Abstract
Hydrogen peroxide, the nonradical 2-electron reduction product of oxygen, is a normal aerobic metabolite occurring at about 10 nm intracellular concentration. In liver, it is produced at 50 nmol/min/g of tissue, which is about 2% of total oxygen uptake at steady state. Metabolically generated H2O2 emerged from recent research as a central hub in redox signaling and oxidative stress. Upon generation by major sources, the NADPH oxidases or Complex III of the mitochondrial respiratory chain, H2O2 is under sophisticated fine control of peroxiredoxins and glutathione peroxidases with their backup systems as well as by catalase. Of note, H2O2 is a second messenger in insulin signaling and in several growth factor-induced signaling cascades. H2O2 transport across membranes is facilitated by aquaporins, denoted as peroxiporins. Specialized protein cysteines operate as redox switches using H2O2 as thiol oxidant, making this reactive oxygen species essential for poising the set point of the redox proteome. Major processes including proliferation, differentiation, tissue repair, inflammation, circadian rhythm, and aging use this low molecular weight oxygen metabolite as signaling compound.
Keywords: Aquaporin, Glutathione Peroxidase, Hydrogen Peroxide, Insulin, Mitochondria, NADPH Oxidase, Peroxiredoxin, Redox, Catalase, Second Messenger
Introduction
One of the surprises in redox biology was the relatively recent appreciation of hydrogen peroxide as a messenger molecule. It is now widely accepted that this low molecular weight molecule is utilized in metabolic regulation in ways similar to diffusible gases such as NO, CO, or H2S. Even more so, H2O2 is recognized as being in the forefront of transcription-independent signals, in one line with Ca2+ and ATP (1). H2O2 diffuses through tissues to initiate immediate cellular effects, such as cell shape changes, the formation of functional actomyosin structures, and the recruitment of immune cells (1). Among the various reactive oxygen species, H2O2 has been identified as a suitable second messenger molecule, in part because of its reactions with specific oxidation-prone protein cysteinyl residues in local environments that lower the pKa to provide specificity in time and space, required in signaling (2, 3). However, until recently, assessing the precise amount of hydrogen peroxide in cellular and subcellular locations under in vivo conditions was challenging, but promising progress in methodology has opened a new level of analysis, introducing genetically encoded fluorescent indicators as H2O2 reporter molecules (4).
Against this background, the present minireview will address the following questions. 1) How can H2O2 be assayed in the biological setting? 2) What are the metabolic sources and sinks of H2O2? 3) What is the role of H2O2 in redox signaling and oxidative stress?
How Can H2O2 Be Assayed in the Biological Setting?
In his book “On the Catalytic Actions of the Living Substance,” in 1928 Otto Warburg (5) noted that one should “study enzymes under the most natural conditions of action, in the living cell itself. From the standpoint of preparative chemistry they may be looked upon as being of utmost impurity. However, if one finds reactants that selectively react with the enzymes, the rest of the cell interacts as little as the glass wall of a test tube in which a chemical reaction is carried out.” This is the mindset behind the current use of proteins selectively sensing and reporting ligands or reactants such as H2O2.
Organ Spectrophotometry of Catalase Compound I
The first demonstration that H2O2 is present as a normal attribute of aerobic metabolism in mammalian cells was by spectrophotometry of catalase Compound I, which is formed in the reaction of catalase with H2O2 (6). Catalase minus catalase Compound I (7) has an optical difference spectrum in the near infrared amenable to specific spectrophotometry in biological systems because there is negligible interference from other components and little light scattering. The absorbance difference between 640 and 660 nm was identified to selectively monitor the steady-state level of catalase Compound I in intact liver (6), enabling readout of H2O2 by using Compound I as a molecular beacon and proving the existence of H2O2 under normal metabolic conditions. As illustrated in Fig. 1, the continuous endogenous production of H2O2 was demonstrated by its reaction with the hydrogen donor, methanol. There is increased formation of Compound I upon infusion of substrate for enhanced production of H2O2, e.g. glycolate (8). Methanol can be used as hydrogen donor for titrations in intact tissues because unlike ethanol, it reacts specifically with catalase Compound I. From titrations with methanol, the steady-state rate of H2O2 production was quantified to be 50 nmol/min/g of liver, which is about 2% of the respiration rate of the liver (9). Supply of medium-chain fatty acids such as octanoate increased the rate of H2O2 generation to 170 nmol/min/g of liver (Table 1). The concentration of H2O2 was estimated to be about 10 nm (10). Exposed liver of anesthetized rats in situ is amenable to this H2O2 assay as well (11). These data represent H2O2 detected by catalase in the liver, a tissue rich in peroxisomes (see Ref. 10). Rates and concentrations of H2O2 in other cell types may be different. Isolated mitochondria had an upper estimate of the proportion of electron flow giving rise to H2O2 with palmitoyl carnitine as substrate of 0.15% (12), an order of magnitude lower than the 2% mentioned above for the intact liver. Thus, either there is an artifactually low rate after isolation of the organelles, or the contribution by extramitochondrial sources is considerable, or there is an overestimation by the hydrogen donor titration method. Conversely, in addition to the H2O2 detected with the catalase Compound I method (Table 1), additional H2O2 flux occurs through the peroxiredoxins, thioredoxins, and GSH peroxidases (see below). These issues need to be addressed in further studies as methodology advances.
FIGURE 1.
