Abstract
Innate lymphoid cells (ILCs) are a group of lymphocytes that promote rapid cytokine-dependent innate immunity, inflammation and tissue repair. In addition, a growing body of evidence suggests ILCs can influence adaptive immune cell responses. During fetal development a subset of ILCs orchestrate the generation and maturation of secondary lymphoid tissues. Following birth, ILCs continue to modulate adaptive immune cell responses indirectly through interactions with stromal cells in lymphoid tissues and epithelial cells at barrier surfaces. In this review we summarize the current understanding of how ILCs modulate the magnitude and quality of adaptive immune cell responses, and in particular focus on recent evidence suggesting that ILCs can also directly regulate CD4+ T cells. Further, we discuss the implications that these pathways may have on human health and disease.
The innate lymphoid cell family
Recent evidence has implicated innate lymphoid cells (ILCs) as critical regulators of innate immunity and inflammation at mammalian barrier surfaces, including the skin, airways and gastrointestinal tract [1-4]. Although ILCs lack antigen-specific receptor rearrangement they exhibit strikingly similar transcription factor profiles and cytokine-producing capabilities as CD4+ T cells, suggesting that ILCs may act as an innate counterpart to the CD4+ T helper (Th) cell arm of the adaptive immune system. In line with this, both ILCs and T cells develop from common lymphoid progenitors in a process dependent upon the transcriptional regulator T cell factor-1 (TCF-1) and the common γ-chain cytokine receptor [5-9]. Further mirroring CD4+ T cells, mature ILCs can be grouped based on expression of lineage-specifying transcription factors and a defined profile of effector cytokines [1,3,4]. Group 1 ILCs parallel Th1 cells in their expression of the transcription factor T-bet, production of IFN-γ in response to interleukin (IL)-12, and ability to mediate immunity to intracellular pathogens and tumors [3,10,11]. Group 2 ILCs parallel Th2 cells in their expression of the transcription factor GATA-3, production of the cytokines IL-5, IL-9 and IL-13 in response to IL-25, IL-33 and thymic stromal lymphopoietin (TSLP), and ability to mediate allergic inflammation and immunity to helminth infection [9,12-14]. Finally, group 3 ILCs parallel Th17 cells in their expression of retinoic acid-related orphan receptor gamma (RORγt), production of IL-17A and IL-22 in response to IL-23 and IL-1β, and ability to maintain intestinal epithelial barrier function, drive tissue inflammation and mediate immunity to extracellular bacteria [15-21] (reviewed in [1,2,22]). Given the ability of ILCs to respond rapidly to stimulation, it has been hypothesized that ILCs represent a critical early source of cytokines prior to the initiation of an adaptive immune response. For example, RORγt+ group 3 ILC-derived IL-22 is required for innate immunity to the enteric pathogen Citrobacter rodentium [17,18,21], prior to the generation of a robust IL-22+ CD4+ T cell response, which may be required for late-stage resolution of infection [23]. In addition, emerging evidence suggests group 3 ILCs may also play a significant role in modulating the adaptive immune system by promoting the generation, organization and maintenance of secondary lymphoid tissues, maintaining intestinal barrier function, and via direct interactions with adaptive immune cell populations. In this review, we will summarize the current understanding of how group 3 ILCs regulate adaptive immune cell populations through direct and indirect mechanisms, and discuss the implications of these findings for human health and disease.
Regulation of secondary lymphoid tissues by lymphoid tissue inducer cells
Group 3 ILCs encompass a heterogeneous family of RORγt-expressing innate lymphocytes that produce IL-22 and/or IL-17A [16,17,24,25]. One subset of group 3 ILCs, termed lymphoid tissue inducer (LTi) cells, were first described by Mebius and colleagues as CD4+ CD3− hematopoietic cells that collect at sites of lymphoid tissue development prior to birth and were proposed to act as initiators of lymphoid organogenesis [26]. Subsequent studies confirmed that LTi cells were required for the formation of secondary lymphoid tissues during fetal development including peripheral lymph nodes and Peyer's patches in the small intestine [27]. These tissues provide an organized environment for antigen presentation of both foreign and self antigens to adaptive immune cells, permitting the generation of protective immune responses to pathogens, orchestration of affinity maturation and the induction of peripheral tolerance [28].
