Abstract
Purpose of review
The accumulation of macrophages in the vascular wall is a hallmark of atherosclerosis. The biological properties of atherosclerotic plaque macrophages determine lesion size, composition and stability. In atherosclerotic plaques, macrophages encounter a microenvironment that is comprised of a variety of lipid oxidation products, each of which has diverse biological effects. In this review, we summarize recent advances in our understanding of the effects of plaque lipids on macrophage phenotypic polarization.
Recent findings
Atherosclerotic lesions in mice and in humans contain various macrophage phenotypes, which play different roles in mediating inflammation, the clearance of dead cells, and possibly resolution. Macrophages alter their phenotype and biological function in response to plaque lipids through the upregulation of specific sets of genes. Interaction of oxidized lipids with pattern recognition receptors and activation of the inflammasome by cholesterol crystals drive macrophages towards an inflammatory M1 phenotype. A new phenotype, Mox, develops when oxidized phospholipids activate stress response genes via Nrf2. Other lipid mediators such as nitrosylated-fatty acids and omega-3 fatty acid-derived products polarize plaque macrophages towards anti-inflammatory and proresolving phenotypes.
Summary
A deeper understanding of how lipids that accumulate in atherosclerotic plaques affect macrophage phenotype and function and thus atherosclerotic lesion development and stability will help to devise novel strategies for intervention.
Keywords: Macrophages, oxidized lipids, atherosclerosis, inflammation
Introduction
Distinguishable macrophage phenotypes, M1 and M2, have been observed in the course of inflammatory reactions [1]. Upon activation by IFNγ and LPS, M1 macrophages secrete inflammatory factors TNFα, IL1β, IL6, and IL12 that incite a Th1 immune response. M1 macrophages also produce reactive oxygen and nitrogen species and upregulate COX2 which leads to the production of eicosanoids. As inflammation proceeds, IL10, IL4, and IL13 alternatively activate macrophages towards an M2 phenotype with anti-inflammatory functionality, including the production of IL10, TGFβ1, and arginase 1 [1]. A macrophage phenotype isolated during the resolution phase of inflammation (Mres) has been characterized by elevated cAMP levels and shares attributes of both M1 and M2 [2]. The development of macrophage phenotypes is under transcriptional control. The NFκB pathway directs activation of M1 while PPARγ directs M2. The recently discovered macrophage phenotype, Mox, develops in response to oxidized phospholipids involving the redox-sensitive transcription factor NF-E2-related factor 2 (Nrf2) [3].
Macrophages play a key role in the development of atherosclerotic lesions [4]. The presence of distinguishable macrophage phenotypes in atherosclerotic plaques has been reviewed recently [5;6]. Subpopulations of pro-and anti-inflammatory macrophages were identified in human lesions [7;8] and in advanced atherosclerotic lesions of LDL-R null mice, where the relative abundance of macrophage phenotypes was found to be 40 % M1, 30% Mox, and 20% M2 [3]. However, mechanisms that control macrophage polarization in atherosclerosis are just beginning to be understood.
Priming for phenotypic polarization can occur in circulating monocytes: In mice, a monocyte subset characterized by expression of Ly6C and CCR2 was shown to be readily recruited to atherosclerotic lesions [9;10] where development into M1 macrophages ensued. Interestingly, C-reactive protein was shown to induce polarization of monocytes towards M1 [11]. Studies in humans have shown that treatment with ligands specific for PPARγ primes monocytes to become M2 macrophages in atherosclerotic lesions [8]. Notably, low-intensity exercise by sedentary individuals also primed circulating monocytes towards an M2 phenotype by a mechanism that involved PPARγ [12].
Macrophage phenotypic polarization also occurs in response to changes in the tissue microenvironment: A macrophage phenotype characterized by elevated expression of CD163, a scavenger receptor for hemoglobin-haptoglobin complexes, was shown to develop in response to intraplaque hemorrhage perhaps as part of a mechanism to protect against atherosclerotic lesion destabilization [13]. The importance for controlled heme-catabolism is further underlined by the finding that iron overload induced an unrestrained proinflammatory M1 phenotype [14].
In addition to interleukins, atherosclerotic plaques contain a plethora of bioactive lipid species that potentially regulate macrophage phenotypic polarization and function. Cholesterol has been observed to crystallize in the core of plaques and induce pro-inflammatory signaling pathways in this form. Sphingolipids can be released from cells or are found associated with HDL. Lysophospholipids and fatty acids can be released from LDL via phospholipases. Prostaglandins and leukotrienes [15] are formed from arachidonic acid and omega-3 fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are precursors to resolvins and protectins. Moreover, the microenvironment in atherosclerotic lesions is characterized by increased oxidative tissue damage and accumulation of oxidatively modified lipids. Lipoproteins and dying cells are main sources of oxidized lipids. Isoprostanes, which are formed by free radical-induced oxidation of arachidonic acid, are useful biomarkers and potentially modulate inflammation in atherosclerotic plaques [16]. Oxysterols can be present as a byproduct of cholesterol metabolism which can be quite dysfunctional in lipid laden foam cells [17]. Additionally, oxidized phospholipids can be presented on MM-LDL and on the membranes of apoptotic cells and shed as microvesicles and apoptotic blebs.
