Background: AgtA mediates terminal α-galactosylation of Skp1, but genetic studies show an unexpectedly profound role in oxygen sensing.
Results: AgtA exhibits salt-sensitive Skp1-binding activity that competes with other Skp1 interactions but subsequently activates α-galactosylation that alters Skp1 binding. AgtA regulates development through non-enzymatic mechanisms.
Conclusion: AgtA modulates Skp1 interactions by a self-limiting glycosylation mechanism.
Significance: Polypeptide recognition by a glycosyltransferase mediates non-enzymatic functions.
Keywords: Development, Dictyostelium, E3 Ubiquitin Ligase, Glycosylation, Glycosyltransferases, Skp1, WD40 Repeat, Cellular Slime Mold, Oxygen Sensing, Prolyl Hydroxylation
Abstract
The role of Skp1 as an adaptor protein that links Cullin-1 to F-box proteins in E3 Skp1/Cullin-1/F-box protein (SCF) ubiquitin ligases is well characterized. In the social amoeba Dictyostelium and probably many other unicellular eukaryotes, Skp1 is modified by a pentasaccharide attached to a hydroxyproline near its C terminus. This modification is important for oxygen-sensing during Dictyostelium development and is mediated by a HIF-α type prolyl 4-hydroxylase and five sequentially acting cytoplasmic glycosyltransferase activities. Gene disruption studies show that AgtA, the enzyme responsible for addition of the final two galactose residues, in α-linkages to the Skp1 core trisaccharide, is unexpectedly critical for oxygen-dependent terminal development. AgtA possesses a WD40 repeat domain C-terminal to its single catalytic domain and, by use of domain deletions, binding studies, and enzyme assays, we find that the WD40 repeats confer a salt-sensitive second-site binding interaction with Skp1 that mediates novel catalytic activation in addition to simple substrate recognition. In addition, AgtA binds similarly well to precursor isoforms of Skp1 by a salt-sensitive mechanism that competes with binding to an F-box protein and recognition by early modification enzymes, and the effect of binding is diminished when AgtA modifies Skp1. Genetic studies show that loss of AgtA is more severe when an earlier glycosylation step is blocked, and overexpressed AgtA is deleterious if catalytically inactivated. Together, the findings suggest that AgtA mediates non-enzymatic control of unmodified and substrate precursor forms of Skp1 by a binding mechanism that is normally relieved by switch-like activation of its glycosylation function.
Introduction
Skp1, an adaptor protein for the Skp1/Cullin-1/F-box protein (SCF)3 class of E3 ubiquitin ligases (1), is modified by a novel pentasaccharide attached to a hydroxyproline residue near its C terminus in the social amoebae Dictyostelium discoideum and likely other protists (2–4). AgtA was previously characterized as the α-galactosyltransferase catalyzing addition of the 4th sugar, resulting in formation of Galα1,3Fucα1,2Galβ1,3GlcNAcα1–4O-Pro-Skp1 (5). Each of the enzymes required to build the trisaccharide form of Skp1 that serves as the AgtA substrate are known (Fig. 1A) (2). Recent studies have indicated that AgtA also mediates the addition of the final sugar, another α-linked Gal residue, to complete the glycan.4
FIGURE 1.