Central to their ability to orchestrate the development, maturation and maintenance of secondary lymphoid tissues, LTi cells express multiple members of the tumor necrosis factor (TNF)-family of proteins, including the lymphotoxin-α and -β subunits and RANKL (TRANCE) which confer the unique lymphoid tissue inducing function of this ILC subset [29-32]. In particular, expression of the surface-bound lymphotoxin heterotrimer (LTα1β2) on LTi cells allows for interactions with lymphotoxin-β receptor (LTβR) on stromal organizer cells and induces stromal cell expression of chemokines and adhesion markers, including CXC-chemokine ligand 13, CC-chemokine ligand 19 (CCL19), CCL21, MAdCAM-1 and VCAM-1. These stromal cell responses act to recruit innate and adaptive immune cells expressing the cognate receptors (CXCR5, CCR7) and integrins (α4β7) to the site of the primitive lymph node [28]. The chemokine gradients induced by LTi cells during secondary lymphoid organogenesis further act to segregate the incoming lymphocytes, allowing for formation of discrete B- and T- cell zones, required for optimal humoral immune responses [33]. Indeed, transfer of LTα1β2-expressing LTi cells to LTα−/− mice is sufficient to induce segregation of B- and T- cell zones [33], confirming that LTi cells are an essential source of lymphotoxin and act to orchestrate lymph node architecture. Thus, prior to birth, LTi cells promote the generation of defined lymphoid environments that are necessary for optimal adaptive immune cell responses throughout life.
Indirect regulation of adaptive immune cell responses by group 3 ILCs
In addition to their role during fetal development, group 3 ILCs play equally critical roles in the maturation and maintenance of secondary lymphoid tissues during adult life (Figure 1). For example, LTα1β2-expressing group 3 ILCs in the intestine of adult mice indirectly regulate the function of the adaptive immune system via interactions with VCAM-1 and IL-7 expressing stromal cells, resulting in the formation and retention of discrete group 3 ILC clusters known as cryptopatches and subsequent chemokine-dependent recruitment of innate and adaptive immune cell populations to form mature intestinal lymphoid structures known as isolated lymphoid follicles (ILFs) [34,35]. Interestingly, this process is dependent upon the presence of commensal bacteria in the intestine, suggesting that bacteria-derived signals promote ILC-mediated formation and maturation of secondary lymphoid structures in the adult intestine [34]. Furthermore, the maturation of ILFs is required for optimal production of IgA by intestinal B cells [36], which is important for mucosal host defense through regulation of commensal bacteria populations [37-39]. One recent study demonstrated that RORγt+ ILCs act to orchestrate the adaptive immune system through two distinct pathways in order to promote production of intestinal IgA (Figure 1). First, expression of the membrane-bound lymphotoxin heterotrimer (LTα1β2) by RORγt+ ILCs was required for T-cell independent IgA production via modulation of dendritic cell function. Second, RORγt+ ILC secretion of soluble lymphotoxin-α trimers (sLTα3) was found to be required for T cell-dependent IgA production by lamina propria B cells through the regulation of T cell homing to the intestine [40]. Further, LTα1β2-expressing group 3 ILCs also promote the restoration of secondary lymphoid tissue architecture following the disruption of immune cell homeostasis during viral or pathogenic bacterial infections [41,42]. Collectively, these studies suggest that group 3 ILCs drive the maturation and maintenance of secondary lymphoid organs during adult life, and that these pathways are critical to orchestrate adaptive immune cell responses to both pathogens and commensal microorganisms.