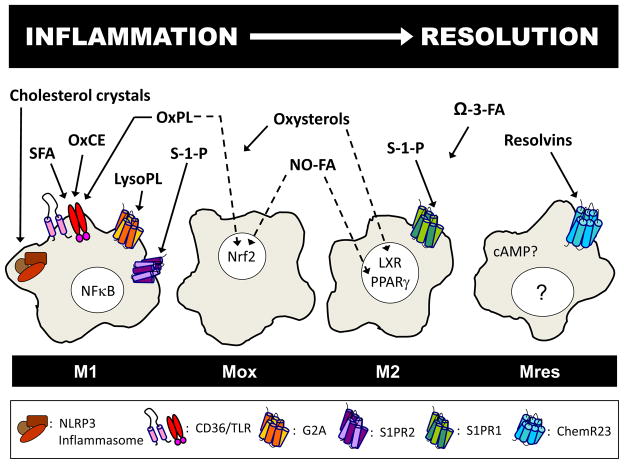
This review will focus on how bioactive lipids in the plaque microenvironment affect pro-and anti-inflammatory signaling to determine phenotypic polarization and consequently biological function of macrophages in atherosclerosis (Figure 1).
Figure 1.
Modulation of macrophage phenotype and function occurs during the progression and resolution of inflammation. In atherosclerosis, phenotypic polarization of macrophages is affected by bioactive plaque lipids. In a simple model, saturated fatty acids (SFA), oxidized cholesteryl esters (OxCE), and oxidized phospholipids (OxPL) induce inflammatory gene expression via scavenger receptor CD36, toll-like receptors (TLR), and NFκB activation, as seen in M1 macrophages. Crystallized cholesterol activates the NLRP3 inflammasome to sustain inflammation, and lysophospholipids (LysoPL) act on the G-protein coupled receptor G2A. Some macrophages are transformed by oxidized lipid signaling via Nrf2 into Mox macrophages. Other bioactive lipids such as nitrosylated fatty acids (NO-FA), sphingosine-1-phosphate (S1P), and oxysterols activate PPARγ, sphingosine-1-phosphate receptors, and liver X receptors (LXR), respectively, to modulate towards an anti-inflammatory M2 phenotype. However, oxysterols can also induce an inflammatory response, as can activation of S1P receptor 2. Omega-3 fatty acids and resolvins have anti-inflammatory or proresolving effects. Increase in cAMP or activation of the G protein-coupled receptor, ChemR23, may drive macrophages toward a resolving phenotype, Mres.
Macrophage phenotypic polarization in response to pro-inflammatory lipid mediators
Oxidatively modified lipids and lipoproteins resemble “danger signals” that are recognized by pattern recognition receptors such as TLR2 and TLR4. Moreover, CD36, a scavenger receptor that binds oxidized LDL, has been shown to act in combination with certain TLR heterodimers, mediating pro-inflammatory effects of oxidized lipoproteins and lipids [18;19]. Accumulating evidence supports a role for toll-like receptors (TLRs) in the pathogenesis of atherosclerosis, and TLR activation in macrophages by lipids induces NFκB-, MAP kinase- and ROS-dependent inflammatory gene expression, producing an M1-like phenotype. In addition, activation of the inflammasome and other stress-response mechanisms result in a pro-inflammatory phenotype in plaque macrophages.
Oxidized lipoproteins
In atherosclerosis, peripheral blood monocytes are recruited to the vessel wall where they differentiate into macrophages and develop into foam cells through the ingestion of lipids [4]. Accumulation of oxidized LDL (oxLDL) in M2 macrophages drives these cells toward a pro-inflammatory state accompanied by downregulation of the anti-inflammatory transcription factor KLF2 [20]. Minimally modified LDL (MM-LDL), was recently shown to activate macrophages via TLR4, inducing recruitment and activation of spleen tyrosine kinase (Syk) and protein kinase C, and to intracellular generation of ROS by NADPH oxidase 2 (gp91phox/Nox2) [21]. Nox2 was required for the expression of inflammatory cytokines including IL-1β and RANTES/CCL5. MM-LDL was shown to activate TLR signaling by inducing a redistribution of CD14, TLR4, and TLR2 in the macrophage membrane [22] and MM-LDL cooperatively activated TLR4 with low-dose LPS [23]. Further evidence for an involvement of TLR4 in mediating effects of modified lipoproteins comes from a study where lipoproteins isolated from zebrafish larvae that had been fed a high fat diet, induced MAP kinase signaling and cell spreading in wild type, but not in TLR4-deficient murine macrophages [24]. Interestingly, phenotypic polarization of macrophages in fish largely resembles that observed in mammalian systems [25].
Oxidized phospholipids
Oxidized phospholipids induce inflammatory gene expression in macrophages and several studies have implied oxidized phospholipids as activators as well as inhibitors of TLR signaling [26–29]. The exact mechanisms of how oxidized phospholipids activate TLRs are not known, but dependent on the experimental system, different combinations of pattern recognition receptor multimers as well as accessory receptors such as CD14 or LBP may be required [30;31].
