Background: Receptor of advanced glycation end product (RAGE) mediates not only proinflammatory signaling, but also stimulates cell proliferation and survival-related signaling.
Results: Inhibiting RAGE resulted in slowed cyst growth in an autosomal dominant polycystic kidney disease (ADPKD) mouse model and improved renal function.
Conclusion: RAGE inhibition is highly effective against cyst enlargement in vivo.
Significance: RAGE signaling may play a role in cystogenesis and could be a new therapeutic target for PKD.
Keywords: Adenovirus, Inflammation, Kidney, Proliferation, Receptor for Advanced Glycation End Products (RAGE), Transgenic Mice, Cystic Disease, Cystogenesis
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disorder. Although a myriad of research groups have attempted to identify a new therapeutic target for ADPKD, no drug has worked well in clinical trials. Our research group has focused on the receptor for advanced glycation end products (RAGE) gene as a novel target for ADPKD. This gene is involved in inflammation and cell proliferation. We have already confirmed that blocking RAGE function attenuates cyst growth in vitro. Based on this previous investigation, our group examined the effect of RAGE on cyst enlargement in vivo. PC2R mice, a severe ADPKD mouse model that we generated, were utilized. An adenovirus containing anti-RAGE shRNA was injected intravenously into this model. We observed that RAGE gene knockdown resulted in loss of kidney weight and volume. Additionally, the cystic area that originated from different nephron segments decreased in size because of down-regulation of the RAGE gene. Blood urea nitrogen and creatinine values tended to be lower after inhibiting RAGE. Based on these results, we confirmed that the RAGE gene could be an effective target for ADPKD treatment.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD)3 is caused by a mutation in the PKD1 and PKD2 genes and is characterized by abnormal epithelial cell growth, fluid-filled cyst formation, and interstitial fibrosis (1). Many reports have indicated that cystogenesis results from multiple mechanisms, such as cAMP accumulation, aberrant MAPK signaling, or altered activation of mammalian target of rapamycin (mTOR), Jak2-STAT1/3, nuclear factor (NF)-κB, and vascular endothelial growth factor signaling (2, 3).
Many studies have attempted to discover therapeutic targets to cure ADPKD. Rapamycin (sirolimus) is one of the most widely used anticancer drugs. It specifically inhibits mTORC1, suppressing cell proliferation. Rapamycin has been applied to various animal models to evaluate its therapeutic effect on ADPKD. It had a dramatic curative effect on ADPKD in preclinical trials (3, 4). However, two independent clinical trials showed a failure to curtail cyst growth (5, 6). Besides these studies with rapamycin, various other target genes have been the focus of new candidate studies. Roscovitine, a cyclin-dependent kinase inhibitor, has been tested in jck and cpk mice. Roscovitine administration effectively induces cell cycle arrest, which results in cyst regression (7). In addition, metformin has been suggested as a new inhibitor against the mTOR pathway. Metformin activates AMP-activated protein kinase, which consequently suppresses mTORC signaling (8). Additionally, accumulation of glucosylceramide is observed in ADPKD mouse models and human patients. This finding indicates that inhibiting glucosylceramide synthase could be an effective way to treat ADPKD (9). Furthermore, an epigenetic approach is emerging as a new therapy. Inhibiting histone deacetylase could prevent cyst progression (10). Although several target genes for ADPKD therapy have been suggested, no effective treatment is currently available.
In our previous study, the receptor for advanced glycation end products (RAGE) was proposed as a potential therapeutic target for polycystic kidney disease (PKD) (11). RAGE is a transmembrane receptor and mediates activation of intracellular signal transduction pathways after binding diverse ligands such as AGEs, high mobility group box 1 protein (HMGB1), and S100/calgranulins (12, 13). Activation of RAGE triggers intracellular signaling that promotes the proinflammatory transcriptional factor NF-κB (13, 14) and the mitogen-activated protein kinase (MAPK) pathway (15, 16). Given the recent studies reporting that AGEs and RAGE are involved in diabetic nephropathy, several methods have been developed to cure chronic kidney disease by interfering with the pathophysiological effect of AGE (12). Accumulation of collagen and secretion of renal profibrotic cytokines could be reduced by preventing AGE formation or blocking RAGE-dependent signaling (17, 18). Blocking RAGE-amphoterin decreases growth and metastases of tumors by suppressing p44/p42, p38, and SAP/JNK MAPK signaling in cancer cells (19).
Numerous mouse models of PKD, such as cpk, bpk, jck, and Pkd2WS25/− mice, are currently available (20), but a more suitable mouse model is needed to test experimental therapies against PKD. Nullizygosity of Pkd2 results in embryonic lethality (21), but our PKD2 transgenic mouse partially rescued the lethality in Pkd2 knock-out mouse embryos. These mice have enlarged kidneys with numerous cysts, but they live for only 1 month (22). We considered that the Pkd2 knock-out rescue mouse (PC2R) to be the new PKD mouse model.
Many cytokines and growth factors in cyst fluid affect the inflammatory response, but some molecules are specifically related to cyst enlargement in PKD (23). For example, overexpression of neutrophil gelatinase-associated lipocalin (NGAL) carried by an adenoviral delivery system attenuates cystic growth in Pkd1flox/−:Ksp-Cre mice (24). In the present study, we injected adenovirus containing anti-RAGE shRNA into PC2R mice to determine the effect of RAGE on cystogenesis. The reduction in RAGE resulted in slowed cyst growth in vitro and in vivo by decreasing phosphorylated extracellular regulated kinase (ERK) signaling. Furthermore, renal function was improved in the anti-RAGE adenovirus-treated group compared with that in the adenovirus-treated control group by checking the renal function markers blood urea nitrogen (BUN) and creatinine. We suggest that RAGE-related signaling may be closely associated with PKD and that RAGE could be a new potential therapeutic target for PKD.
EXPERIMENTAL PROCEDURES
In Vitro Test for Adenovirus in the WT 9-12 Cell Line
We performed an in vitro assay in WT 9-12 cells to confirm whether the generated adenovirus works well. WT 9-12 cells are renal epithelial cells isolated from proximal and distal tubules of human patients with ADPKD. This cell line was purchased from ATCC, and it grows on bovine collagen-coated culture dishes. Approximately 3.5 × 105 cells were seeded in 100-mm dishes, and the adenovirus was applied in a dose-dependent and time-dependent manner.