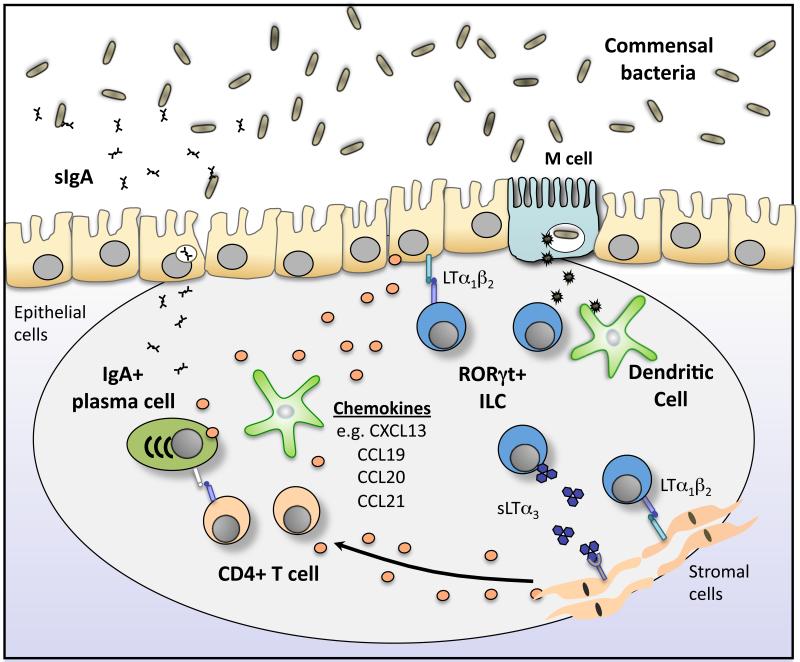
Figure 1. RORγt+ ILCs orchestrate the formation of gut associated lymphoid tissue and the induction of IgA.
RORγt+ ILCs orchestrate the formation of secondary lymphoid tissues in the intestine in response to commensal bacteria-derived signals. Lymphotoxin-mediated interactions with epithelial cells and stromal cells induce the production of chemokines, including CCL19, CCL20 CCL21 and CXCL13, which attract DCs, B cells and T cells to the intestine to form Peyer's patches and isolated lymphoid follicles (ILFs). This process is critical for the induction of IgA production by resident B cells. In addition M cells present in these lymphoid tissues sample antigen from the lumen and deliver to antigen-presenting cells in the underlying lymphoid tissue, possibly including MHCII+ ILCs.
Group 3 ILCs may also modulate host adaptive immune cell responses towards commensal bacteria via cytokine-mediated regulation of intestinal barrier function (Figure 2). For example, depletion of ILCs or neutralization of IL-22 in immunodeficient Rag1−/− mice results in impaired anatomical containment of specific commensal bacteria species resident in lymphoid-tissues, and the onset of pro-inflammatory adaptive immune cell responses directed against commensal bacteria that disseminated to systemic tissues [19]. In addition, it has recently been shown that loss of RORγt+ ILC-intrinsic aryl hydrocarbon receptor (Ahr) expression results in the ablation of IL-22 production by ILCs and an outgrowth of segmented filamentous bacteria (SFB), a strain of commensal bacteria attached to the epithelial surface of the small intestine that has previously shown to induce robust Th17 cell responses [43-45]. Consistent with this, the increased abundance of SFB in the intestinal tract resulted in elevated frequencies of pro-inflammatory Th17 cells and the onset of spontaneous colitis [45]. Thus, in line with previous studies demonstrating that ILC-derived IL-22 is a crucial innate regulator of intestinal barrier integrity and bacterial containment [17-19,21], IL-22-producing ILCs also appear to indirectly regulate CD4+ T cell responses and intestinal homeostasis by modulating the composition and anatomical containment of commensal bacteria.
