrophages (AA-AM ) and this is inhibited by HO-1 overexpression2. Hypoxia also induces the expression of HIMF in the lung epithelium24 and HIMF mitogenic action on the vasculature requires Th2 cytokines, such as IL-444 to result in PH. Consequences of the shift towards proliferation include the hypoxic activation of STAT3 signaling and the upregulation of the miR-17 family of microRNAs. Treatment with MEX interferes with an early hypoxic signal in the lung, suppressing inflammation, HIMF transcriptional upregulation, and alternative macrophage activation. It addition, MEX treatment may directly suppress STAT3 activation in lung vascular cells and also upregulate miR-204 levels, thus breaking the STAT3-miR-204-STAT3 feed-forward loop and shifting the balance to an anti-proliferative state.
The ability to secrete microparticles that contain not only proteins but RNA or miRNA species which can modulate the expression of multiple genes make these packaging vesicles an attractive and quite plausible means for MSCs to regulate multiple pathways and produce a robust therapeutic effect in vivo. Indeed, exosomes are lipid vesicles of endocytic origin released by many cell types including vascular cells, dendritic cells, and mast cells13 that function to mediate intercellular communication through the exchange of protein and RNA moieties. Of possible physiologic relevance is the differential distribution of tetraspanins CD63 (abundant in mouse MEX) and CD81 (abundant in mouse FEX). Although this differential distribution is not apparent between human MEX and human FEX (results not shown), these are molecules that may play a role in target cell specificity of exosomes and could participate in signaling pathways. Full molecular characterization of exosomal preparations produced by mouse bone marrow MSCs and human umbilical cord stroma MSCs is the focus of ongoing work. Exosomes isolated from a mast-cell line or from primary bone marrow-derived mast cells were reported to contain mRNAs and microRNAs that were transferable to other mast cells, and, in the case of mRNAs, to be translated into new proteins13. The authors identified different miRNAs within exosomes and, in a more recent study, pre- and mature miRNAs, but not larger species, were identified within MSC-derived exosomes51. Given the robust, long-lasting anti-inflammatory and cytoprotective effects of MEX demonstrated for the first time in the present study, it is reasonable to postulate that one or more miRNA species that are unique to or highly enriched within MEX, serve as master regulator(s) of several genes and pathways underlying the development of PH.
In summary, in this study we isolated, identified, and characterized exosomes from mouse and human MSC-CM and demonstrated a robust biologic effect that is unique to MEX versus exosomes derived from other cells, such as fibroblasts. Importantly, we demonstrate for the first time that MEX are the major paracrine anti-inflammatory and therapeutic mediators of MSC action on the lung, acting, at least in part, through inhibition of hypoxic STAT3 signaling. Further work is required to identify the critical components of MEX, be they protein, lipid, or nucleic acid species. Although the applicability of our findings to a human disease model need to be verified, the efficacy of this treatment in preventing PH makes these microvesicles an attractive candidate for exploring models of therapeutic interventions in PH and other diseases with no definitive therapy to date.
Supplementary Material
CLINICAL PERSPECTIVE.
Pulmonary arterial hypertension (PAH) remains without cure despite significant progress in our understanding of its pathophysiology. Given the complex molecular and cellular pathways underlying the development of PAH, therapies aimed at multiple pathways and cellular targets may prove to be more efficacious. Stem cell-based therapies hold such a promise, as they can simultaneously target diverse signaling pathways and have long lasting effects. Accumulating studies support an important cytoprotective, anti-inflammatory role for mesenchymal stem cells (MSCs) with demonstrated efficacy against pulmonary hypertension in animal models of disease. We previously reported both prevention and reversal of severe pulmonary hypertension and right heart failure in a mouse model of hypoxia-induced pulmonary hypertension and have postulated a paracrine mode of MSC protective functions. In this report, we show that MSCs secrete microvesicles (exosomes) which are the vectors of their action, being both necessary and sufficient to confer cytoprotection on the lung vasculature. We show that, potentially through epigenetic mechanisms involving microRNA signaling, MEX can have long-lasting therapeutic effects on pulmonary hypertension. These findings may lead to the development of alternative strategies in the field of stem cell - based therapies, at least for certain lung diseases, as delivery of in vitro purified exosomes could substitute the delivery of intact cells. Exosome treatment, in addition to its enhanced practicality in terms of storage and administration, does not carry the danger of oncogenic potential of donor cells, an important consideration in all stem cell – based therapies.
Acknowledgements
The authors would like to thank Xianlan Liu for technical expertise, Sarah Gately for assistance in preparing the manuscript, and Dr. Georg Hansmann for critical review of the manuscript.