Cell Proliferation Assay
WT 9-12 cells were infected by adenovirus (multiplicity of infection (m.o.i.) = 200). At 24 h after infection, 5 × 103 cells were seeded in each well of a 24-well culture plate. We used the XTT cell proliferation kit (11465015001; Roche Applied Science) and conducted experiments following the manufacturer's instructions. The absorbance was measured at 470 nm. The total number of adenovirus-treated cells was counted using a hemocytometer. The counting experiments were performed three times independently.
Mouse Care
We adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals or the equivalent. Mice were cared for under optimal conditions of 12-h light and 12-h dark at 20 °C and 50% humidity, and they had free access to sterilized water and food. PC2R mice were generated by mating human PKD2 transgenic mice with Pkd2+/− mice. Human PKD2 transgenic mice were generated by our laboratory (22), and Pkd2+/− mice were obtained from Dr. Stefan Somlo. The PC2R mice were C57BL/6. Experiments using mice were approved by Sookmyung Women's University (SMU-IACUC-2011-0516-008).
Genotyping
The PC2R genotyping primers were as follows: human PKD2 forward primer, 5′-ACTGACCGAACATGAACATCA-3′ and reverse primer, 5′-AACCATGACTGATCTGGGAAG-3′; mouse Pkd2, 5′-GCGCCGGCCTAGCTGTCCC-3′, 5′-GTTGTCGCGGCTCCACG-3′, and 5′-GTGCTACTTCCATTTGTCACGTCCTGC-3′. The PCR product of the human PKD2 gene was 500 bp, the wild-type mouse Pkd2 gene was 150 bp, and the knock-out was 350 bp. The genetic status of the PC2R mice was characterized by complete loss of the mouse Pkd2 gene (−/−) but the presence of the human PKD2 gene (+).
Adenovirus Injection into PC2R Mice
Genenmed Inc. (Seoul, South Korea) produced control adenovirus and anti-mRAGE shRNA encoding adenovirus. The pAd lox vector has a CMV promoter, and the construct produces adenovirus under the control of the cre-lox recombination system (25). For in vivo study, generated adenovirus in HEK293 cells was purified and concentrated (final concentration: 3∼8 × 1011/ml). PC2R mice were utilized at P6 for adenovirus injection. We injected adenovirus (3 × 108 pfu/day) intravenously three times (at P6, P8, and P10) into PC2R mice, and the mice were sacrificed at P15.
Immunohistochemistry and H&E Staining
Kidneys were isolated from mice and fixed in 10% formalin (HT-5011; Sigma). Paraffin-embedded kidneys were sliced into 5-μm width sections. The sections were stained with hematoxylin and eosin (H&E) to observe the entire shape and size of the kidney. Kidney morphology was imaged with an Olympus microscope (Olympus IX81, Tokyo, Japan) and a scan slide module with MetaMorph software (Molecular Devices Corp., Downingtown, PA). Unstained paraffin sections were incubated at 60 °C for 1 h for immunohistochemistry and were consecutively rehydrated in ethanol. Antigen retrieval was performed in a 0.01 m citric acid solution (pH 6.0). After blocking, anti-PCNA primary antibody (FL-261; Santa Cruz Biotechnology) and anti-p-ERK primary antibody (4377; Cell Signaling Technology) were added to the sections, and the sections were incubated at 4 °C overnight. Sections were stained with an ABC kit (PK-6200; Vector Laboratories) and a NovaRed kit (SK-4800; Vector Laboratories). H&E staining was performed as a counterstain.
Tubule Marker Staining and Analysis of Cyst Area
Paraffin-embedded kidney sections were rehydrated in serially diluted ethanol, and a 0.01 m citric acid solution was used for antigen retrieval. The following renal tubule markers were used: anti-LTA antibody (FL-1321; Vector Laboratories) for proximal tubules, anti-THP antibody (H-135; Santa Cruz Biotechnology) for distal tubules, and anti-DBA antibody (FL-1031; Vector Laboratories) for the collecting duct tubules. DAPI staining was performed to label the nuclei. Fluorescent signals were detected with a fluorescence microscope, and the cystic area was measured automatically using MetaMorph software.
Renal Function Test
Blood was collected from the posterior vena cava and the heart. Serum was isolated in Vacutainers (368972; BD Biosciences), and the BUN and creatinine analysis was performed by Dr. Chulho Lee's laboratory using a Hitachi 7150 Clinical Analyzer (KRIBB, Daejeon, South Korea).
Real-time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
A NucleoSpin (Macherey-Nagel, Dueren, Germany) kit was used for isolating mRNA from samples. cDNA was synthesized from 2 μg of mRNA with M-MLV reverse transcriptase. A Rotor-Gene PCR machine and software (RG-3000A; Qiagen) were utilized for real time RT-PCR. The diluted cDNA was mixed with SYBR Green (HiTaq SYBR, Q100220; GenePole, South Korea) and the following primers: human RAGE forward, 5′-GAAGGAACAGACCAGGAGACACC-3′ and reverse, 5′-TGCCACAAGATGACCCCAATG-3′; human β-actin forward, 5′-AAGGCCAACCGCGAGAAGAT-3′ and reverse, 5′-CCAGAGGCGTACAGGGATAGCAC-3′.
Western Blotting
Total protein was extracted from mouse kidneys using Nucleospin (Macherey-Nagel), and protein concentration was measured using a BCA assay (bicinchoninic acid solution, copper II sulfate; Sigma). Protein (30 μg) was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to PVDF membranes (Pall Corp., Port Washington, NY). The primary antibodies used were RAGE (ab3611; Abcam, Cambridge, MA), β-actin (A300-491A; Bethyl Laboratories, Montgomery, TX), p-ERK (4377; Cell Signaling Technology), ERK (9102; Cell Signaling Technology), p-AKT (4060; Cell Signaling Technology), AKT (4691; Cell Signaling Technology), p-MEK (2338; Cell Signaling Technology), MEK (4694; Cell Signaling Technology), p-p38 (9216; Cell Signaling Technology), and p-38 (9212; Cell Signaling Technology). Chemiluminescent signals were detected on an LAS-3000 imager (Fujifilm, Tokyo, Japan). A stripping solution (0.2 m glycine (pH 2.8) and 0.5 m NaCl) was utilized to remove antibodies used previously.