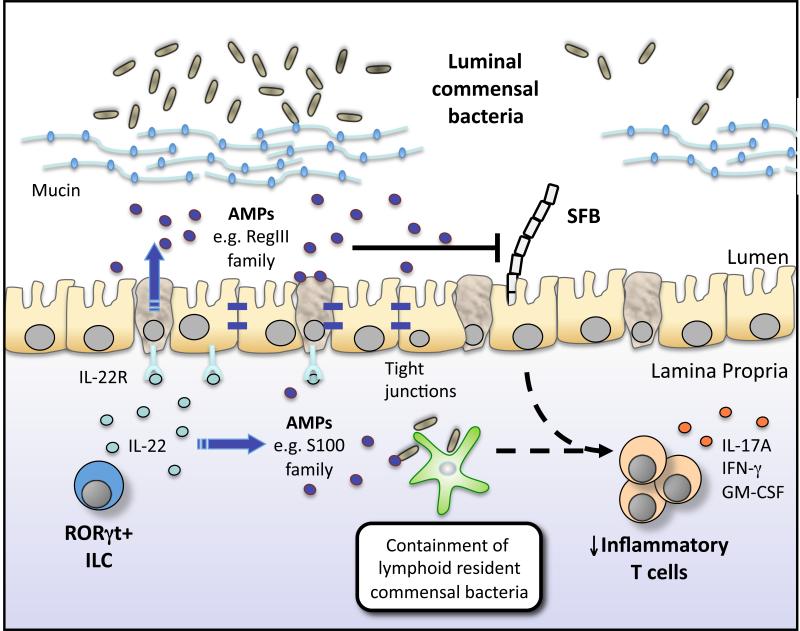
Figure 2. RORγt+ ILCs regulate intestinal adaptive immune responses via IL-22.
RORγt+ ILCs regulate epithelial barrier function and immune homeostasis in the intestine via the production of interleukin (IL)-22. ILC-derived IL-22 acts on epithelial cells and secretory cells to regulate epithelial barrier integrity and induce the production of antimicrobial peptides (AMPs) such as RegIIIγ and S100 family proteins, as well as mucins, which spatially segregate commensal bacteria from the epithelial barrier. ILC-derived IL-22-dependent pathways further regulate the growth of specific commensal bacteria species that are intimately associated with the host, such as segmented filamentous bacteria (SFB) and Alcaligenes/Achromobacter spp. Collectively, these responses limit the development of pathologic CD4+ T helper cell responses and intestinal inflammation.
Direct regulation of adaptive immune cell responses by group 3 ILCs
In addition to their roles in indirectly regulating the adaptive immune system, emerging evidence suggests that RORγt+ ILCs may also act to directly regulate the adaptive immune system through multiple pathways (Figure 3). For example, human IL-22-producing ILCs have been shown to co-produce B-cell activating factor (BAFF) [46], a positive regulator of B cell function, suggesting ILCs located in lymphoid tissue may directly promote B cell responses. Furthermore, a series of studies by Lane and colleagues demonstrated that RORγt+ ILCs express CXCR5 and CCR7 and respond to cognate chemokines produced by stromal cells by forming distinct clusters at intra-follicular regions of secondary lymphoid tissues [47,48], which was hypothesized to facilitate their ability to regulate adaptive immune cell responses at these sites. Indeed adult group 3 ILCs expressing the TNF-family members OX40L and CD30L were found to be present in these ILC clusters and acted to maintain memory CD4+ T cells following Listeria monocytogenes infection or protein immunization, via direct interactions with the corresponding receptors OX40 and CD30 on memory CD4+ T cells [48-51]. Consistent with a failure to maintain memory CD4+ T cell responses, mice lacking both CD30L and OX40L are unable to sustain germinal center formation or long-lived antibody responses, and exhibit a loss of memory CD4+ T cells in the intestinal tract [48,50,52].