Moreover, oxidized phospholipids induce the development of the Mox phenotype via activation of Nrf2-dependent gene expression [3]. The unique expression pattern of antioxidative and detoxifying genes in Mox macrophages sharply distinguishes this phenotype from conventional M1 and M2 macrophages and implies specific functions in atherosclerotic lesions. For instance, Mox macrophages demonstrate decreased phagocytotic capacity and reduced migratory properties [3]. In any case, it remains to be shown whether the Mox phenotype plays a beneficial or detrimental role in the development of atherosclerotic lesions.
It was shown that Nrf2-dependent anti-oxidant signaling counteracts LPS-induced inflammation in foam cells [32] via mechanisms that included upregulation of heme-oxygenase-1 (HO-1), a marker for Mox macrophages [3]. However, recent findings point to a role for Nrf2 in mediating inflammatory responses in addition to anti-inflammatory processes. In support of this concept, it was shown that Nrf2 deficiency was protective in models of experimental atherosclerosis [33–35]. A possible mechanism underlying these seemingly paradoxical findings may involve a role for Nrf2 in activation of the inflammasome [33]. In fact, the proatherogenic activity of Nrf2 may be caused by cholesterol crystals which have been shown to activate the NLRP3 inflammasome in a Nrf2-dependent manner [33].
Cholesterol crystals
Crystallization of cholesterol was thought to be a late event in atherosclerosis and thus would not contribute to the initiating inflammatory events. However, a recent study showed that minute cholesterol crystals can be detected in early lesions after only 2 weeks of HFD feeding in ApoE null mice as well as in human lesions [36]. Engulfed cholesterol crystals were shown to cause damage to the phagolysosome of macrophages, allowing leakage of mediators like cathepsin into the cytoplasm which can activate the NLRP3 inflammasome [36;37]. Inflammasome activation leads to activation of caspase-1 and release of IL-1 and IL-18 and LDL-R null mice transplanted with NLRP3-deficient bone marrow showed decreased atherosclerosis [36]. Together, these findings strongly support a role for inflammasome activation by cholesterol crystals in early atherogenesis.
Oxidized cholesteryl esters
In the core of LDL, cholesterol is found esterified with fatty acids. Cholesteryl esters containing unsaturated acyl chains can be oxidatively modified, leading to the formation of biologically active compounds that activate macrophages [38;39]. Cholesteryl linoleate induces inflammatory signals in macrophages in a MAP kinase pathway-dependent fashion [24;40]. Another cholesteryl ester, 7-ketocholesteryl-9-carboxynonanoate induces NFκB signaling in macrophages [41]. Recent studies have shown that oxidized cholesteryl esters can trigger inflammatory signaling and foam cell formation via pinocytosis by a process that involves TLR4 [21;42]. Clearly, cholesteryl esters are capable of inducing inflammatory signals; however, other cholesteryl esters such as 9-oxononanoyl-cholesterol are able to induce expression of anti-inflammatory mediators such as TGFβ [43].
Oxidized cholesteryl esters and oxidized phospholipids may play an important combined role in the regulation of foam cell formation. Cholesteryl ester hydroperoxides increase expression of the scavenger receptor CD36 by macrophages [44]. Certain oxidized phospholipids that are ligands for CD36 and also the scavenger receptor class B, type 1 (SR-B1) were shown to interfere with SR-B1-mediated selective uptake of cholesteryl esters [45]. A link between phenotypic polarization and foam cell formation was provided by a recent study that showed that activation of αMβ2 integrins prevented alternative macrophage activation and foam cell formation by inhibiting CD36 expression [46].
Oxysterols
Originally believed to be cytotoxic by-products of cholesterol metabolism, recent findings demonstrate that oxysterols, as ligands of liver X receptors (LXR), potently modulate lipid metabolism and inflammation (recently reviewed in [17]). Oxysterols may also have effects on macrophages that are independent of LXR. ORP9 is an oxysterol binding protein that is upregulated in oxLDL-stimulated peripheral blood monocytes [47]. Moreover, treatment of Thp-1 macrophages with 7-keto-cholesterol or a mixture of oxysterols induced expression of MCP-1 [48]. Additionally, oxysterols caused expression of the scavenger receptor CD36 via a GPCR/Src/PLC/PKCδ/ERK-dependent mechanism that resulted in PPARγ upregulation [49].
Lysophospholipids and fatty acids
The action of phospholipases on lipoproteins and membrane phospholipids results in release of lysophospholipids and free fatty acids. A recent study showed that modification of LDL by group V PLA2 results in products that activate macrophages to secrete TNFα and IL6 via NFκB activation in addition to enhancing lipid uptake and foam cell formation [50]. An acidic environment, as would be found in advanced atherosclerotic plaques, enhances the inflammatory activity of lysophosphatidylcholine (lysoPC) and free fatty acids by impairing the ability of albumin to sequester those products [51]. The G-protein coupled receptor G2A mediates the ability of lysoPC to induce signaling and chemotaxis in macrophages [52–54]. Moreover, G2A may be a receptor for oxidized fatty acids [55]. Recently, two splice variants of G2A were described, both of which recognized 9-HODE [56]. Interestingly, deficiency of G2A was shown to promote an inflammatory phenotype in macrophages, resembling an M1 macrophage. This was characterized by increased nuclear translocation of NFκB, and IL6 and IL12 expression, as well as reduced Arginase 1 [57]. Global and myeloid cell-specific G2A deficiency were shown to have profound effects on atherosclerosis development [57–59].