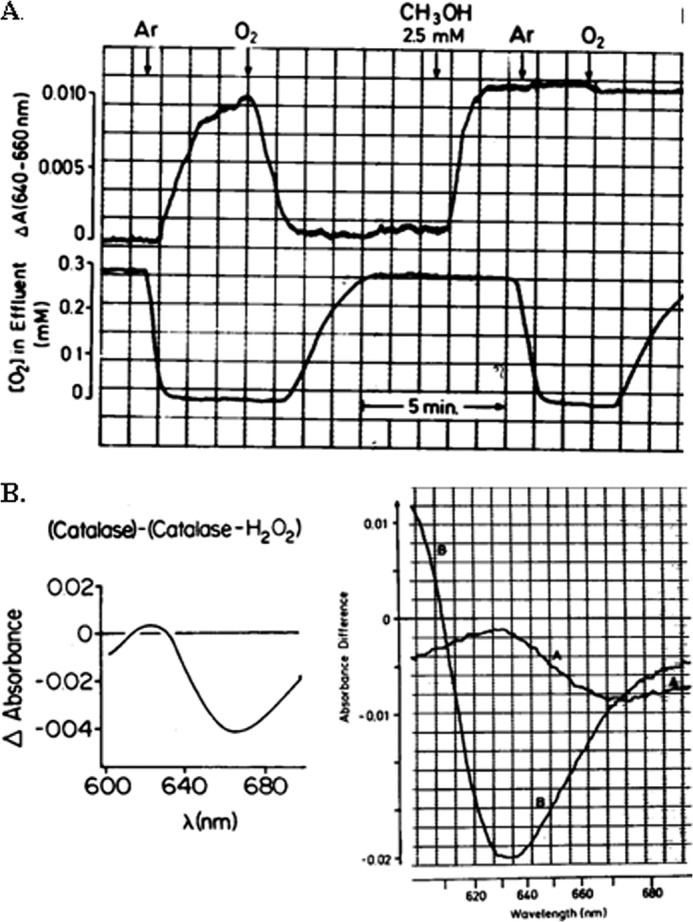
Demonstration of steady-state H2O2 generation in intact liver by organ spectrophotometry. A, the absorbance difference between 640 and 660 nm is used for monitoring catalase Compound I (top) and oxygen concentration in effluent perfusate (bottom). Anoxia and reoxygenation (argon and oxygen, arrows) and methanol (arrow) as hydrogen donor modulate, and thereby prove the existence of, H2O2 steady states; from Sies and Chance (6) with permission. B, catalase minus catalase Compound I difference spectra. Left, isolated enzyme. Right, organ difference spectrum (trace A) and cyanide difference spectrum (trace B); from Sies et al. (8) with permission.
TABLE 1.
H2O2 production rates in intact organ
Isolated hemoglobin-free perfused liver data were obtained by methanol titration of catalase Compound I; from Oshino et al. (9). For discussion, see Refs. 10 and 32.
| Substrate or inhibitor | H2O2 production rate |
|---|---|
| nmol of H2O2/min/g of liver wet wt | |
| l-Lactate, 2 mm; pyruvate, 0.3 mm | 49 |
| + Antimycin, 8 μm | 75 |
| + Octanoate, 0.3 mm | 170 |
| + Oleate, 0.1 mm | 66 |
| + Glycolate, 3 mm | 490 |
Genetically Encoded Fluorescent Protein Indicators of H2O2
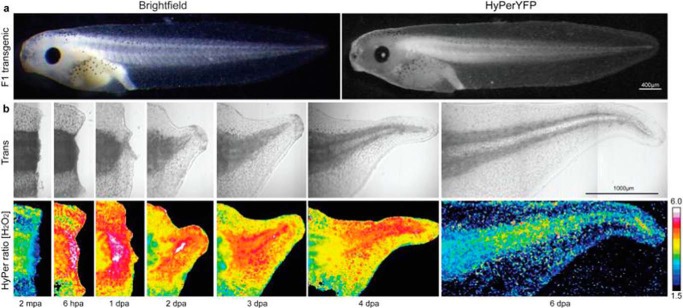
The fluorescent probe HyPer (4) consists of circularly permuted yellow fluorescent protein (cpYFP) inserted into the regulatory domain of the prokaryotic H2O2-sensing protein, OxyR (hydrogen peroxide-inducible gene regulator). An illustration of the type of imaging of H2O2 in intact organisms is given in Fig. 2, where the time course and color intensity ascribed to H2O2 generation in a model of tissue injury and repair as well as proliferation are indicated (13). Several types of redox-sensitive proteins have been developed, as reviewed in Refs. 14 and 15). Major issues concern specificity and sensitivity. Nonetheless, progress in the development of these techniques has enormous potential in noninvasive investigation of physiological and pathophysiological processes. The use of H2O2-generating enzymes fused to HyPer is one such example; the HyPer-d-amino acid oxidase construct enables calibration and intercellular as well as subcellular analysis noninvasively (16).
FIGURE 2.
Production of H2O2 during tadpole tail regeneration. Images on the bottom show the false color representation of [H2O2] at 2 min post amputation (mpa) of the tadpole tail and in hours (hpa) or days (dpa) post amputation. From Love et al. (13), with permission.
“Nonredox” Exogenous Probes
Using boronate-based chemistry (17, 18), an exogenous probe compound is administered to the intact cell or organism that is then to be transformed in vivo to a diagnostic fluorescent compound or an ”exomarker,“ which is analyzable by e.g. mass spectrometry. One such example is the use of the compound, MitoB ((3-hydroxybenzyl)triphenylphosphonium bromide), to infer levels of mitochondrial H2O2 (19). Peroxynitrite can also react with the boronate-based probes. Possibilities and pitfalls in using available methods to detect hydrogen peroxide in living cells were examined (20, 21).
What Are the Metabolic Sources and Sinks of H2O2?