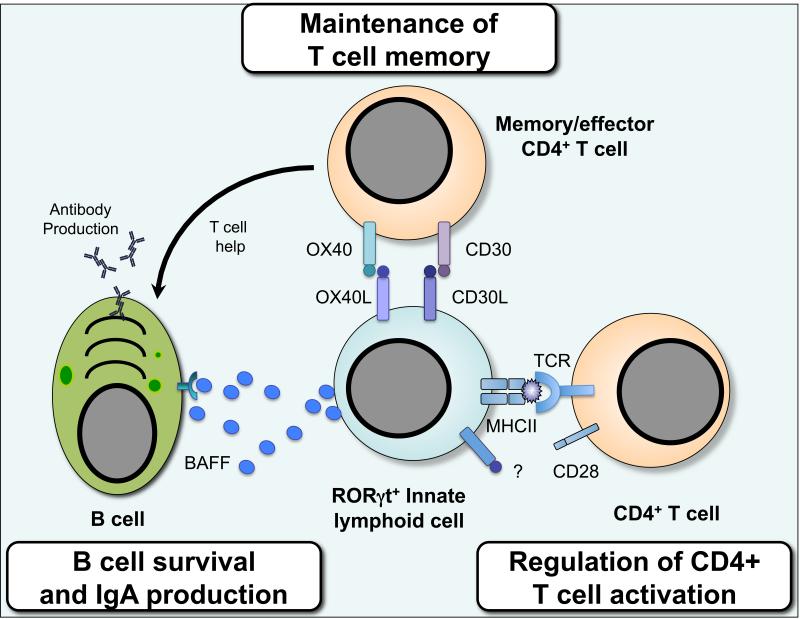
Figure 3. Direct regulation of adaptive immunity by RORγt+ ILCs.
Group 3 ILCs expressing the transcription factor RORγt directly interact with CD4+ T cells through receptor mediated cell-cell contact. ILCs regulate the magnitude and quality of the CD4+ T cell response via presentation of antigen in the context of MHC class II. In the steady state ILCs lack co-stimulatory molecule expression and appear to limit CD4+ T cell responses, however it remains to be tested whether ILCs can promote T cell proliferation under inflammatory settings. Furthermore, ILCs are located at distinct sites within the spleen and lymph nodes and act to critically regulate the survival of recirculating memory CD4+ T cells via interactions between ILC-expressed OX40L and CD30L and cognate receptors expressed by activated T cells. Finally, the ability of ILCs to regulate adaptive immune cell responses is not limited to T cells alone as ILCs may also produce cytokines and growth factors, including B cell-activating factor (BAFF), which support the function of B cells in lymphoid tissues.
In addition, we have recently shown that RORγt+ group 3 ILCs may also act to directly regulate immune cell homeostasis in the intestine through the expression of major histocompatability complex class II (MHCII) and direct interactions with commensal bacteria-responsive CD4+ T cells. Mice with an RORγt+ ILC-intrinsic deletion in MHCII developed dysregulated CD4+ T cell responses towards commensal bacteria and spontaneous intestinal inflammation [53]. Interestingly, although MHCII+ RORγt+ ILCs were able to efficiently pick up, process and present exogenous antigens in vitro, they were unable to induce CD4+ T cell proliferation, possibly due to their lack of co-stimulatory molecule expression, suggesting MHCII+ RORγt+ ILCs may act to inhibit rather than promote T cell responses in this context [53]. Recent evidence also suggests interactions between RORγt+ ILCs and CD4+ T cells may be bidirectional. Rag1−/− mice were found to have higher numbers of group 3 ILCs and increased levels of IL-22 and anti-microbial peptides in the intestine [54]. This phenomenon could be reversed upon transfer of purified-CD4+ T cells, suggesting T cells may also act to regulate ILC numbers and function [54].
Despite these advances, it remains unclear when or where group 3 ILCs act to modulate CD4+ T cell responses. Previous studies have shown that RORγt+ ILCs are found clustered at the interface of the B- and T- cell zones in lymph nodes and spleen and that these cells interact with CD4+ T cells following priming by dendritic cells [33,48,51]. Further work is needed to determine whether group 3 ILCs preferentially regulate antigen-specific CD4+ T cells or whether these pathways are active in the context of infectious, inflammatory or autoimmune diseases. Interestingly, in regard to their regulation of CD4+ T cell responses to commensal bacteria, RORγt+ group 3 ILCs are constitutively present at intestinal sites where luminal antigen is sampled, such as Peyer's patches, ILFs and cryptopatches. These lymphoid structures are organized around specialized epithelial cells called microfold cells (M cells), which play critical roles in the immunosurveillance of intestinal luminal microorganisms (Reviewed in [55]), provoking the hypothesis that commensal bacterial antigens sampled through M cells may be taken up by neighboring MHCII+ RORγt+ ILCs. Additionally, subcapsular sinus macrophages, which filter circulating antigen in the spleen, were recently shown to transfer antigen-containing blebs to IL-17A-expressing innate-like lymphocytes [56], although it remains to be seen whether RORγt+ ILCs may acquire antigen in this manner.