Pro-atherogenic effects have been attributed to saturated fatty acids. Palmitic acid promotes foam cell formation by macrophages by the upregulation of oxLDL receptor 1 (LOX1), possibly by inducing ER stress [60], an effect counteracted by unsaturated fatty acids. Several studies indicate that saturated free fatty acids may be ligands for TLRs 2 and 4, causing NFκB-dependent inflammatory gene expression and thereby contributing to atherosclerosis, inflamed adipose tissue, and insulin resistance [61–63]. Moreover, palmitate and stearate in the presence of high glucose increase expression of TLR2 and TLR4 on monocytes, causing ROS generation and increased NFκB activity [61]. Debate continues as to whether saturated fatty acids directly bind to and activate TLRs [64].
Macrophage phenotypic polarization in response to anti-inflammatory and proresolving lipid mediators
Several lipid mediators that are present in atherosclerotic plaques were shown to polarize macrophages towards anti-inflammatory and proresolving phenotypes. For instance, oxidized lipids were described to have numerous anti-inflammatory effects [65] and omega-3 fatty acid-derived products, resolvins and protectins, have been shown to play important roles in the resolution of inflammation [66].
Polyunsaturated fatty acids and nitro-fatty acids
Unlike saturated fatty acids, polyunsaturated fatty acids (PUFAs) can evoke anti-inflammatory mechanisms in macrophages. What is more, unsaturated fatty acids often counteract inflammatory pathways induced by saturated fatty acids; for example, in macrophages PUFAs suppress palmitic acid-induced increases in LOX1 and FABP expression [66;67]. Conjugated linoleic acid (CLA) has been shown to cause regression of preestablished murine atherosclerotic plaques, accompanied by a decrease in lesion macrophage accumulation and suppressed COX2, cPLA2, MCP1, and MMP9 expression in macrophages [68].
Another class of anti-inflammatory unsaturated fatty acid products are electrophilic nitro-fatty acids which are formed via nitric oxide or nitrite-dependent redox reactions [69]. Nitro-fatty acids have been shown to activate Nrf2 and PPARγ [70–72]. The anti-inflammatory activity of nitro-fatty acids has been shown in vitro as well as in a cardiac ischemia reperfusion model in vivo [69;73;74]. Recently, it was shown that nitro-fatty acids also exert anti-atherosclerotic effects: subcutaneous injection of nitro-oleic acid resulted in a significant reduction of lesion formation in apoE null mice without affecting serum lipid levels. Furthermore, atherosclerotic lesions of nitro-oleic-acid-treated mice showed increased collagen content and stability [75].
Omega-3 fatty acids and resolvins
Omega-3 fatty acids have long been known to have anti-atherogenic effects. A recent study of carotid endarterectomy patients who consumed omega-3-polyunsaturated fatty acid capsules for 21 days leading up to surgery had more stable plaques that were less inflamed with fewer T cells and fewer foam cells [76]. Macrophages in atherosclerotic plaques of obese/LDL-R−/− mice showed defective phagocytosis of apoptotic cells that was reversed by supplementation of omega-3 fatty acids EPA and DHA [77]. Moreover, action of COX-2 on omega-3-fatty acids was shown to produce potent anti-inflammatory products [78].
Macrophages themselves can produce a class of DHA-derived anti-inflammatory and proresolving products, the maresins (macrophage mediator in resolving inflammation) [79]. Lipoxin A4, aspirin-triggered lipoxin A4, resolvin E1/D1/D2, and maresin 1 all enhance phagocytosis of apoptotic neutrophils by macrophages, and they activate macrophages in a way that does not cause the induction of inflammatory cytokines [80]. Effects of these mediators in atherosclerotic plaques have been implied [81;82]. Peripheral artery disease (PAD) patients had significantly lower plasma levels of aspirin-triggered lipoxin than healthy volunteers. Further investigation showed that aspirin-triggered lipoxin and resolvin E1 likely effect the migration of vascular smooth muscle cells in PAD [81].
Sphingosine-1-phosphate
Sphingosine-1-phosphate (S1P) has been shown to induce an anti-inflammatory phenotype in macrophages via S1P receptor 1 [83]. Additionally, some anti-atherogenic effects of high-density lipoproteins are mediated by S1P via S1P receptor 1 (S1PR1) [84]. However, recent evidence suggests that other S1P receptors (S1PR2) may mediate proatherogenic effects. ApoE/S1PR2 null mice have smaller plaques, decreased macrophage density, and decreased proinflammatory cytokines. ApoE/S1P2 null macrophages show decreased activity of ROCK/NFκB, reduced cytokine expression, and decreased oxLDL uptake [85;86].
Open questions: Functional consequences of lipid-induced macrophage phenotypic polarization in plaques
With so many bioactive lipids present in atherosclerotic plaques, it is important to more clearly define their effects on plaque macrophages. Moreover, elucidating the time course of the appearance and action of different lipid mediators will help us better understand the progress of atherosclerosis as well as the mechanisms that are involved in resolution.