Sources
A major source of hydrogen peroxide comes from the dismutation of the superoxide anion radical, formed by 1-electron reduction of oxygen. Although there is spontaneous dismutation, superoxide dismutases catalyze the reaction. Among several types of superoxide source, NAD(P)H oxidases are prominent, operating under the control of growth factors and cytokines (22). Activated monocytes or macrophages release superoxide (23), and neutrophils and eosinophils utilize oxidants in antibacterial defense (oxidative burst). Important for signaling, other cell types also exhibit controlled release of superoxide, as shown for human dermal fibroblasts treated with the proinflammatory cytokines interleukin-1 or tumor necrosis factor-α (24). Spatial and temporal analysis of NADPH oxidase-generated H2O2 signaling became amenable using novel fluorescence resonance energy transfer (FRET)-based reporter proteins, OxyFRET and PerFRET (25).
Another major cellular source of H2O2 resides in the mitochondria (26). Respiratory chain-linked H2O2 production (27) was attributed to superoxide radicals (28), and the mechanism of mitochondrial superoxide production by the cytochrome bc1 complex (Complex III) has been elucidated (29). It is noteworthy that Complex I is another major source of mitochondrial superoxide production and that the release of superoxide is directed toward the mitochondrial matrix space, whereas Complex III produces it toward the intermembrane space. Transitory reactivation of Complex I is a central pathological feature in ischemia-reperfusion injury. Prevention of this reactivation by modification of a cysteine switch (S-nitrosation of Cys-29 in the ND3 subunit) was shown to be a robust cardioprotective mechanism (30). Mitochondrial Complex II is a further independent source of mitochondrial reactive oxygen species (31). Direct production of H2O2 by enzymatic sources occurs by a number of oxidases, many of which operate in specific cell types and in specific subcellular compartments, such as xanthine oxidase, monoamine oxidases, or d-amino acid oxidase, to name a few (32).
Sinks
Metabolic sinks of H2O2 include the catalatic reaction, carried out by catalase, as well as the various peroxidatic reactions, performed as well by catalase, but importantly also by numerous peroxidases. Furthermore, in organs, the diffusion of H2O2 away from its source, even across membranes to the extracellular space or to other cells, is a possibility. The catalatic reaction, i.e. the dismutation of H2O2 to H2O and O2, may be regarded as a safety valve, occurring at higher ranges of H2O2 concentration, e.g. under toxic conditions. Catalase can also reduce H2O2 in the presence of metabolic hydrogen donors in the peroxidatic reaction (33). As shown in Fig. 1, external hydrogen donors such as methanol can be used to ”titrate“ catalase Compound I (8, 9). Peroxidases reduce H2O2 in usually highly specialized reactions. Although the flux in these peroxidase reactions may be low, their metabolic significance is considerable, in view of temporal and spatial regulation (see below).
Peroxidases of various nature are susceptible to regulation by metabolic signals. A foremost example emerged with the discovery of the peroxiredoxins (34), as reviewed (35). The 106-fold higher rate constant of the reaction of H2O2 with the cysteine thiolate (Cp) in peroxiredoxins as compared with most other deprotonated thiols (36–38) makes for a special role. Thus, under normal cellular conditions, eukaryotic peroxiredoxins were predicted to be responsible for the reduction of up to 90% of mitochondrial H2O2 and even more than that of cytosolic H2O2 (39, 40). On the other hand, cysteine residues in peroxiredoxins can become hyperoxidized to cysteine sulfinic acid, which results in an inactivation of the peroxidase. This is crucial for the sensitivity in H2O2 redox signaling. As a result, there is a subsequent local buildup of H2O2, allowing the oxidation of specific target proteins, likened to the opening of a “floodgate” (41). The functional loop is closed by sulfiredoxins, which reduce the hyperoxidized peroxiredoxins (Fig. 3) (42, 43).
FIGURE 3.
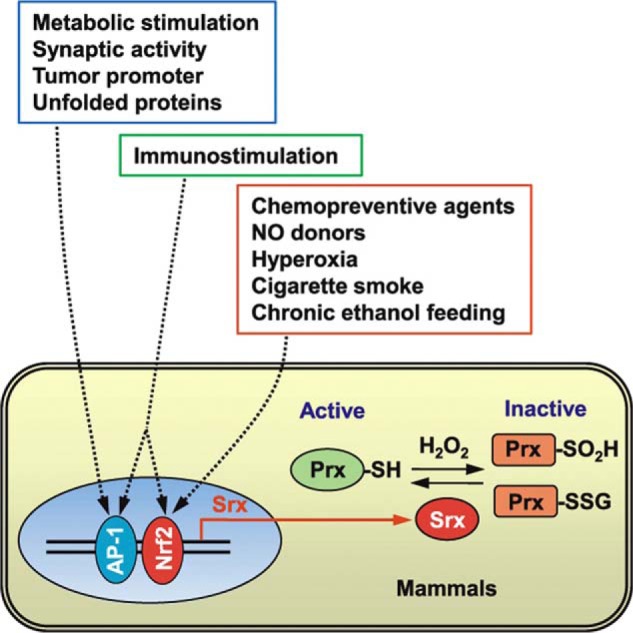
Role of sulfiredoxin (Srx) as a regulator of peroxiredoxin (Prx) function and regulation of its expression. Relationship to external stimuli is also shown. From Jeong et al. (43), with permission.
Glutathione peroxidases in various subcellular compartments and cell types have a major function in the control of H2O2 and of other hydroperoxides (see Refs. 44 and 45). Glutathione disulfide reductase activity allows for maintenance of flux, and GSSG efflux from cells is another option. Using external H2O2 as challenge, the rate of GSSG efflux from liver, for example, was 3 nmol of GSSG/min/gram of wet weight at a steady-state rate of H2O2 infusion of 100 nmol/min/gram of wet weight (46).