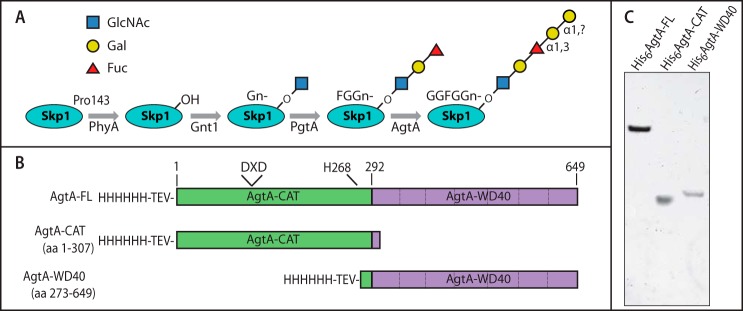
Skp1 modification pathway and AgtA expression constructs. A, schematic of the Skp1-hydroxylation/glycosylation pathway in Dictyostelium. The modification occurs on Pro-143 near the C terminus, and is initiated by the activity of the prolyl hydroxylase PhyA. Five glycosyltransferase reactions mediated by 3 proteins follow sequentially to yield a Hyp-linked linear pentasaccharide. B, diagram of full-length His6AgtA and the His6-tagged domain constructs expressed in E. coli and Dictyostelium. The N-terminal catalytic domain construct (His6AgtA-CAT) terminates 33 amino acids after the end of sequence similarity (codon 274) to other CAZy GT77 sequences (2), and the C-terminal expression construct (His6AgtA-WD40) initiates 19 amino acids upstream of this boundary. The TEV protease site inserted after the His6 tag, the candidate canonical DXD motif, and the conserved His-268, are indicated. C, purified His6AgtA-FL, His6AgtA-CAT, and His6AgtA-WD40 were analyzed by SDS-PAGE and staining with Coomassie Blue for total protein.
AgtA regulates the execution of a developmental process in Dictyostelium known as culmination. Culmination refers to the transition in the starvation-induced developmental program in which the migrating slug reorganizes into a fruiting body consisting of dormant spores perched atop a cellular stalk. Environmental O2 is one of several regulators of culmination, and substantial evidence indicates that the Skp1 prolyl 4-hydroxylase, an O2-dependent enzyme required for formation of the 4-hydroxyproline to which the glycan is attached, is an O2-sensor for culmination. Further genetic analysis has revealed that PgtA, a bifunctional diglycosyltransferase that catalyzes addition of the second and third sugars, and AgtA, modulate the response to Skp1 prolyl hydroxylation (see pathway scheme in Fig. 1A). Remarkably, the effect of interrupting the AgtA locus is nearly as severe as blockade of hydroxylation itself as opposed to disruption of PgtA, which shows only a modest effect (6). Because biochemical and genetic evidence indicates that Skp1 is the only protein substrate of the hydroxylation/glycosylation pathway (2, 4, 7, 8), modification of Skp1 by PgtA to convert the monosaccharide to the trisaccharide was proposed to reverse the effect of hydroxylation, and terminal α-galactosylation by AgtA was proposed to liberate Skp1 from this inhibition (2). However, the mechanism by which AgtA regulates Skp1 function remains unclear because the protein contains, in addition to its N-terminal catalytic domain, a C-terminal sequence of 7 predicted WD40 repeats, expected to fold into a β-propeller (5, 9, 10). Furthermore, the initial purification of AgtA from Dictyostelium suggested partial co-purification with Skp1 and similar levels of expression, which is unusual for an enzyme/substrate pair (11). Although the overexpressed catalytic domain rescued Skp1 α-galactosylation and O2-sensing in the agtA deletion strain (6), the high level of overexpressed protein might mask a critical function of the WD40 repeats, which represent one of the most common protein domains in nature and often mediate protein-protein interactions (9, 12).