Statistical Analysis
The one-tailed t test was performed using GraphPad Prism6 software. A p value < 0.05 was considered significant.
RESULTS
Inhibiting RAGE Suppresses Cell Growth in Vitro
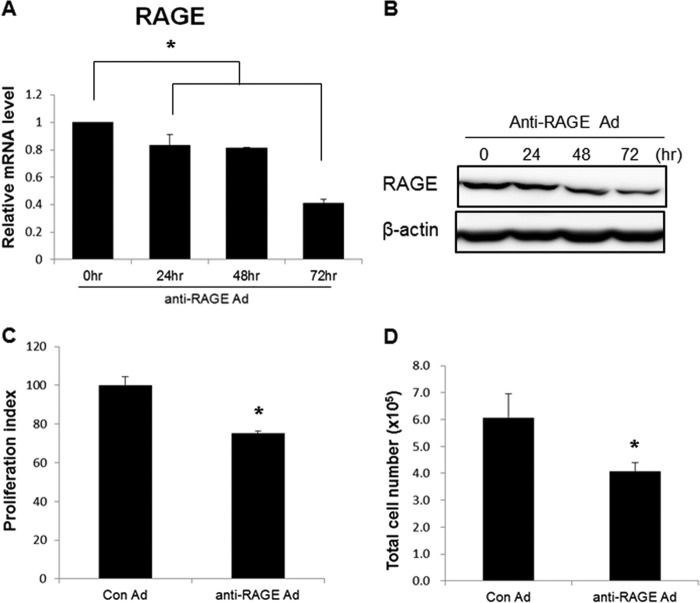
We previously demonstrated that inhibiting RAGE expression decreases cyst growth under three-dimensional culture conditions (11). Because of the long lasting effects of inhibiting RAGE in vitro and in vivo, we generated an adenovirus containing shRNA against RAGE to block downstream RAGE signaling. To confirm the effect of the generated virus, we infected the WT 9-12 cell line with the anti-RAGE adenovirus. Expression levels of RAGE decreased in a dose-dependent manner (data not shown). The anti-RAGE adenovirus (m.o.i. = 200) was added to the cells and reduced RAGE expression in a time-dependent manner (Fig. 1, A and B).
FIGURE 1.
Anti-RAGE adenovirus inhibited proliferation of WT 9-12 cells. A, reduction of the RAGE transcript in a time-dependent manner. Approximately 3.5 × 105 cells were seeded in 100-mm dishes, and anti-RAGE adenovirus (m.o.i. = 200) was added. Fresh media were added after 24 h. The cells were harvested at the indicated time points. B, decrease in RAGE protein in a time-dependent manner. C, relative viability of infected cells was measured using the XTT assay (m.o.i. = 200, 72 h after infection). D, total number of cells in each group (m.o.i. = 200, 72 h after infection). Cells were stained with trypan blue and counted. Data are mean ± S.D. (error bars). *, p < 0.05.
To determine the effect of RAGE on regulating cell proliferation, WT 9-12 cells were infected with control or anti-RAGE adenovirus (m.o.i. = 200). As a result, down-regulation of RAGE caused a reduction in both viable cell number (Fig. 1C) and total cell number (Fig. 1D) compared with control adenovirus-treated cells at 72 h after infection. These data demonstrate that suppressing RAGE inhibited cell growth in PKD cells.
Inhibiting RAGE Suppresses Cyst Growth in PC2R Mice
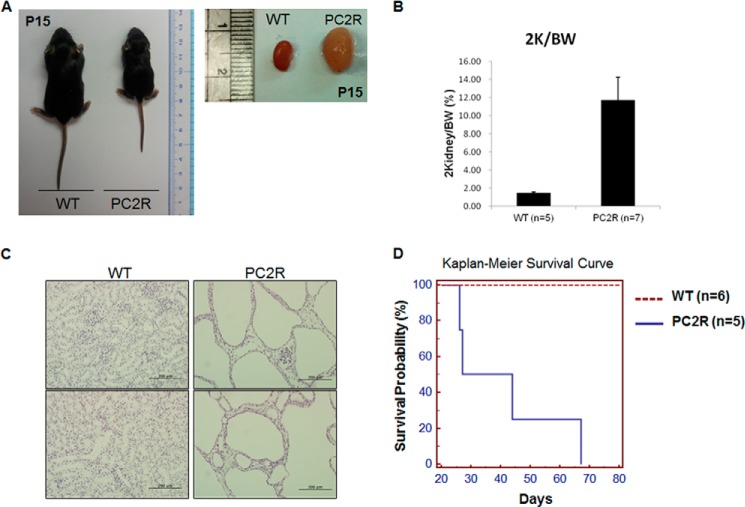
Conditional Pkd1 and Pkd2 knock-out mice have been used previously (24, 26) for PKD-related research because the homozygous loss of either Pkd1 or Pkd2 results in embryonic lethality (21, 27). The PC2R mouse is a partially rescued Pkd2 knock-out mouse with the human PKD2 transgene, and it has a average lifespan of 28–36 days, similar to Pkd1 or Pkd2 conditional mice (22) (Fig. 2). PC2R mice exhibited a smaller body size and lower body weight at P15 than those of wild-type littermates (Fig. 2, A and B, PC2R mice, 5.66 ± 0.98 g, n = 7; wild-type mice, 7.18 ± 1.06 g, n = 5). Further examination revealed that PC2R mice had severe bilateral polycystic kidneys (Fig. 2C). Thus, we thought that PC2R mice were suitable for an experimental therapeutic test.
FIGURE 2.
Characteristic of PC2R mouse. A, comparison of body and kidney size between WT and PC2R mice. Kidneys were isolated from both mice at P15. The PC2R mouse exhibited a smaller body size than that of wild-type littermate. The PC2R mouse kidney was enlarged and harbored a myriad of cysts. B, PC2R mice (n = 7) showing higher ratio rate of kidney weight to body weight than wild-type mice (n = 5). Error bars, S.D. C, overall structure of renal tissue. Isolated kidneys were fixed in 10% formalin and embedded in paraffin. Sliced paraffin sections were stained with hematoxylin and eosin. The PC2R mouse kidneys were filled with large cysts, and renal tissue was completely disrupted (magnification, ×200), Scale bars, 200 μm. D, Kaplan-Meier survival curve in wild-type (n = 6) and PC2R (n = 5) mice.