Regulation of adaptive immune cell responses by group 1 or group 2 ILCs
Although less well-defined, emerging evidence suggests that group 1 or group 2 ILCs may also influence adaptive immune cell responses. Group 2 ILCs can induce B cell proliferation and enhance IgA production via the secretion of IL-5 and IL-6 [9], and regulate barrier function in the lung via production of amphiregulin [57]. Group 2 ILCs have also been reported to express MHCII under some circumstances [13,53], and transfer of group 2 ILCs can enhance Th2 responses in recipient mice [13,58], although the contribution of MHCII in this process is currently unknown. In addition, classical NK cells, a subset of group 1 ILCs, can modulate the magnitude of CD4+ T cell responses via both direct and indirect mechanisms dependent on their expression of NK-cell receptors, such as NKp46 [59-62]. Of note, a subset of group 3 ILCs also express NKp46, although it is currently not known whether NKp46+ group 3 ILCs can also regulate CD4+ T cell responses via similar mechanisms. Taken together, these data suggest that the broader ILC family may act to regulate CD4+ T cell and B cell responses during infectious or inflammatory settings. Further work is needed to determine whether other ILC subsets can also modulate CD4+ T cells via direct interactions and to determine whether the ability of ILCs to inhibit or enhance adaptive immune responses occurs in a context-dependent manner.
Implications for human health and disease
Recent evidence suggests that altered ILC populations in humans are associated with the pathogenesis and progression of numerous chronic infectious and inflammatory diseases [1,2,10,11,58,63-70]. While the potential contribution of dysregulated ILC-derived cytokine production to innate immunity, inflammation and tissue repair in these diseases is relatively well appreciated [2,22], it is also possible that altered ILC responses may contribute to disease progression by significantly influencing adaptive immune cell responses. For example, alterations in group 3 ILC responses have recently been associated with both inflammatory bowel disease (IBD) and HIV infection [10,63-66,69,70]. In the context of IBD, dysregulated ILC responses may directly contribute to intestinal inflammation through impaired IL-22 production and increased IL-17A and IFN-γ production [1,10,11,66,69,70], although these altered ILC responses may also contribute to disease progression through impaired regulation of pro-inflammatory CD4+ T cell responses to commensal bacteria, perhaps via MHCII. In the context of HIV infection, a loss of ILCs may also result in increased pro-inflammatory CD4+ T cell responses to commensal bacteria at mucosal sites, which would increase the availability of activated CD4+ T cells for viral infection and replication, thus contributing to disease progression.
In support of an important role for ILCs in modulating adaptive immunity in human disease, a recent study reported that therapeutic administration of a monoclonal antibody directed against CD25 targeted group 3 ILCs, resulting in reduced pathologic adaptive immune cell responses [67]. This report provides compelling evidence of a role for ILCs in human disease pathogenesis, and further implicates ILCs as therapeutic targets due to their ability to modulate adaptive immune cell responses. Further interrogation of the interactions between ILCs and the adaptive immune system may also inform the design of next generation vaccine strategies, given the potential importance of ILCs in maintaining CD4+ T cell memory responses and high affinity antibody production [48,50-52]. Taken together, these studies highlight the need for a better understanding of how ILCs regulate the adaptive immune system in order to develop novel therapeutic strategies aimed at boosting immunity to pathogens while limiting human disease associated with inappropriate inflammatory responses to commensal bacteria, allergens or self-antigens.
Highlights.