H2O2 Compartmentation
As discussed above, the local concentration of H2O2 is governed by the control of its generation and of its removal. Concerning removal, the diffusion of this uncharged molecule away from the site of generation and across biomembranes leads to H2O2 gradients (47). High capacity of removal, e.g. by catalase in the peroxisomes, will generate intracellular gradients. Importantly, the local activity of peroxiredoxins near signaling sites, e.g. caveolae areas of the plasma membrane, will govern steady-state concentrations. Use of techniques for cell culture studies with the glucose oxidase/catalase system (48) yielded the insight that the peroxiredoxin-2 dimer-to-monomer ratio is suitable to follow the H2O2 steady-state concentration down to physiological levels (49).
Aquaporins as Peroxiporins
H2O2, a molecule with chemical and physicochemical properties close to those of H2O, was shown to use water channels, the aquaporins, to cross the cell membrane more rapidly than by simple diffusion (50). This discovery opened an exciting field on membrane transport of hydrogen peroxide (51). Specific aquaporins facilitate the diffusion of H2O2 across membranes, which is why they are also referred to as peroxiporins (52). Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells caused loss of viability (53). Silencing of aquaporin-8 inhibited H2O2 entry into HeLa cells (54). Aquaporin-3 was shown to mediate H2O2 uptake to regulate downstream signaling (55). There are multiple interactions of aquaporins and H2O2 in cells, both at the intracellular-extracellular spaces, but also within subcellular compartments (56). Aquaporin-8 is able to modulate Nox (NAD(P)H oxidase)-produced H2O2 transport through the plasma membrane in leukemia cells (57), an interesting aspect for potential therapeutic strategies addressing H2O2 transport.
What Is the Role of H2O2 in Redox Signaling and Oxidative Stress?
Mechanism
The oxidative modification of amino acid side chains in proteins by H2O2 involves, in decreasing order of reactivity and biological reversibility, cysteine, methionine, proline, histidine, and tryptophan (see Ref. 58). Thiol modification is key in H2O2 sensing and perception in proteins (59). Transmission of a redox signal to protein thiols initiated by H2O2 can occur in several ways (see Ref. 37): (i) by direct oxidation of a target protein, (ii) by oxidation via a highly reactive sensor protein, (iii) by activation of a target protein upon dissociation of an oxidized inhibitor, (iv) by oxidation of a target protein via a secondary product generated through e.g. thioredoxin, (v) by inactivation of a scavenging protein such as peroxiredoxin to allow the oxidation of the target protein (floodgate model, see Ref. 41 above), and (vi) by association of the target protein with an H2O2-generating protein to allow site-directed oxidation. In addition to direct oxidation, protein glutathionylation and other modifications can occur and serve in redox signaling.
Targets
Insulin signaling was probably the first transduction chain in which H2O2 was invoked as a second messenger (60); H2O2 was called an “insulinomimetic” (61). Growth factors such as platelet-derived growth factor (PDGF) (62), through H2O2 production, induce downstream effects on tyrosine phosphorylation, as do other important growth factors such as epidermal growth factor (EGF) (63), fibroblast growth factor (FGF) (64), or vascular endothelial growth factor (VEGF) (65). A major mechanism is the inactivation of protein phosphatases by H2O2, thereby increasing the level of protein phosphorylation. Also, direct modification of the EGF receptor by H2O2 at a critical active site cysteine (Cys-797) was shown to enhance tyrosine kinase activity (66).
Regarding nonreceptor kinases, signal-mediated H2O2 production increases Akt (also known as protein kinase B (PKB)) activation (67). Another group of serine/threonine kinases, the MAP kinases, mediate redox modulation of Erk1/2, JNK, and p38. As comprehensively reviewed in Ref. 68, many studies documented H2O2-induced activation of MAPK pathways, and the redox-based inactivation of upstream components also serves to modulate MAPK signal duration. Critical thiols are centrally involved in activation of essential switches in defense reactions, namely in the NF-κB (69) and Nrf2/Keap1 (70) systems, important in chemoprevention and cytoprotection (71). The nature of targets extends from the specific ones mentioned above to reactive cysteines in general, a wide open field of research on sulfur switches, governing the set point in the protein-cysteine redox proteome (72–74).
Processes
The functional consequences of H2O2 signaling concern fundamental biological processes. The role of mitochondrial H2O2 was recently discussed (75) for hypoxia, inflammation, apoptosis, and autophagy. Concepts of the inflammasome (76) and redoxosome (77) have evolved. In wound healing, H2O2 signaling has been established as a prominent early feature (1, 78, 79), shown for the wound healing/proliferation model in Fig. 2. H2O2 acts as a chemoattractant (78, 80). New horizons have been opened in understanding the intricate relationships of reactive oxygen species in immunology (81).
Much has to be learned for better understanding the role of redox signaling in metabolism, in insulin signaling in particular (82). Although reactive oxygen species enhance insulin signaling (83), excessive levels may cause diabetic complications, so that these opposing actions constitute a “peroxide dilemma” (84, 85).
The current perception of the aging process includes a role of metabolic alterations such as dysregulated nutrient sensing and mitochondrial dysfunction, all of which encompass alterations in H2O2 signaling. Intracellular H2O2 concentration in skeletal muscle rises by about 100 nm during contractions (86). This response is weakened in aging, which may contribute to age-related loss of muscle mass and to frailty (86). An interesting aspect of redox regulation in aging is the cellular polarity, mediated by the activity of AMP-activated protein kinase (AMPK) in controlling the cytoskeleton (87). Peroxiredoxins are conserved markers of circadian rhythm (88), and chronobiological research has revealed a tight coupling of redox reactions to circadian rhythmicity (89).