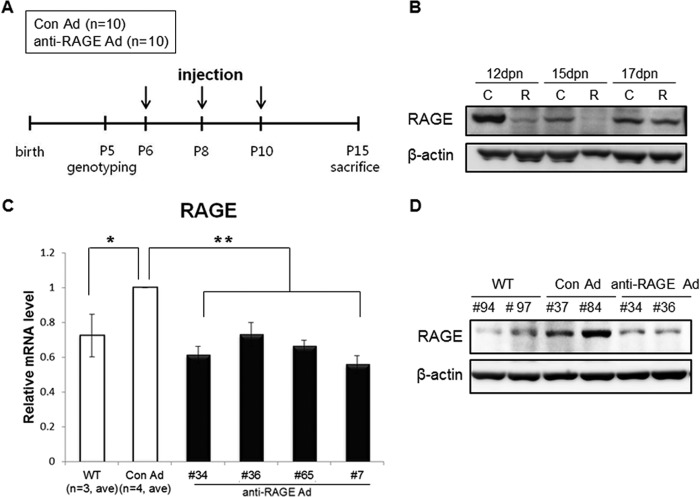
To determine whether the effect of inhibiting RAGE was reproduced in vivo, we utilized PC2R mice in the present study. These mice were injected in the tail vein on P6, P8, and P10 with anti-RAGE adenovirus or control adenovirus (Fig. 3A). The effect of anti-RAGE adenovirus in vivo was sustained for a maximum of 5 days after injection (Fig. 3B). Thus, the mice were killed at P15, and the kidneys were examined. PC2R mice had a high level of RAGE expression compared with WT littermate mice. The anti-RAGE adenovirus-injected pups exhibited down-regulated RAGE expression compared with control pups in terms of mRNA and protein levels (Fig. 3, C and D). RAGE expression was down-regulated by ∼30–40% following anti-RAGE adenovirus treatment in kidney tissues (Fig. 3C).
FIGURE 3.
Injection of anti-RAGE adenovirus into PC2R mice. A, schematic view of adenovirus injection. Genotyping was performed at 5 days postnatal (dpn). Control or anti-RAGE adenovirus was injected three times intravenously into PC2R mice. Injected mice were sacrificed at P15, and organs and blood were collected. Arrows indicate time points when mice were injected. B, duration of adenovirus effect in PC2R. After finishing the intravenous injection at P10, we sacrificed mice at three time points (P12, P15, and P17) and isolated the kidneys. RAGE protein remained low until 5 days after the final injection. However, protein levels at P17 (7 days after the last injection) were restored. C, control adenovirus-injected PC2R group; R, anti-RAGE adenovirus injected PC2R group. C, real-time polymerase chain reaction analysis to check RAGE mRNA level in the injected PC2R mice and WT littermate mice. RAGE mRNA levels (30–40%) decreased in the treated anti-RAGE adenovirus-treated group (WT; n = 3, Con Ad (control adenovirus-injected PC2R mice), n = 4; anti-RAGE Ad (anti-RAGE adenovirus-injected PC2R mice), n = 4. D, Western blot analysis to check RAGE protein level in the injected PC2R mice. Western blotting was performed to confirm efficiency of the adenovirus infection. Total protein was extracted from PC2R mice and WT littermate mice. The numbers of PC2R mice are listed on the blot.
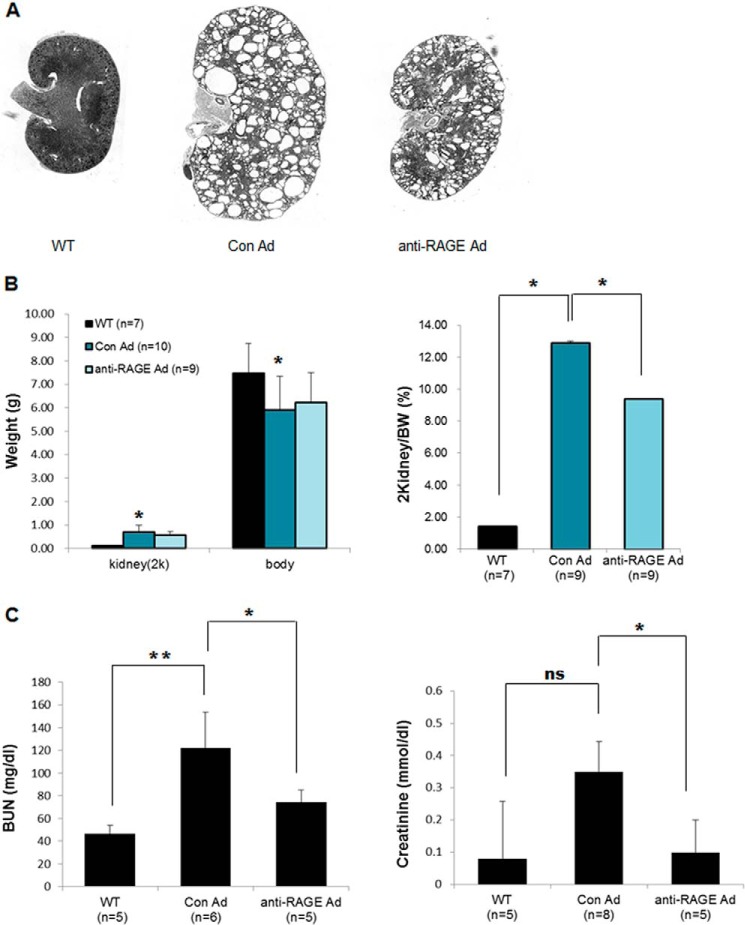
Furthermore, the kidneys of anti-RAGE adenovirus-injected mice had smaller cysts compared with those in control adenovirus-injected mice (Fig. 4A). The RAGE knock-down group showed a significant decrease in the ratio of kidney weight to body weight (Fig. 4B, right). Down-regulated RAGE led to a significant suppression not only in cystogenesis, but also in BUN and creatinine serum levels (Fig. 4C). These results demonstrate that inhibiting RAGE using an adenovirus system suppressed cyst formation in vivo.