Funding Sources: This work was supported by NIH RO1 HL055454 and NIH RO1 HL085446 (SK & SAM).
Footnotes
Conflict of Interest Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early Macrophage Recruitment and Alternative Activation Are Critical for the Later Development of Hypoxia-Induced Pulmonary Hypertension. Circulation. 2011;123:1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8:223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 7.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K, Abley D, Korbutt G, Archer SL, Thebaud B. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 10.Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, Kourembanas S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99–107. doi: 10.1002/stem.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, Kourembanas S. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170–81. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Aoki N, Jin-no S, Nakagawa Y, Asai N, Arakawa E, Tamura N, Tamura T, Matsuda T. Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology. 2007;148:3850–3862. doi: 10.1210/en.2006-1479. [DOI] [PubMed] [Google Scholar]
- 15.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33:441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 17.Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179:1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 18.Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One. 2008;3:e2461. doi: 10.1371/journal.pone.0002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 21.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitali SH, Mitsialis SA, Liang OD, Liu X, Fernandez-Gonzalez A, Christou H, Wu X, McGowan FX, Kourembanas S. Divergent cardiopulmonary actions of heme oxygenase enzymatic products in chronic hypoxia. PLoS One. 2009;4:e5978. doi: 10.1371/journal.pone.0005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zampetaki A, Minamino T, Mitsialis SA, Kourembanas S. Effect of heme oxygenase-1 overexpression in two models of lung inflammation. Exp Biol Med. 2003;228:442–446. doi: 10.1177/15353702-0322805-02. [DOI] [PubMed] [Google Scholar]
- 24.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–1067. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- 25.Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest. 2004;113:28–37. doi: 10.1172/JCI200419491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38:699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548–554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 28.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, Cote J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123:1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 30.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 33.Cloonan N, Brown MK, Steptoe AL, Wani S, Chan WL, Forrest AR, Kolle G, Gabrielli B, Grimmond SM. The miR-17-5p microRNA is a key regulator of the G1/S phase cell cycle transition. Genome Biol. 2008;9:R127. doi: 10.1186/gb-2008-9-8-r127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe T, Sato T, Amano T, Kawamura Y, Kawamura N, Kawaguchi H, Yamashita N, Kurihara H, Nakaoka T. Dnm3os, a non-coding RNA, is required for normal growth and skeletal development in mice. Dev Dyn. 2008;237:3738–3748. doi: 10.1002/dvdy.21787. [DOI] [PubMed] [Google Scholar]
- 36.Chan SY, Loscalzo J. MicroRNA-210: A unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072–1083. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 38.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Champion HC, Crow MT, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) induces the vascular and hemodynamic changes of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L582–593. doi: 10.1152/ajplung.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egashira K, Koyanagi M, Kitamoto S, Ni W, Kataoka C, Morishita R, Kaneda Y, Akiyama C, Nishida KI, Sueishi K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy inhibits vascular remodeling in rats: blockade of MCP-1 activity after intramuscular transfer of a mutant gene inhibits vascular remodeling induced by chronic blockade of NO synthesis. FASEB J. 2000;14:1974–1978. doi: 10.1096/fj.00-0141com. [DOI] [PubMed] [Google Scholar]
- 42.Itoh T, Nagaya N, Ishibashi-Ueda H, Kyotani S, Oya H, Sakamaki F, Kimura H, Nakanishi N. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology. 2006;11:158–163. doi: 10.1111/j.1440-1843.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 43.Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 44.Yamaji-Kegan K, Su Q, Angelini DJ, Myers AC, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J Immunol. 2010;185:5539–5548. doi: 10.4049/jimmunol.0904021. [DOI] [PubMed] [Google Scholar]
- 45.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Teng X, Hassoun PM, Yang SC, Champion HC, Tuder RM, Johns RA. Resistin-like molecule-beta in scleroderma-associated pulmonary hypertension. Am J Respir Cell Mol Biol. 2009;41:553–561. doi: 10.1165/rcmb.2008-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bromberg J, Darnell JE., Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 47.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yahata Y, Shirakata Y, Tokumaru S, Yamasaki K, Sayama K, Hanakawa Y, Detmar M, Hashimoto K. Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation. J Biol Chem. 2003;278:40026–40031. doi: 10.1074/jbc.M301866200. [DOI] [PubMed] [Google Scholar]
- 49.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 50.Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation. 2004;110:1499–1506. doi: 10.1161/01.CIR.0000141576.39579.23. [DOI] [PubMed] [Google Scholar]
- 51.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.