Beyond the development of different capacities to regulate inflammation, little is known about functional properties resulting from macrophage phenotypic polarization. What are the migratory properties and phagocytotic capacities of the different phenotypes? In the context of atherosclerosis, it is important to further elucidate the capabilities of different macrophage phenotypes to form foam cells. How might phenotypic polarization alter reverse cholesterol transport and lipid metabolism? Future studies will shed more light on these important properties of different macrophage phenotypes that are present in atherosclerotic lesions.
Conclusions/future outlook, therapeutic potential
The plaque environment contains many different lipid mediators that drive macrophage phenotypic polarizaion. Some lipids activate the inflammasome or act via pattern recognition receptors to induce inflammatory programs in macrophages or enhance the formation of foam cells. Other lipids incite stress responses in macrophages that enhance antioxidant and detoxifying actions. Yet other lipids such as S1P and oxysterols have both inflammatory and anti-inflammatory effects.
The relative abundance of various macrophage phenotypes may determine the outcome of an inflammatory reaction [5]. Chronic inflammation in atherosclerosis resembles an unresolved inflammatory response where M1 and Mox macrophages outnumber M2 macrophages [3;87]. Shifting the balance from pro-inflammatory towards anti-inflammatory, possibly “proresolving”, macrophages could be a therapeutic strategy to initiate resolution in atherosclerotic plaques and in chronically inflamed tissues in general. In support of this hypothesis, it was shown that during plaque regression, induced by increased HDL or decreased serum cholesterol levels, macrophages switch their phenotype into the anti-inflammatory M2 type [88;89].
Among compounds or treatment strategies that can either prime circulating monocytes or induce the transition of a pro-inflammatory macrophage into M2 macrophages are thiazolidinediones (TZDs) [8], adiponectin [90], FK 506 (tacrolimus) [91], the S1P analogue FTY 720 [92], cordycepin (a component of cordyceps militaris) and adenosine [93], inhibition of CD40-CD40 ligand signaling [94], as well as exercise [12]. Some studies suggest that LXR ligands, conjugated linoleic acid, and PUFAs could switch macrophages into an anti-inflammatory, possibly pro-resolving phenotype. Drugs commonly taken by patients suffering from cardiovascular disease include aspirin and statins which modify COX2 via acetylation or S-nitrosylation, respectively, and this process drives the generation of aspirin-triggered lipoxins, namely 15-epi-LXA4, which may establish and carry forward the resolution of inflammation [80;95].
Macrophage phenotypic polarization and its connection to plaque lipids is an important aspect of lesion development and progression. A better understanding of the complex microenvironment and the multiple biological processes in atherosclerotic lesions that control macrophage function will lead to novel treatment strategies.
Key points.
Macrophages in atherosclerotic lesions change their phenotype and function in response to plaque lipids.
The plaque microenvironment determines the relative abundance of pro-inflammatory M1, various forms of M2 and the newly identified Mox macrophage phenotypes.
Changing lesion macrophages into a pro-resolving phenotype may be a promising strategy to attain plaque regression.
Acknowledgments
This work was supported by NIH grant R01-HL-084422.
Reference List
- 1.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 2.Bystrom J, Evans I, Newson J, Stables M, Toor I, Van RN, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *3.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. This report established the existence of a novel macrophage phenotype that develops in response to oxidized phospholipids and documented that Mox accounts for about 30% of macrophages present in mouse lesions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *4.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. This review discusses macrophage function throughout the course of atherosclerosis including monocyte entry, inflammatory activation, cholesterol loading, fibrous cap thinning, and plaque necrosis. It includes an interesting discussion on the limitations of current mouse models of the disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Garlanda C, Locati M. Macrophage Diversity and Polarization in Atherosclerosis. A Question of Balance. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 6.Shimada K. Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ J. 2009;73:994–1001. doi: 10.1253/circj.cj-09-0277. [DOI] [PubMed] [Google Scholar]
- 7.Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, Van RN, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devaraj S, Jialal I. C-Reactive Protein Polarizes Human Macrophages to an M1 Phenotype and Inhibits Transformation to the M2 Phenotype. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.225508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yakeu G, Butcher L, Isa S, Webb R, Roberts AW, Thomas AW, Backx K, James PE, Morris K. Low-intensity exercise enhances expression of markers of alternative activation in circulating leukocytes: roles of PPARgamma and Th2 cytokines. Atherosclerosis. 2010;212:668–673. doi: 10.1016/j.atherosclerosis.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies SS, Roberts LJ. F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med. 2011;50:559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata N, Glass CK. Macrophages, oxysterols and atherosclerosis. Circ J. 2010;74:2045–2051. doi: 10.1253/circj.cj-10-0860. [DOI] [PubMed] [Google Scholar]