Oxidative Stress
The initial concept of oxidative stress focused on the damage of biomolecules such as DNA, lipids, and proteins (58). It was extended to include the emerging role of biologically generated oxidants in redox signaling (90): “Oxidative stress is an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage.” With the recognition of the role of low level oxidant stimuli for altering the set point of gene expression for batteries of enzymes, known as hormesis (91), physiological oxidative stress came into focus on a spatial and temporal dimension. Tissue-scale gradients and regional specificity are being identified (78, 92).
Concluding Remarks
Retrospective
The occurrence of H2O2 in normal aerobic metabolism was heavily contested in the early days of research in bioenergetics, with the quote from the 1920s in the Warburg-Wieland dispute “that even after killing a whole dog there was not one drop of H2O2 detectable.” In addition, Keilin and Hartree in 1945 (33) stated: “Contrary to the view that H2O2 is generally formed in cells and tissues during respiratory processes are the following two facts … ” and Britton Chance in 1951 (93) concluded: “Quantitative evidence for the existence of significant amounts of … H2O2 … in tissue is lacking, since catalase, by virtue of its peculiar capacity for catalatic reactions literally ‘destroys the evidence’ of free hydrogen peroxide in the cell.” It was not until the continuous detection of catalase Compound I in intact tissue under steady-state conditions that H2O2 production was proven in 1970 (6). It might be appropriate to quote the final sentence in the review on hydroperoxide metabolism in mammalian organs from 1979 (10): “Finally, recent understanding of the beneficial action of H2O2 in phagocytosis and in ethanol oxidation suggests caution in condemning any metabolite as useless until its functions in toto are thoroughly understood.”
Prospective
The advent of novel converging techniques from cell biology, noninvasive imaging for H2O2 detection, and metabolic studies opened a new vista. Hopefully, there will be real-time spatially resolved quantitative monitoring of H2O2 as a versatile and innocuous oxygen metabolite functioning in redox signaling. Appropriate control is provided by the powerful generators, scavengers, and switches discussed above. H2O2 serves as a central hub for information flow in plant cells as well (94), and there is indication that waves of H2O2 transmit information in plant cells (95). At present, it still appears puzzling how local fine-tuning is orchestrated in the simultaneous presence of a multitude of potential reactants. Shaping the microenvironment for the recruitment of target proteins to the site of H2O2 production, and vice versa, is one of the strategies. A concept has been proposed (96) of ”redox optimization“ between mitochondrial respiration and formation of reactive oxygen species. More refinement of methodology for noninvasive detection of H2O2 production by cellular NADPH oxidases is required (97). The threshold from signaling to excessive toxic levels will be challenging to further identify. The precise transition points for these cellular responses may vary due to cell type and metabolic conditions (see Ref. 2).
Note: This minireview focused on aspects of metabolic H2O2 generation. Xenobiotic and toxicological sources such as in ”redox cycling“ and lipid peroxidation (98) were not considered here. Further, it should be mentioned that redox signaling extends to other large and important sectors, only one example being that of peroxynitrite biology and the field of protein tyrosine nitration (99, 100). It will be another challenging area of research to analyze the cross-talk and interrelationships between different modalities of redox signaling.
Acknowledgments
Fruitful discussions with Dr. Wilhelm Stahl and Dr. Holger Steinbrenner are gratefully acknowledged.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany and by the National Foundation for Cancer Research (NFCR), Bethesda, MD. This minireview forms part of the Trevor Slater Award Lecture at the Society for Free Radical Research International (SFRRI) meeting at Kyoto, Japan, March 23, 2014.
REFERENCES
- 1. Cordeiro J. V., Jacinto A. (2013) The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat. Rev. Mol. Cell Biol. 14, 249–262 [DOI] [PubMed] [Google Scholar]
- 2. Stone J. R., Yang S. (2006) Hydrogen peroxide: a signaling messenger. Antioxid. Redox. Signal. 8, 243–270 [DOI] [PubMed] [Google Scholar]
- 3. Forman H. J., Maiorino M., Ursini F. (2010) Signaling functions of reactive oxygen species. Biochemistry 49, 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belousov V. V., Fradkov A. F., Lukyanov K. A., Staroverov D. B., Shakhbazov K. S., Terskikh A. V., Lukyanov S. (2006) Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 3, 281–286 [DOI] [PubMed] [Google Scholar]
- 5. Warburg O. H. (1928) [On the Catalytic Actions of the Living Substance] Julius Springer, Berlin [Google Scholar]
- 6. Sies H., Chance B. (1970) The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 11, 172–176 [DOI] [PubMed] [Google Scholar]
- 7. Chance B. (1947) An intermediate compound in the catalase-hydrogen peroxide reaction. Acta Chem. Scand. 1, 236–267 [Google Scholar]
- 8. Sies H., Bücher T., Oshino N., Chance B. (1973) Heme occupancy of catalase in hemoglobin-free perfused rat liver and of isolated rat liver catalase. Arch. Biochem. Biophys. 154, 106–116 [DOI] [PubMed] [Google Scholar]