Innate lymphoid cells (ILCs) are critical regulators of immune responses in lymphoid and barrier tissues.
ILCs modulate adaptive immune cell responses indirectly through cytokine-mediated regulation of stromal cells and epithelial cells.
ILCs can also directly regulate adaptive immune cells via MHCII or other cell-surface bound molecules.
ILC regulation of adaptive immune cells may be of clinical relevance in a wide range of chronic human inflammatory diseases.
Acknowledgements
We thank members of the Sonnenberg and Artis laboratories for discussions and critical reading of the manuscript. Research in the Sonnenberg laboratory is supported by the National Institutes of Health (DP5OD012116), the NIAID Mucosal Immunology Studies Team (MIST) Scholar Award in Mucosal Immunity and the Molecular Studies in Digestive and Liver Disease Molecular Pathology and Imaging Core (P30DK50306). MRH is supported by a research fellowship from the Crohn's and Colitis Foundation of America (CCFA).
Abbreviations used
- NK
Natural killer
- MHCII
major histocompatibility complex class II
- IL
Interleukin
- IFN-γ
Interferon gamma
- Th
T helper
- TSLP
thymic stromal lymphopoietin
- IgA
Immunoglobulin A
- HIV
Human immunodeficiency virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sonnenberg GF, Artis D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity. 2012;37:601–610. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 3.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 4.Sonnenberg GF, Mjosberg J, Spits H, Artis D. SnapShot: Innate Lymphoid Cells. Immunity. 2013;39:622–622. e621. doi: 10.1016/j.immuni.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mielke LA, Groom JR, Rankin LC, Seillet C, Masson F, Putoczki T, Belz GT. TCF-1 Controls ILC2 and NKp46+RORgammat+ Innate Lymphocyte Differentiation and Protection in Intestinal Inflammation. J Immunol. 2013;191:4383–4391. doi: 10.4049/jimmunol.1301228. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Monticelli LA, Saenz SA, Chi AW, Sonnenberg GF, Tang J, De Obaldia ME, Bailis W, Bryson JL, Toscano K, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. doi: 10.1016/j.immuni.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 10.Bernink JH, Peters CP, Munneke M, Te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 16.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 17.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 23.Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d'Hargues Y, Goppert N, Croxford AL, Waisman A, Tanriver Y, et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 25.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A: RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 27.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 28.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 29.White A, Carragher D, Parnell S, Msaki A, Perkins N, Lane P, Jenkinson E, Anderson G, Caamano JH. Lymphotoxin a-dependent and -independent signals regulate stromal organizer cell homeostasis during lymph node organogenesis. Blood. 2007110:1950–1959. doi: 10.1182/blood-2007-01-070003. [DOI] [PubMed] [Google Scholar]
- 30.Vondenhoff MF, Greuter M, Goverse G, Elewaut D, Dewint P, Ware CF, Hoorweg K, Kraal G, Mebius RE. LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol. 2009;182:5439–5445. doi: 10.4049/jimmunol.0801165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 32.Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, Rho J, Wong BR, Josien R, Kim N, et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MY, McConnell FM, Gaspal FM, White A, Glanville SH, Bekiaris V, Walker LS, Caamano J, Jenkinson E, Anderson G, et al. Function of CD4+CD3- cells in relation to B- and T-zone stroma in spleen. Blood. 2007;109:1602–1610. doi: 10.1182/blood-2006-04-018465. [DOI] [PubMed] [Google Scholar]
- 34.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 35.Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 2005;5:413–420. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Macpherson AJ. IgA adaptation to the presence of commensal bacteria in the intestine. Curr Top Microbiol Immunol. 2006;308:117–136. doi: 10.1007/3-540-30657-9_5. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki K, Fagarasan S. How host-bacterial interactions lead to IgA synthesis in the gut. Trends Immunol. 2008;29:523–531. doi: 10.1016/j.it.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji M, Suzuki K, Kinoshita K, Fagarasan S. Dynamic interactions between bacteria and immune cells leading to intestinal IgA synthesis. Semin Immunol. 2008;20:59–66. doi: 10.1016/j.smim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Kruglov AA, Grivennikov SI, Kuprash DV, Winsauer C, Prepens S, Seleznik GM, Eberl G, Littman DR, Heikenwalder M, Tumanov AV, et al. Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science. 2013;342:1243–1246. doi: 10.1126/science.1243364. [DOI] [PubMed] [Google Scholar]