- 18.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, Khoury JE, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis. 2011;214:345–349. doi: 10.1016/j.atherosclerosis.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–8. 21p. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavez-Sanchez L, Madrid-Miller A, Chavez-Rueda K, Legorreta-Haquet MV, Tesoro-Cruz E, Blanco-Favela F. Activation of TLR2 and TLR4 by minimally modified low-density lipoprotein in human macrophages and monocytes triggers the inflammatory response. Hum Immunol. 2010;71:737–744. doi: 10.1016/j.humimm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Wiesner P, Choi SH, Almazan F, Benner C, Huang W, Diehl CJ, Gonen A, Butler S, Witztum JL, Glass CK, Miller YI. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, Pattison J, Deer E, Sayaphupha T, Dennis EA, Witztum JL, Tsimikas S, Miller YI. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high cholesterol diet: macrophage binding and activation. J Biol Chem. 2010;285:32343–32351. doi: 10.1074/jbc.M110.137257. By utilizing an interesting model of high fat feeding of zebrafish, this paper shows that oxidized cholesteryl esters induce an inflammatory macrophage phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forlenza M, Fink IR, Raes G, Wiegertjes GF. Heterogeneity of macrophage activation in fish. Dev Comp Immunol. 2011 doi: 10.1016/j.dci.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Walton KA, Hsieh X, Gharavi N, Wang S, Wang G, Yeh M, Cole AL, Berliner JA. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J Biol Chem. 2003;278:29661–29666. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 27.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van LG, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, Gutierrez MA, Navab M, Reddy ST, Witztum JL, Fogelman AM, Rea TH, Eisenberg D, Berliner J, Modlin RL. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008;118:2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oskolkova OV, Afonyushkin T, Preinerstorfer B, Bicker W, von SE, Hainzl E, Demyanets S, Schabbauer G, Lindner W, Tselepis AD, Wojta J, Binder BR, Bochkov VN. Oxidized phospholipids are more potent antagonists of lipopolysaccharide than inducers of inflammation. J Immunol. 2010;185:7706–7712. doi: 10.4049/jimmunol.0903594. [DOI] [PubMed] [Google Scholar]
- 30.Erridge C, Webb DJ, Spickett CM. Toll-like receptor 4 signalling is neither sufficient nor required for oxidised phospholipid mediated induction of interleukin-8 expression. Atherosclerosis. 2007;193:77–85. doi: 10.1016/j.atherosclerosis.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 31.vonSchlieffen E, Oskolkova OV, Schabbauer G, Gruber F, Bluml S, Genest M, Kadl A, Marsik C, Knapp S, Chow J, Leitinger N, Binder BR, Bochkov VN. Multi-hit inhibition of circulating and cell-associated components of the toll-like receptor 4 pathway by oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2009;29:356–362. doi: 10.1161/ATVBAHA.108.173799. [DOI] [PubMed] [Google Scholar]
- 32.Kuhn AM, Tzieply N, Schmidt MV, von KA, Namgaladze D, Yamamoto M, Brune B. Antioxidant signaling via Nrf2 counteracts lipopolysaccharide-mediated inflammatory responses in foam cell macrophages. Free Radic Biol Med. 2011;50:1382–1391. doi: 10.1016/j.freeradbiomed.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 33.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011 doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 34.Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS ONE. 2008;3:e3791. doi: 10.1371/journal.pone.0003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, Wang X, Castellani LW, Reue K, Lusis AJ, Araujo JA. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. This paper provides evidence that not only are cholesterol crystals present in early atherosclerotic lesions, but they play an important role in inflammation by activating the inflammasome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leitinger N. Cholesteryl ester oxidation products in atherosclerosis. Mol Aspects Med. 2003;24:239–250. doi: 10.1016/s0098-2997(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 39.Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized low density lipoprotein. J Biol Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber J, Boechzelt H, Karten B, Surboeck M, Bochkov VN, Binder BR, Sattler W, Leitinger N. Oxidized cholesteryl linoleates stimulate endothelial cells to bind monocytes via the extracellular signal-regulated kinase 1/2 pathway. Arterioscler Thromb Vasc Biol. 2002;22:581–586. doi: 10.1161/01.atv.0000012782.59850.41. [DOI] [PubMed] [Google Scholar]
- 41.Huang Z, Li W, Wang R, Zhang F, Chi Y, Wang D, Liu Z, Zhang Y, Matsuura E, Liu Q. 7-ketocholesteryl-9-carboxynonanoate induced nuclear factor-kappa B activation in J774A.1 macrophages. Life Sci. 2010;87:651–657. doi: 10.1016/j.lfs.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 42.Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sottero B, Gamba P, Longhi M, Robbesyn F, Abuja PM, Schaur RJ, Poli G, Leonarduzzi G. Expression and synthesis of TGFbeta1 is induced in macrophages by 9-oxononanoyl cholesterol, a major cholesteryl ester oxidation product. Biofactors. 2005;24:209–216. doi: 10.1002/biof.5520240125. [DOI] [PubMed] [Google Scholar]