FIGURE 4.
Knockdown of RAGE suppressed cystogenesis in PC2R mice. A, morphological comparison of the kidneys in each group. Representative data are presented. B, differences in kidney weight/total body weight between PC2R mice in each group. The weight of both kidneys and total body weight of the mice were measured. Then, the kidney weight/total body weight was calculated. Normal, WT littermates (n = 7); PC2R + Con Ad, control adenovirus-injected PC2R mice (n = 9); PC2R + anti-RAGE Ad, anti-RAGE adenovirus-injected PC2R mice (n = 9). C, analysis of BUN and creatinine levels. Serum was harvested from blood, and the BUN and creatinine levels were measured. WT littermates (n = 5); control adenovirus-injected (BUN; n = 6, Cr; n = 8); anti-RAGE adenovirus-injected (n = 5). Data are mean ± S.D. (error bars). **, p < 0.001; *, p < 0.05; ns, nonsignificant.
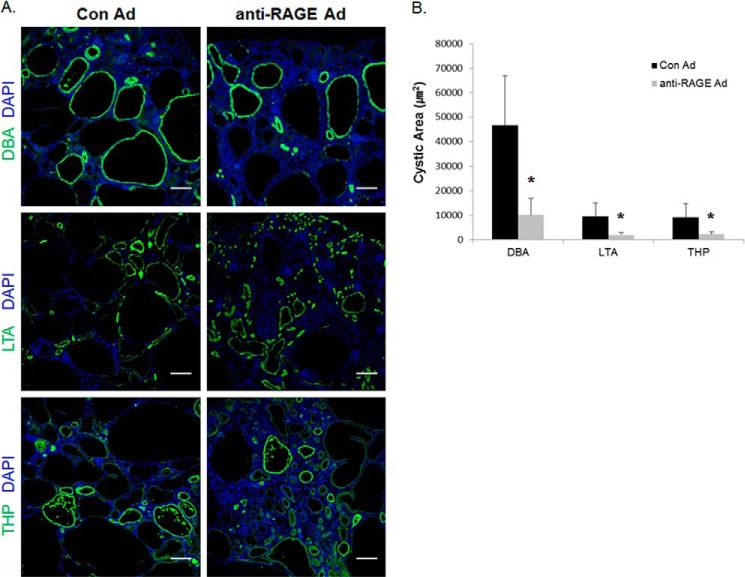
Renal tubular cysts arise from multiple tubule segments, and the origins of the cysts can be determined by staining with specific antibodies, such as LTA (proximal tubule), DBA (collecting tubule), and THP (distal tubule) (21, 22). We were able to verify the change in size and the origin of the cysts by using immunohistochemistry with segment-specific antibodies in the anti-RAGE adenovirus-treated and control groups. The PC2R mice developed cysts in multiple parts of the nephron, and the majority of large cysts were stained positively with the collecting duct marker, DBA. In Fig. 5, A and B, we showed that suppressing RAGE by the adenovirus system led to attenuated cyst formation in different nephron segments. Therefore, our data indicate that RAGE inhibition is highly effective against cyst enlargement in PC2R mice.
FIGURE 5.
Silencing RAGE reduced the cystic area originating from each nephron segment in PC2R mice. A, kidney sections stained with tubule-specific markers. Kidney sections were stained with tubule-specific antibodies to classify cyst origin. The antibodies used were anti-DBA (green) for collecting duct staining, anti-LTA (green) for the proximal tubule, and anti-THP antibody (green) for the distal tubule. The nuclei were stained with DAPI (blue). Representative data are presented (magnification, ×100). Scale bars, 200 μm. B, statistical analysis of cystic area in each case. The IMA module of MetaMorph software was utilized to calculate cystic area (each >40 cysts). Data are mean ± S.D. (error bars). *, p < 0.001.
Inhibition of RAGE Reduces Proliferation in PC2R Mice
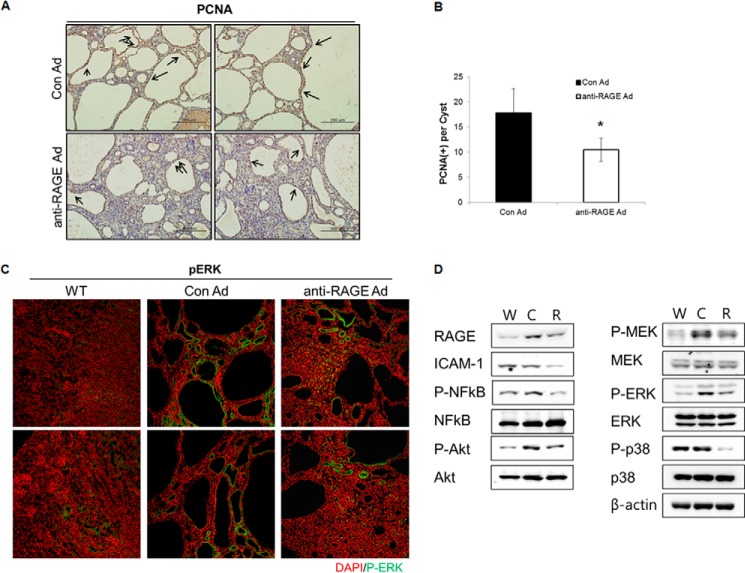
RAGE interacts directly with ERK1 and ERK2, leading to activation of p38 MAPK and NF-κB (28, 29). Moreover, blocking RAGE with a neutralizing antibody reduces connective tissue growth factor and phosphorylated ERK expression in diabetic nephropathy (30). As the main feature in cystic tissues is aberrant proliferation, we analyzed the effect of RAGE silencing on the proliferation of cystic epithelial regions. First, the number of PCNA-positive cells decreased in cystic epithelia of anti-RAGE adenovirus-injected mice kidneys (Fig. 6A, quantified in Fig. 6B, Con Ad, 17.81 ± 4.8; RAGE Ad, 10.5 ± 2.2). Activation of MAPK/ERK is important for cyst enlargement in PKD and is a cell proliferation marker in PKD (31, 32). Next, we conducted immunostaining and Western blot analyses to evaluate ERK phosphorylation more precisely. Phosphorylated ERK decreased in cyst-lined epithelial cells of anti-RAGE adenovirus-injected mice (Fig. 6C). However, cyst-surrounding tubules showed a positive signal for phosphorylated ERK in both adenovirus-treated PC2R groups and WT group. RAGE associates with multiple signaling pathways, including the PI3K/Akt, MAPK pathways, and NF-κB signaling (33). To evaluate the effects of inhibiting RAGE on proliferation and inflammation, we examined the activation of RAGE-mediated signaling pathway molecules. The phosphorylation of Akt, MEK, ERK, and p38 were not only down-regulated but also ICAM-1 and phosphorylated NF-kB decreased following injection of anti-RAGE adenovirus compared with control adenovirus (Fig. 6D). These results suggest that inhibiting RAGE attenuated cyst growth by suppressing RAGE-mediated signaling.