- 9. Oshino N., Chance B., Sies H., Bücher T. (1973) The role of H2O2 generation in perfused rat liver and the reaction of catalase compound I and hydrogen donors. Arch. Biochem. Biophys. 154, 117–131 [DOI] [PubMed] [Google Scholar]
- 10. Chance B., Sies H., Boveris A. (1979) Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59, 527–605 [DOI] [PubMed] [Google Scholar]
- 11. Oshino N., Jamieson D., Sugano T., Chance B. (1975) Optical measurement of the catalase-hydrogen peroxide intermediate (Compound I) in the liver of anaesthetized rats and its implication to hydrogen peroxide production in situ. Biochem. J. 146, 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. St-Pierre J., Buckingham J. A., Roebuck S. J., Brand M. D. (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 277, 44784–44790 [DOI] [PubMed] [Google Scholar]
- 13. Love N. R., Chen Y., Ishibashi S., Kritsiligkou P., Lea R., Koh Y., Gallop J. L., Dorey K., Amaya E. (2013) Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 15, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lukyanov K. A., Belousov V. V. (2014) Genetically encoded fluorescent redox sensors. Biochim. Biophys. Acta 1840, 745–750 [DOI] [PubMed] [Google Scholar]
- 15. Ezerina D., Morgan B., Dick T. P. (2014) Imaging dynamic redox processes with genetically encoded probes. J. Mol. Cell. Cardiol. 10.1016/j.yjmcc.2013.12.023 [DOI] [PubMed] [Google Scholar]
- 16. Matlashov M. E., Belousov V. V., Enikolopov G. (2014) How much H2O2 is produced by recombinant D-amino acid oxidase in mammalian cells? Antioxid. Redox Signal. 20, 1039–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin V. S., Dickinson B. C., Chang C. J. (2013) Boronate-based fluorescent probes: imaging hydrogen peroxide in living systems. Methods Enzymol. 526, 19–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zielonka J., Sikora A., Hardy M., Joseph J., Dranka B. P., Kalyanaraman B. (2012) Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem. Res. Toxicol. 25, 1793–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Logan A., Cochemé H. M., Li Pun P. B., Apostolova N., Smith R. A., Larsen L., Larsen D. S., James A. M., Fearnley I. M., Rogatti S., Prime T. A., Finichiu P. G., Dare A., Chouchani E. T., Pell V. R., Methner C., Quin C., McQuaker S. J., Krieg T., Hartley R. C., Murphy M. P. (2014) Using exomarkers to assess mitochondrial reactive species in vivo. Biochim. Biophys. Acta 1840, 923–930 [DOI] [PubMed] [Google Scholar]
- 20. Grisham M. B. (2013) Methods to detect hydrogen peroxide in living cells: Possibilities and pitfalls. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 165, 429–438 [DOI] [PubMed] [Google Scholar]
- 21. Winterbourn C. (2014) The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Biophys. Acta 1840, 730–738 [DOI] [PubMed] [Google Scholar]
- 22. Griendling K. K., Sorescu D., Ushio-Fukai M. (2000) NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 86, 494–501 [DOI] [PubMed] [Google Scholar]
- 23. Babior B. M., Kipnes R. S., Curnutte J. T. (1973) Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 52, 741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. (1989) Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem. J. 263, 539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Enyedi B., Zana M., Donkó Á., Geiszt M. (2013) Spatial and temporal analysis of NADPH oxidase-generated hydrogen peroxide signals by novel fluorescent reporter proteins. Antioxid. Redox Signal. 19, 523–534 [DOI] [PubMed] [Google Scholar]
- 26. Boveris A., Oshino N., Chance B. (1972) The cellular production of hydrogen peroxide. Biochem. J. 128, 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loschen G., Flohé L., Chance B. (1971) Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Lett. 18, 261–264 [DOI] [PubMed] [Google Scholar]
- 28. Loschen G., Azzi A., Richter C., Flohé L. (1974) Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 42, 68–72 [DOI] [PubMed] [Google Scholar]
- 29. Dröse S., Brandt U. (2008) The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J. Biol. Chem. 283, 21649–21654 [DOI] [PubMed] [Google Scholar]
- 30. Chouchani E. T., Methner C., Nadtochiy S. M., Logan A., Pell V. R., Ding S., James A. M., Cochemé H. M., Reinhold J., Lilley K. S., Partridge L., Fearnley I. M., Robinson A. J., Hartley R. C., Smith R. A., Krieg T., Brookes P. S., Murphy M. P. (2013) Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 19, 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siebels I., Dröse S. (2013) Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates. Biochim. Biophys. Acta 1827, 1156–1164 [DOI] [PubMed] [Google Scholar]
- 32. Sies H. (1974) Biochemistry of the peroxisome in the liver cell. Angew. Chem. Int. Ed. Engl. 13, 706–718 [DOI] [PubMed] [Google Scholar]
- 33. Keilin D., Hartree E. F. (1945) Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem. J. 39, 293–301 [PMC free article] [PubMed] [Google Scholar]
- 34. Kim K., Kim I. H., Lee K. Y., Rhee S. G., Stadtman E. R. (1988) The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J. Biol. Chem. 263, 4704–4711 [PubMed] [Google Scholar]
- 35. Rhee S. G., Woo H. A., Kil I. S., Bae S. H. (2012) Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem. 287, 4403–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flohé L. (2010) Changing paradigms in thiology: from antioxidant defense toward redox regulation. Methods Enzymol. 473, 1–39 [DOI] [PubMed] [Google Scholar]
- 37. Winterbourn C. C. (2013) The biological chemistry of hydrogen peroxide. Methods Enzymol. 528, 3–25 [DOI] [PubMed] [Google Scholar]