- 44.Jedidi I, Couturier M, Therond P, Gardes-Albert M, Legrand A, Barouki R, Bonnefont-Rousselot D, Aggerbeck M. Cholesteryl ester hydroperoxides increase macrophage CD36 gene expression via PPARalpha. Biochem Biophys Res Commun. 2006;351:733–738. doi: 10.1016/j.bbrc.2006.10.122. [DOI] [PubMed] [Google Scholar]
- 45.Kar NS, Ashraf MZ, Valiyaveettil M, Podrez EA. Mapping and characterization of the binding site for specific oxidized phospholipids and oxidized low density lipoprotein of scavenger receptor CD36. J Biol Chem. 2008;283:8765–8771. doi: 10.1074/jbc.M709195200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yakubenko VP, Bhattacharjee A, Pluskota E, Cathcart MK. alphaMbeta integrin activation prevents alternative activation of human and murine macrophages and impedes foam cell formation. Circ Res. 2011;108:544–554. doi: 10.1161/CIRCRESAHA.110.231803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen T, Huang Z, Wang L, Wang Y, Wu F, Meng S, Wang C. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res. 2009;83:131–139. doi: 10.1093/cvr/cvp121. [DOI] [PubMed] [Google Scholar]
- 48.Leonarduzzi G, Gamba P, Sottero B, Kadl A, Robbesyn F, Calogero RA, Biasi F, Chiarpotto E, Leitinger N, Sevanian A, Poli G. Oxysterol-induced up-regulation of MCP-1 expression and synthesis in macrophage cells. Free Radic Biol Med. 2005;39:1152–1161. doi: 10.1016/j.freeradbiomed.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 49.Leonarduzzi G, Gargiulo S, Gamba P, Perrelli MG, Castellano I, Sapino A, Sottero B, Poli G. Molecular signaling operated by a diet-compatible mixture of oxysterols in up-regulating CD36 receptor in CD68 positive cells. Mol Nutr Food Res. 2010;54 (Suppl 1):S31–S41. doi: 10.1002/mnfr.200900493. [DOI] [PubMed] [Google Scholar]
- 50.Boyanovsky BB, Li X, Shridas P, Sunkara M, Morris AJ, Webb NR. Bioactive products generated by group V sPLA(2) hydrolysis of LDL activate macrophages to secrete pro-inflammatory cytokines. Cytokine. 2010;50:50–57. doi: 10.1016/j.cyto.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lahdesmaki K, Plihtari R, Soininen P, Hurt-Camejo E, la-Korpela M, Oorni K, Kovanen PT. Phospholipase A(2)-modified LDL particles retain the generated hydrolytic products and are more atherogenic at acidic pH. Atherosclerosis. 2009;207:352–359. doi: 10.1016/j.atherosclerosis.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 52.Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Radu CG, Yang LV, Bentolila LA, Riedinger M, Witte ON. Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol Biol Cell. 2005;16:2234–2247. doi: 10.1091/mbc.E04-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang LV, Radu CG, Wang L, Riedinger M, Witte ON. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood. 2005;105:1127–1134. doi: 10.1182/blood-2004-05-1916. [DOI] [PubMed] [Google Scholar]
- 55.Obinata H, Hattori T, Nakane S, Tatei K, Izumi T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem. 2005;280:40676–40683. doi: 10.1074/jbc.M507787200. [DOI] [PubMed] [Google Scholar]
- 56.Ogawa A, Obinata H, Hattori T, Kishi M, Tatei K, Ishikawa O, Izumi T. Identification and analysis of two splice variants of human G2A generated by alternative splicing. J Pharmacol Exp Ther. 2010;332:469–478. doi: 10.1124/jpet.109.158758. [DOI] [PubMed] [Google Scholar]
- 57.Bolick DT, Skaflen MD, Johnson LE, Kwon SC, Howatt D, Daugherty A, Ravichandran KS, Hedrick CC. G2A Deficiency in Mice Promotes Macrophage Activation and Atherosclerosis. Circ Res. 2009;104:318–327. doi: 10.1161/CIRCRESAHA.108.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parks BW, Srivastava R, Yu S, Kabarowski JH. ApoE-dependent modulation of HDL and atherosclerosis by G2A in LDL receptor-deficient mice independent of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2009;29:539–547. doi: 10.1161/ATVBAHA.108.179937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parks BW, Lusis AJ, Kabarowski JH. Loss of the lysophosphatidylcholine effector, G2A, ameliorates aortic atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2703–2709. doi: 10.1161/01.ATV.0000246774.02426.71. [DOI] [PubMed] [Google Scholar]
- 60.Ishiyama J, Taguchi R, Yamamoto A, Murakami K. Palmitic acid enhances lectin-like oxidized LDL receptor (LOX-1) expression and promotes uptake of oxidized LDL in macrophage cells. Atherosclerosis. 2010;209:118–124. doi: 10.1016/j.atherosclerosis.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab. 2011;300:E145–E154. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, Stienstra R, van d V, Stalenhoef AF, Giamarellos-Bourboulis EJ, Kanneganti TD, Van der Meer JW. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1beta production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 2010;62:3237–3248. doi: 10.1002/art.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 65.Bochkov VN, Leitinger N. Anti-inflammatory properties of lipid oxidation products. J Mol Med. 2003;81:613–626. doi: 10.1007/s00109-003-0467-2. [DOI] [PubMed] [Google Scholar]