FIGURE 6.
Cell proliferation in PC2R mice attenuated by down-regulation of RAGE. A, PCNA staining of PC2R kidney sections. Kidneys were isolated from PC2R mice in both the control and anti-RAGE groups. Kidneys were embedded in paraffin and sliced into 5-μm wide sections. Immunohistochemical staining was performed using an anti-PCNA antibody. Scale bar = 200 μm. B, PCNA-positive cells surrounding the cyst (200–500-μm diameter) were counted in 10 random, nonoverlapping fields, and the number of proliferating cells/cyst was calculated. Data are mean ± S.D. (error bars). *, p < 0.001. C, immunostaining for p-ERK of WT kidney and PC2R kidney sections. Anti-phosphorylated ERK antibody was applied to the sections to observe localization of p-ERK (green). Magnification, ×200. D, Western blot was performed for checking the phosphorylation level of indicated proteins. W, wild-type mice; C, control adenovirus-injected PC2R mice; R, anti-RAGE adenovirus-injected PC2R mice.
DISCUSSION
Cystogenesis is characterized mainly by an inflammatory response through the secretion of cytokines and fibrosis, as well as proliferative cystic growth in PKD. Progression of the fibrotic phenotype through cellular matrix exchange leads to end stage renal disease (34). Many types of cytokines and growth factors in cyst fluid are related not only to renal function but also to cyst formation (11). For example, the inflammatory cytokine tumor necrosis factor-α (TNF-α) in Pkd2+/− mice is directly related to cyst enlargement and its inhibitor, etanercept, reduces cyst formation (35). Activating RAGE is also associated with proinflammatory cytokines, such as transforming growth factor-β, TNF-α, insulin-like growth factor-1, and monocyte chemotactic protein-1 (12). These diverse cytokines are similar to those found in cystic fluid in PKD. In addition, cells stimulated by RAGE release these cytokines to recruit adjacent cells into the kidney inflammatory response (12). Because the inflammatory response mechanisms during cystogenesis have not been determined, we suggest that activating RAGE may be associated with the inflammation and fibrosis observed in PKD.
In a previous study, we confirmed that inhibiting RAGE has the potential to regulate cyst formation (11) and that RAGE RNAi blocks cyst development by inhibiting ERK signaling in a three-dimensional culture system (11). In the present study, we generated adenovirus encoding anti-RAGE shRNA for stable reduction in an in vivo experiment. The results showed that suppressing RAGE slowed adenoviral delivery, which led to slower cyst growth and reduced ERK activation in PC2R mice (Figs. 4 and 6). RAGE binds ERK1/2 and is involved in PI3K and MAPK signaling in cells (28, 33). We concluded that RAGE signaling is involved in both inflammation and cyst formation by modulating ERK in PKD.
The majority of renal cysts evolved from the collecting duct in PC2R mice. In Pkd2WS25/WS25 mice, 40% of cysts originated from the collecting tubule, and 42% were of distal tubular origin (21). We do not know the exact origin of renal tubular cysts in Pkd2 knock-out mice, but we suggest that the Pkd2 gene is related to the collecting duct-originated cysts. Renal cysts that originated from different nephron segments may react differently to therapy (7). Injection with anti-RAGE adenovirus resulted in suppressed cyst formation in different nephron segments (Fig. 5). Thus, targeting RAGE may affect cystogenesis in cystic diseases.
The PKD2 transgene delays lethality in the Pkd2 knock-out mouse, and rescued mice remain viable for 1 month (22). The PC2R mice in the present study had numerous cysts in bilateral kidneys and had a small body size. Pkd1flox/−:Ksp-Cre mice developed severe polycystic kidneys and died between P14 and P17 (26); therefore, these mice were used for testing PKD experimental therapies in vivo. We speculated that PC2R mice would also be a suitable mouse model for PKD research because the PC2R mice and Pkd1 conditional knock-out mice share similar lifespans and phenotypes.
RAGE-related signaling is implicated in a number of pathological diseases, including inflammatory diseases such as diabetes, arthritis, cancers, and neurodegenerative disorders (33). Therefore, several tools for blocking RAGE-mediated signaling have been developed, such as soluble RAGE (sRAGE), RAGE-blocking antibodies, small molecule inhibitors, and a dominant negative mutant form of RAGE (14, 33). In cancer, inhibiting RAGE using an sRAGE or anti-RAGE F(ab′)2 fragment decreases tumor proliferation and invasion (19). Moreover, treatment with sRAGE and an adenoviral vector expressing dominant negative RAGE provides protection against glomerulosclerosis (36). Although adenovirus primarily infects the liver in PKD, supplemental neutrophil gelatinase-associated lipocalin increases circulating levels and suppresses kidney cyst growth in vivo (24). In the present study, adenovirus encoding RAGE shRNA was injected intravenously into PC2R mice, and 62.5% of the pups (five of eight pups) showed down-regulated RAGE in the kidney.