- 38. Hall A., Nelson K., Poole L. B., Karplus P. A. (2011) Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid. Redox Signal. 15, 795–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cox A. G., Winterbourn C. C., Hampton M. B. (2010) Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 425, 313–325 [DOI] [PubMed] [Google Scholar]
- 40. Winterbourn C. C. (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 [DOI] [PubMed] [Google Scholar]
- 41. Wood Z. A., Poole L. B., Karplus P. A. (2003) Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300, 650–653 [DOI] [PubMed] [Google Scholar]
- 42. Biteau B., Labarre J., Toledano M. B. (2003) ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425, 980–984 [DOI] [PubMed] [Google Scholar]
- 43. Jeong W., Bae S. H., Toledano M. B., Rhee S. G. (2012) Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression. Free Radic. Biol. Med. 53, 447–456 [DOI] [PubMed] [Google Scholar]
- 44. Brigelius-Flohé R., Maiorino M. (2013) Glutathione peroxidases. Biochim. Biophys. Acta 1830, 3289–3303 [DOI] [PubMed] [Google Scholar]
- 45. Sies H. (1999) Glutathione and its role in cellular functions. Free Radic. Biol. Med. 27, 916–921 [DOI] [PubMed] [Google Scholar]
- 46. Sies H., Summer K. H. (1975) Hydroperoxide-metabolizing systems in rat liver. Eur. J. Biochem. 57, 503–512 [DOI] [PubMed] [Google Scholar]
- 47. Antunes F., Cadenas E. (2000) Estimation of H2O2 gradients across biomembranes. FEBS Lett. 475, 121–126 [DOI] [PubMed] [Google Scholar]
- 48. Mueller S., Millonig G., Waite G. N. (2009) The GOX/CAT system: a novel enzymatic method to independently control hydrogen peroxide and hypoxia in cell culture. Adv. Med. Sci. 54, 121–135 [DOI] [PubMed] [Google Scholar]
- 49. Sobotta M. C., Barata A. G., Schmidt U., Mueller S., Millonig G., Dick T. P. (2013) Exposing cells to H2O2: a quantitative comparison between continuous low-dose and one-time high-dose treatments. Free Radic. Biol. Med. 60, 325–335 [DOI] [PubMed] [Google Scholar]
- 50. Henzler T., Steudle E. (2000) Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J. Exp. Bot. 51, 2053–2066 [DOI] [PubMed] [Google Scholar]
- 51. Bienert G. P., Schjoerring J. K., Jahn T. P. (2006) Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta. 1758, 994–1003 [DOI] [PubMed] [Google Scholar]
- 52. Bienert G. P., Møller A. L., Kristiansen K. A., Schulz A., Møller I. M., Schjoerring J. K., Jahn T. P. (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192 [DOI] [PubMed] [Google Scholar]
- 53. Marchissio M. J., Francés D. E., Carnovale C. E., Marinelli R. A. (2012) Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicol. Appl. Pharmacol. 264, 246–254 [DOI] [PubMed] [Google Scholar]
- 54. Bertolotti M., Bestetti S., García-Manteiga J. M., Medraño-Fernandez I., Dal Mas A., Malosio M. L., Sitia R. (2013) Tyrosine kinase signal modulation: a matter of H2O2 membrane permeability? Antioxid. Redox Signal. 19, 1447–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miller E. W., Dickinson B. C., Chang C. J. (2010) Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. U.S.A. 107, 15681–15686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bienert G. P., Chaumont F. (2013) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 10.1016/j.bbagen.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 57. Vieceli Dalla Sega F., Zambonin L., Fiorentini D., Rizzo B., Caliceti C., Landi L., Hrelia S., Prata C. (2014) Specific aquaporins facilitate Nox-produced hydrogen peroxide transport through plasma membrane in leukaemia cells. Biochim. Biophys. Acta 1843, 806–814 [DOI] [PubMed] [Google Scholar]
- 58. Sies H. (1986) Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engl. 25, 1058–1071 [Google Scholar]
- 59. Hancock J., Desikan R., Harrison J., Bright J., Hooley R., Neill S. (2006) Doing the unexpected: proteins involved in hydrogen peroxide perception. J. Exp. Bot. 57, 1711–1718 [DOI] [PubMed] [Google Scholar]
- 60. May J. M., de Haen C. (1979) Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J. Biol. Chem. 254, 2214–2220 [PubMed] [Google Scholar]
- 61. Heffetz D., Bushkin I., Dror R., Zick Y. (1990) The insulinomimetic agents H2O2 and vanadate stimulate protein tyrosine phosphorylation in intact cells. J. Biol. Chem. 265, 2896–2902 [PubMed] [Google Scholar]
- 62. Sundaresan M., Yu Z. X., Ferrans V. J., Irani K., Finkel T. (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 270, 296–299 [DOI] [PubMed] [Google Scholar]
- 63. Bae Y. S., Kang S. W., Seo M. S., Baines I. C., Tekle E., Chock P. B., Rhee S. G. (1997) Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 217–221 [PubMed] [Google Scholar]
- 64. Lo Y. Y., Cruz T. F. (1995) Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J. Biol. Chem. 270, 11727–11730 [DOI] [PubMed] [Google Scholar]
- 65. Ushio-Fukai M., Tang Y., Fukai T., Dikalov S. I., Ma Y., Fujimoto M., Quinn M. T., Pagano P. J., Johnson C., Alexander R. W. (2002) Novel role of gp91phox-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 91, 1160–1167 [DOI] [PubMed] [Google Scholar]
- 66. Paulsen C. E., Truong T. H., Garcia F. J., Homann A., Gupta V., Leonard S. E., Carroll K. S. (2012) Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 8, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ushio-Fukai M., Alexander R. W., Akers M., Yin Q., Fujio Y., Walsh K., Griendling K. K. (1999) Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 274, 22699–22704 [DOI] [PubMed] [Google Scholar]