- 41.Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, Ouyang W. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol. 2011;12:941–948. doi: 10.1038/ni.2089. [DOI] [PubMed] [Google Scholar]
- 42.Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 43.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, Fu YX, Zhou L. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim MY, Rossi S, Withers D, McConnell F, Toellner KM, Gaspal F, Jenkinson E, Anderson G, Lane PJ. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology. 2008;124:166–174. doi: 10.1111/j.1365-2567.2007.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Withers DR, Gaspal FM, Mackley EC, Marriott CL, Ross EA, Desanti GE, Roberts NA, White AJ, Flores-Langarica A, McConnell FM, et al. Cutting edge: lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J Immunol. 2012;189:2094–2098. doi: 10.4049/jimmunol.1201639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MY, Anderson G, White A, Jenkinson E, Arlt W, Martensson IL, Erlandsson L, Lane PJ. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3- inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J Immunol. 2005;174:6686–6691. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 50.Gaspal FM, Kim MY, McConnell FM, Raykundalia C, Bekiaris V, Lane PJ. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol. 2005;174:3891–3896. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- 51.Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 52.Withers DR, Jaensson E, Gaspal F, McConnell FM, Eksteen B, Anderson G, Agace WW, Lane PJ. The survival of memory CD4+ T cells within the gut lamina propria requires OX40 and CD30 signals. J Immunol. 2009;183:5079–5084. doi: 10.4049/jimmunol.0901514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korn LL, Thomas HH, Hubbeling HG, Spencer SP, Sinha R, Simkins HMA, Salzman NH, Bushman FD, Laufer TM. Conventional CD4+ T cells regulate IL-22-producing intestinal innate lymphoid cells. Mucosal Immunol. 2014 doi: 10.1038/mi.2013.121. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray EE, Friend S, Suzuki K, Phan TG, Cyster JG. Subcapsular sinus macrophage fragmentation and CD169+ bleb acquisition by closely associated IL-17-committed innate-like lymphocytes. PLoS One. 2012;7:e38258. doi: 10.1371/journal.pone.0038258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narni-Mancinelli E, Jaeger BN, Bernat C, Fenis A, Kung S, De Gassart A, Mahmood S, Gut M, Heath SC, Estelle J, et al. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science. 2012;335:344–348. doi: 10.1126/science.1215621. [DOI] [PubMed] [Google Scholar]
- 60.Ghadially H, Horani A, Glasner A, Elboim M, Gazit R, Shoseyov D, Mandelboim O. NKp46 regulates allergic responses. Eur J Immunol. 2013 doi: 10.1002/eji.201343388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LY, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012;5:670–680. doi: 10.1038/mi.2012.72. [DOI] [PubMed] [Google Scholar]
- 64.Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 2012;5:658–669. doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perry JS, Han S, Xu Q, Herman ML, Kennedy LB, Csako G, Bielekova B. Inhibition of LTi Cell Development by CD25 Blockade Is Associated with Decreased Intrathecal Inflammation in Multiple Sclerosis. Sci Transl Med. 2012;4:145ra106. doi: 10.1126/scitranslmed.3004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 69.Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, Chang J, Matuzaki Y, Suzuki S, Sugita A, Koganei K, et al. Imbalance of NKp44(+)NKp46(−) and NKp44(−)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterology. 2010;139:882–892. 892, e881–883. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 70.Ciccia F, Accardo-Palumbo A, Alessandro R, Rizzo A, Principe S, Peralta S, Raiata F, Giardina A, De Leo G, Triolo G. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis Rheum. 201264:1869–1878. doi: 10.1002/art.34355. [DOI] [PubMed] [Google Scholar]