The effect of RAGE signaling has been well characterized in diabetic phenotypes such as diabetic nephropathy. AGE-RAGE signaling induces abnormal changes in glomeruli, which lead to glomerulosclerosis (37). Interestingly, several studies on the renin-angiotensin system (RAS) in diabetes have reported that suppression of RAS attenuates the progression of diabetic nephropathy because of a decrease in RAGE-dependent signaling and a simultaneous increase in endogenous soluble RAGE (esRAGE) and sRAGE. The results suggest that the levels of esRAGE or sRAGE are negatively correlated with diabetic nephropathy severity. This phenomenon has been confirmed in both rodent models and human patients (38–40). These reports suggest that it may be a good therapeutic approach to induce the secretion of esRAGE or to supply sRAGE in diabetic nephropathy. It can be postulated that directly introducing esRAGE in vivo will have a curative effect on diabetic nephropathy and ADPKD. Our results shed light on the beneficial effect of anti-RAGE therapy in ADPKD. Therefore, we decided to apply sRAGE to ADPKD animal models for further investigation.
In conclusion, inhibiting RAGE using an adenoviral delivery system resulted in slower cyst enlargement in all nephron segments of PC2R mice. In addition, we found that suppressing RAGE led to inhibition of proliferation and ERK activation. Decreases in cyst growth rate because of anti-RAGE adenovirus and levels of serum BUN and creatinine revealed that renal function was improved by inhibiting RAGE. We propose that RAGE may play a role in cystogenesis and could be a new therapeutic target for PKD.
Acknowledgments
We thank Dr. Chulho Lee for the BUN and Cr analyses and official agencies for financial support.
This work was supported by the Bio & Medical Technology Development Programs 2010-0019867 and 2012M3A9D12012054518, Basic Science Research Program 2012 R1A1A2043225, and Mid-career Researcher Program 2012-043084 of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology of Korea.
- ADPKD
- autosomal dominant polycystic kidney disease
- AGE
- advanced glycation end product
- BUN
- blood urea nitrogen
- DBA
- Dolichos biflorus antigen
- esRAGE
- endogenous soluble RAGE
- LTA
- Lotus tetragonolobus antigen
- m.o.i.
- multiplicity of infection
- mTOR
- mammalian target of rapamycin
- PC2R
- Pkd2 knock-out rescued mouse
- PCNA
- proliferating cell nuclear antigen
- RAGE
- receptor for advanced glycation end products
- THP
- Tamm-Horsfall protein.
REFERENCES
- 1. Chapin H. C., Caplan M. J. (2010) The cell biology of polycystic kidney disease. J. Cell Biol. 191, 701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bello-Reuss E., Holubec K., Rajaraman S. (2001) Angiogenesis in autosomal-dominant polycystic kidney disease. Kidney Int. 60, 37–45 [DOI] [PubMed] [Google Scholar]
- 3. Novalic Z., van der Wal A. M., Leonhard W. N., Koehl G., Breuning M. H., Geissler E. K., de Heer E., Peters D. J. (2012) Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. J. Am. Soc. Nephrol. 23, 842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zafar I., Ravichandran K., Belibi F. A., Doctor R. B., Edelstein C. L. (2010) Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease. Kidney Int. 78, 754–761 [DOI] [PubMed] [Google Scholar]
- 5. Serra A. L., Poster D., Kistler A. D., Krauer F., Raina S., Young J., Rentsch K. M., Spanaus K. S., Senn O., Kristanto P., Scheffel H., Weishaupt D., Wüthrich R. P. (2010) Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 [DOI] [PubMed] [Google Scholar]
- 6. Walz G., Budde K., Mannaa M., Nürnberger J., Wanner C., Sommerer C., Kunzendorf U., Banas B., Hörl W. H., Obermüller N., Arns W., Pavenstädt H., Gaedeke J., Büchert M., May C., Gschaidmeier H., Kramer S., Eckardt K. U. (2010) Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 [DOI] [PubMed] [Google Scholar]
- 7. Bukanov N. O., Smith L. A., Klinger K. W., Ledbetter S. R., Ibraghimov-Beskrovnaya O. (2006) Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature 444, 949–952 [DOI] [PubMed] [Google Scholar]
- 8. Takiar V., Nishio S., Seo-Mayer P., King J. D., Jr., Li H., Zhang L., Karihaloo A., Hallows K. R., Somlo S., Caplan M. J. (2011) Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. U.S.A. 108, 2462–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Natoli T. A., Smith L. A., Rogers K. A., Wang B., Komarnitsky S., Budman Y., Belenky A., Bukanov N. O., Dackowski W. R., Husson H., Russo R. J., Shayman J. A., Ledbetter S. R., Leonard J. P., Ibraghimov-Beskrovnaya O. (2010) Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat. Med. 16, 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xia S., Li X., Johnson T., Seidel C., Wallace D. P., Li R. (2010) Polycystin-dependent fluid flow sensing targets histone deacetylase 5 to prevent the development of renal cysts. Development 137, 1075–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park E. Y., Seo M. J., Park J. H. (2010) Effects of specific genes activating RAGE on polycystic kidney disease. Am. J. Nephrol. 32, 169–178 [DOI] [PubMed] [Google Scholar]
- 12. Bohlender J. M., Franke S., Stein G., Wolf G. (2005) Advanced glycation end products and the kidney. Am. J. Physiol. Renal Physiol. 289, F645-F659 [DOI] [PubMed] [Google Scholar]
- 13. Bierhaus A., Humpert P. M., Morcos M., Wendt T., Chavakis T., Arnold B., Stern D. M., Nawroth P. P. (2005) Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 83, 876–886 [DOI] [PubMed] [Google Scholar]
- 14. Busch M., Franke S., Rüster C., Wolf G. (2010) Advanced glycation end-products and the kidney. Eur. J. Clin. Invest. 40, 742–755 [DOI] [PubMed] [Google Scholar]
- 15. Arumugam T., Simeone D. M., Schmidt A. M., Logsdon C. D. (2004) S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE). J. Biol. Chem. 279, 5059–5065 [DOI] [PubMed] [Google Scholar]
- 16. Zhu P., Ren M., Yang C., Hu Y. X., Ran J. M., Yan L. (2012) Involvement of RAGE, MAPK, and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Dermatol. 21, 123–129 [DOI] [PubMed] [Google Scholar]