- 66.Ishiyama J, Taguchi R, Akasaka Y, Shibata S, Ito M, Nagasawa M, Murakami K. Unsaturated FAs prevent palmitate-induced LOX-1 induction via inhibition of ER stress in macrophages. J Lipid Res. 2011;52:299–307. doi: 10.1194/jlr.M007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coleman SL, Park YK, Lee JY. Unsaturated fatty acids repress the expression of adipocyte fatty acid binding protein via the modulation of histone deacetylation in RAW 264.7 macrophages. Eur J Nutr. 2010 doi: 10.1007/s00394-010-0140-9. [DOI] [PubMed] [Google Scholar]
- 68.McClelland S, Cox C, O’Connor R, de GM, McCarthy C, Cryan L, Fitzgerald D, Belton O. Conjugated linoleic acid suppresses the migratory and inflammatory phenotype of the monocyte/macrophage cell. Atherosclerosis. 2010;211:96–102. doi: 10.1016/j.atherosclerosis.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Khoo NK, Freeman BA. Electrophilic nitro-fatty acids: anti-inflammatory mediators in the vascular compartment. Curr Opin Pharmacol. 2010;10:179–184. doi: 10.1016/j.coph.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kansanen E, Bonacci G, Schopfer FJ, Kuosmanen SM, Tong KI, Leinonen H, Woodcock SR, Yamamoto M, Carlberg C, Yla-Herttuala S, Freeman BA, Levonen AL. Electrophilic Nitro-fatty Acids Activate NRF2 by a KEAP1 Cysteine 151-independent Mechanism. J Biol Chem. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsujita T, Li L, Nakajima H, Iwamoto N, Nakajima-Takagi Y, Ohashi K, Kawakami K, Kumagai Y, Freeman BA, Yamamoto M, Kobayashi M. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: a study using green fluorescent protein transgenic zebrafish. Genes Cells. 2011;16:46–57. doi: 10.1111/j.1365-2443.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PR, Xu HE, Batthyany CI, Chen YE, Hallis TM, Freeman BA. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. J Biol Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borniquel S, Jansson EA, Cole MP, Freeman BA, Lundberg JO. Nitrated oleic acid up-regulates PPARgamma and attenuates experimental inflammatory bowel disease. Free Radic Biol Med. 2010;48:499–505. doi: 10.1016/j.freeradbiomed.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PR, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res. 2010;85:155–166. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *75.Rudolph TK, Rudolph V, Edreira MM, Cole MP, Bonacci G, Schopfer FJ, Woodcock SR, Franek A, Pekarova M, Khoo NK, Hasty AH, Baldus S, Freeman BA. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:938–945. doi: 10.1161/ATVBAHA.109.201582. This paper highlights the anti-atherogenic effects of recently discovered nitrosylated-fatty acids in a mouse model of atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cawood AL, Ding R, Napper FL, Young RH, Williams JA, Ward MJ, Gudmundsen O, Vige R, Payne SP, Ye S, Shearman CP, Gallagher PJ, Grimble RF, Calder PC. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212:252–259. doi: 10.1016/j.atherosclerosis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 77.Li S, Sun Y, Liang CP, Thorp EB, Han S, Jehle AW, Saraswathi V, Pridgen B, Kanter JE, Li R, Welch CL, Hasty AH, Bornfeldt KE, Breslow JL, Tabas I, Tall AR. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105:1072–1082. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, Rudolph V, Freeman BA, Schopfer FJ. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sato K, Okajima F. Role of sphingosine 1-phosphate in anti-atherogenic actions of high-density lipoprotein. World J Biol Chem. 2010;1:327–337. doi: 10.4331/wjbc.v1.i11.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang F, Okamoto Y, Inoki I, Yoshioka K, Du W, Qi X, Takuwa N, Gonda K, Yamamoto Y, Ohkawa R, Nishiuchi T, Sugimoto N, Yatomi Y, Mitsumori K, Asano M, Kinoshita M, Takuwa Y. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J Clin Invest. 2010;120:3979–3995. doi: 10.1172/JCI42315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, Hla T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:81–85. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS ONE. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci USA. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol. 2010;299:H656–H663. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bai L, Gabriels K, Wijnands E, Rousch M, Daemen MJ, Tervaert JW, Biessen EA, Heeneman S. Low- but not high-dose FK506 treatment confers atheroprotection due to alternative macrophage activation and unaffected cholesterol levels. Thromb Haemost. 2010;104:143–150. doi: 10.1160/TH09-07-0502. [DOI] [PubMed] [Google Scholar]
- 92.Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van BT, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 93.Shin S, Moon S, Park Y, Kwon J, Lee S, Lee CK, Cho K, Ha NJ, Kim K. Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines. Immune Netw. 2009;9:255–264. doi: 10.4110/in.2009.9.6.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lutgens E, Lievens D, Beckers L, Wijnands E, Soehnlein O, Zernecke A, Seijkens T, Engel D, Cleutjens J, Keller AM, Naik SH, Boon L, Oufella HA, Mallat Z, Ahonen CL, Noelle RJ, de Winther MP, Daemen MJ, Biessen EA, Weber C. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010;207:391–404. doi: 10.1084/jem.20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, Martinez JD, Huang MH, Uretsky BF, Perez-Polo JR. Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–935. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]