- 68. Truong T. H., Carroll K. S. (2013) Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 48, 332–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kapahi P., Takahashi T., Natoli G., Adams S. R., Chen Y., Tsien R. Y., Karin M. (2000) Inhibition of NF-κB activation by arsenite through reaction with a critical cysteine in the activation loop of IκB kinase. J. Biol. Chem. 275, 36062–36066 [DOI] [PubMed] [Google Scholar]
- 70. Covas G., Marinho H. S., Cyrne L., Antunes F. (2013) Activation of Nrf2 by H2O2: de novo synthesis versus nuclear translocation. Methods Enzymol. 528, 157–171 [DOI] [PubMed] [Google Scholar]
- 71. Brigelius-Flohé R., Flohé L. (2011) Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 15, 2335–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cremers C. M., Jakob U. (2013) Oxidant sensing by reversible disulfide bond formation. J. Biol. Chem. 288, 26489–26496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Go Y. M., Jones D. P. (2013) The redox proteome. J. Biol. Chem. 288, 26512–26520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jacob C., Giles G. I., Giles N. M., Sies H. (2003) Sulfur and selenium: the role of oxidation state in protein structure and function. Angew. Chem. Int. Ed. Engl. 42, 4742–4758 [DOI] [PubMed] [Google Scholar]
- 75. Finkel T. (2012) Signal transduction by mitochondrial oxidants. J. Biol. Chem. 287, 4434–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tschopp J., Schroder K. (2010) NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 [DOI] [PubMed] [Google Scholar]
- 77. Oakley F. D., Abbott D., Li Q., Engelhardt J. F. (2009) Signaling components of redox active endosomes: the redoxosomes. Antioxid. Redox Signal. 11, 1313–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Niethammer P., Grabher C., Look A. T., Mitchison T. J. (2009) A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sen C. K., Roy S. (2008) Redox signals in wound healing. Biochim. Biophys. Acta 1780, 1348–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Enyedi B., Niethammer P. (2013) H2O2: a chemoattractant? Methods Enzymol. 528, 237–255 [DOI] [PubMed] [Google Scholar]
- 81. Nathan C., Cunningham-Bussel A. (2013) Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 13, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tiganis T. (2011) Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol. Sci. 32, 82–89 [DOI] [PubMed] [Google Scholar]
- 83. Loh K., Deng H., Fukushima A., Cai X., Boivin B., Galic S., Bruce C., Shields B. J., Skiba B., Ooms L. M., Stepto N., Wu B., Mitchell C. A., Tonks N. K., Watt M. J., Febbraio M. A., Crack P. J., Andrikopoulos S., Tiganis T. (2009) Reactive oxygen species enhance insulin sensitivity. Cell Metab. 10, 260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Steinbrenner H. (2013) Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic. Biol. Med. 65, 1538–1547 [DOI] [PubMed] [Google Scholar]
- 85. Szypowska A. A., Burgering B. M. (2011) The peroxide dilemma: opposing and mediating insulin action. Antioxid. Redox Signal. 15, 219–232 [DOI] [PubMed] [Google Scholar]
- 86. Jackson M. (2011) Control of reactive oxygen species production in contracting skeletal muscle. Antioxid. Redox Signal. 15, 2477–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Soares H., Marinho H. S., Real C., Antunes F. (2014) Cellular polarity in aging: role of redox regulation and nutrition. Genes Nutr. 9, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Edgar R. S., Green E. W., Zhao Y., van Ooijen G., Olmedo M., Qin X., Xu Y., Pan M., Valekunja U. K., Feeney K. A., Maywood E. S., Hastings M. H., Baliga N. S., Merrow M., Millar A. J., Johnson C. H., Kyriacou C. P., O'Neill J. S., Reddy A. B. (2012) Peroxiredoxins are conserved markers of circadian rhythms. Nature 485, 459–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bass J. (2012) Circadian topology of metabolism. Nature 491, 348–356 [DOI] [PubMed] [Google Scholar]
- 90. Jones D., Sies H. (2007) Oxidative stress. In: Encyclopedia of Stress (Fink G., ed) Vol. 3, pp. 45–48, Academic Press, San Diego [Google Scholar]
- 91. Calabrese E. J., Baldwin L. A. (2003) Toxicology rethinks its central belief. Nature 421, 691–692 [DOI] [PubMed] [Google Scholar]
- 92. Albrecht S. C., Barata A. G., Grosshans J., Teleman A. A., Dick T. P. (2011) In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 14, 819–829 [DOI] [PubMed] [Google Scholar]
- 93. Chance B. (1951) Enzyme-substrate compounds. Adv. Enzymol. Relat. Subj. Biochem. 12, 153–190 [DOI] [PubMed] [Google Scholar]
- 94. Petrov V. D., Van Breusegem F. (2012) Hydrogen peroxide-a central hub for information flow in plant cells. AoB Plants 2012, pls014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vestergaard C. L., Flyvbjerg H., Møller I. M. (2012) Intracellular signaling by diffusion: can waves of hydrogen peroxide transmit intracellular information in plant cells? Front. Plant Sci. 3, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cortassa S., O'Rourke B., Aon M. A. (2014) Redox-optimized ROS balance and the relationship between mitochondrial respiration and ROS. Biochim. Biophys. Acta 1837, 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nauseef W. M. (2014) Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim. Biophys. Acta 1840, 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kappus H., Sies H. (1981) Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia 37, 1233–1241 [DOI] [PubMed] [Google Scholar]
- 99. Radi R. (2013) Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 46, 550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ullrich V., Kissner R. (2006) Redox signaling: bioinorganic chemistry at its best. J. Inorg. Biochem. 100, 2079–2086 [DOI] [PubMed] [Google Scholar]