- 17. Cohen M. P., Sharma K., Jin Y., Hud E., Wu V. Y., Tomaszewski J., Ziyadeh F. N. (1995) Prevention of diabetic nephropathy in db/db mice with glycated albumin antagonists: a novel treatment strategy. J. Clin. Invest. 95, 2338–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly D. J., Gilbert R. E., Cox A. J., Soulis T., Jerums G., Cooper M. E. (2001) Aminoguanidine ameliorates overexpression of prosclerotic growth factors and collagen deposition in experimental diabetic nephropathy. J. Am. Soc. Nephrol. 12, 2098–2107 [DOI] [PubMed] [Google Scholar]
- 19. Taguchi A., Blood D. C., del Toro G., Canet A., Lee D. C., Qu W., Tanji N., Lu Y., Lalla E., Fu C., Hofmann M. A., Kislinger T., Ingram M., Lu A., Tanaka H., Hori O., Ogawa S., Stern D. M., Schmidt A. M. (2000) Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 405, 354–360 [DOI] [PubMed] [Google Scholar]
- 20. Torres V. E., Harris P. C. (2007) Polycystic kidney disease: genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J. Intern. Med. 261, 17–31 [DOI] [PubMed] [Google Scholar]
- 21. Wu G., D'Agati V., Cai Y., Markowitz G., Park J. H., Reynolds D. M., Maeda Y., Le T. C., Hou H., Jr., Kucherlapati R., Edelmann W., Somlo S. (1998) Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93, 177–188 [DOI] [PubMed] [Google Scholar]
- 22. Park E. Y., Sung Y. H., Yang M. H., Noh J. Y., Park S. Y., Lee T. Y., Yook Y. J., Yoo K. H., Roh K. J., Kim I., Hwang Y. H., Oh G. T., Seong J. K., Ahn C., Lee H. W., Park J. H. (2009) Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J. Biol. Chem. 284, 7214–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cowley B. D., Jr., Ricardo S. D., Nagao S., Diamond J. R. (2001) Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int. 60, 2087–2096 [DOI] [PubMed] [Google Scholar]
- 24. Wei F., Karihaloo A., Yu Z., Marlier A., Seth P., Shibazaki S., Wang T., Sukhatme V. P., Somlo S., Cantley L. G. (2008) Neutrophil gelatinase-associated lipocalin suppresses cyst growth by Pkd1 null cells in vitro and in vivo. Kidney Int. 74, 1310–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hardy S., Kitamura M., Harris-Stansil T., Dai Y., Phipps M. L. (1997) Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 71, 1842–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shibazaki S., Yu Z., Nishio S., Tian X., Thomson R. B., Mitobe M., Louvi A., Velazquez H., Ishibe S., Cantley L. G., Igarashi P., Somlo S. (2008) Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum. Mol. Genet. 17, 1505–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu W., Peissel B., Babakhanlou H., Pavlova A., Geng L., Fan X., Larson C., Brent G., Zhou J. (1997) Perinatal lethality with kidney and pancreas defects in mice with a targeted Pkd1 mutation. Nat. Genet. 17, 179–181 [DOI] [PubMed] [Google Scholar]
- 28. Ishihara K., Tsutsumi K., Kawane S., Nakajima M., Kasaoka T. (2003) The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett. 550, 107–113 [DOI] [PubMed] [Google Scholar]
- 29. Lotze M. T., Tracey K. J. (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342 [DOI] [PubMed] [Google Scholar]
- 30. Chung A. C., Zhang H., Kong Y. Z., Tan J. J., Huang X. R., Kopp J. B., Lan H. Y. (2010) Advanced glycation end products induce tubular CTGF via TGF-β-independent Smad3 signaling. J. Am. Soc. Nephrol. 21, 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Torres V. E., Harris P. C. (2006) Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat. Clin. Pract. Nephrol. 2, 40–55; quiz 55 [DOI] [PubMed] [Google Scholar]
- 32. Omori S., Hida M., Fujita H., Takahashi H., Tanimura S., Kohno M., Awazu M. (2006) Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J. Am. Soc. Nephrol. 17, 1604–1614 [DOI] [PubMed] [Google Scholar]
- 33. Riehl A., Németh J., Angel P., Hess J. (2009) The receptor RAGE: bridging inflammation and cancer. Cell Commun. Signal. 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu N., Glockner J. F., Rossetti S., Babovich-Vuksanovic D., Harris P. C., Torres V. E. (2006) Autosomal dominant polycystic kidney disease coexisting with cystic fibrosis. J. Nephrol. 19, 529–534 [PubMed] [Google Scholar]
- 35. Li X., Magenheimer B. S., Xia S., Johnson T., Wallace D. P., Calvet J. P., Li R. (2008) A tumor necrosis factor-α-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat. Med. 14, 863–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo J., Ananthakrishnan R., Qu W., Lu Y., Reiniger N., Zeng S., Ma W., Rosario R., Yan S. F., Ramasamy R., D'Agati V., Schmidt A. M. (2008) RAGE mediates podocyte injury in adriamycin-induced glomerulosclerosis. J. Am. Soc. Nephrol. 19, 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. D'Agati V., Yan S. F., Ramasamy R., Schmidt A. M. (2010) RAGE, glomerulosclerosis and proteinuria: roles in podocytes and endothelial cells. Trends Endocrinol. Metab. 21, 50–56 [DOI] [PubMed] [Google Scholar]
- 38. Giannini C., D'Adamo E., de Giorgis T., Chiavaroli V., Verrotti A., Chiarelli F., Mohn A. (2012) The possible role of esRAGE and sRAGE in the natural history of diabetic nephropathy in childhood. Pediatr. Nephrol. 27, 269–275 [DOI] [PubMed] [Google Scholar]
- 39. Forbes J. M., Thorpe S. R., Thallas-Bonke V., Pete J., Thomas M. C., Deemer E. R., Bassal S., El-Osta A., Long D. M., Panagiotopoulos S., Jerums G., Osicka T. M., Cooper M. E. (2005) Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 16, 2363–2372 [DOI] [PubMed] [Google Scholar]
- 40. Sourris K. C., Morley A. L., Koitka A., Samuel P., Coughlan M. T., Penfold S. A., Thomas M. C., Bierhaus A., Nawroth P. P., Yamamoto H., Allen T. J., Walther T., Hussain T., Cooper M. E., Forbes J. M. (2010) Receptor for AGEs (RAGE) blockade may exert its renoprotective effects in patients with diabetic nephropathy via induction of the angiotensin II type 2 (AT2) receptor. Diabetologia 53, 2442–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]