Most eukaryotic GTs are type 2 membrane proteins consisting of a single catalytic domain connected by a linker region to an N-terminal transmembrane domain and a short cytoplasmic extension (13), but AgtA and the other Skp1 GTs lack these domains and exist as soluble cytoplasmic proteins. Although the WD40 repeats of AgtA are unprecedented for known GTs, other GTs possess SH3 (14), TPR (15), and lectin (16) domains. In some instances, these so-called “add-on” domains (17) have been implicated in acceptor substrate recognition, as has the linker region of a type 2 membrane GT (18). Thus the AgtA C-terminal domain may contribute to Skp1 recognition, although it is noted that the earlier GTs in the pathway, the αGlcNAcT (Gnt1) and PgtA (see Fig. 1A), do not rely on add-on domains to achieve exquisite selectivity for Skp1 in vitro (7) and in the cell (4, 6, 8). Here we investigate the functionality of the AgtA WD40 repeat domain in the recognition of Skp1 by AgtA. Our investigations show that the modification by AgtA of Skp1 depends on the WD40 repeats by a mechanism that not only involves second site recognition but novel catalytic activation. Furthermore, Skp1 recognition by AgtA is competitive in vitro with F-box protein (FBP) binding required for SCF complex assembly, and interferes with early Skp1 processing steps in cells. The importance of a non-enzymatic function of AgtA is supported by the blockade of culmination that occurs in a double knock-out with the first Skp1 GT Gnt1 in which the substrate form of Skp1 is not assembled. The findings are interpreted in terms of a binding activity that titrates the interaction of Skp1 with FBPs and other binding partners and is relieved by execution of its glycosylation function.
EXPERIMENTAL PROCEDURES
Dictyostelium Growth and Development
Cells were grown axenically in HL-5 medium, and assayed for O2-dependent development as described previously (19). Briefly, cells were deposited on filters in PDF buffer and developed in the dark for 42 h under the indicated O2 concentrations with the balance made up with N2. Development was assessed by fruiting body morphology, and quantification of total spore numbers as counted in a hemacytometer. Stalk morphology was imaged in the DAPI channel of an epifluorescence microscope in the presence of Calcofluor White ST to reveal cellulosic cell walls (19). Cell proliferation was assayed by counting cells during logarithmic growth in HL-5 medium by daily counting in a hemacytometer.
Dictyostelium Strains
Normal (Ax3), agtA− (HW420), and gnt1− (gnt1.3/HW503) strains were described previously (4). The gnt1−/agtA− double disruption strain HW520 was generated by electroporation of gnt1.3-disruption DNA into agtA− HW420 as previously described (4). Disruption of gnt1 was assessed by assaying Gnt1 activity in extracts, and Skp1 isoform accumulation in cells based on Western blotting with Skp1 isoform-specific Abs (4). Complementation was performed by transfer of DpGnt1 cDNA from a pVSE into the pVSC expression plasmid as described previously (4), and electroporation into strain HW520 and selection in the presence of 10 μg/ml of G418 to yield HW522. The plasmid for catalytically inactive mutant DpGnt1(D102A) was derived in the same manner (4) and introduced into HW520 to yield HW524. These pVSC-derived plasmids are designed to stably overexpress DpGnt1 (the ortholog from D. purpureum) under control of the prespore cell-specific cotB promoter.
Overexpression of His6AgtA-FL in agtA− strain HW420 was achieved by electroporation of pVS(His)agtA (5) and selection of clones in the presence of G418, yielding strain HW530. The corresponding plasmid encoding catalytically inactive AgtA(D132A) was constructed as described below, and used to generate HW532. These pVS-derived plasmids are designed to stably overexpress His6AgtA under control of the semi-constitutive discoidin γ promoter.
Preparation and Purification of Recombinant AgtA and AgtA Domains
The protein coding sequences for AgtA-FL (amino acids 2–649) was excerpted from pCR4TOPOcagtA (5) using NheI and BamHI, and ligated into pET15b-TEV predigested with the same restriction enzymes, resulting in a coding sequence preceded by an N-terminal His6 tag and TEV protease cleavage site. The D132A mutation was generated by site-directed mutagenesis, using mutagenesis primers 5′-tggactgctactgatatcgtttggaaaagagatccattcattc and 5′-tttccaaacgatatcagtagcagtccataatacattataacc. The H268A mutation was generated similarly using 5′-ccattcattattgctaacaattgtataattggtcatagaagt and 5′-ccaattatacaattgttagcaataatgaatggtgtaatattatctct. The coding sequence for the N-terminal catalytic domain (amino acids 2–307) was excised from pVS(His)NagtA (5) using NheI and BamHI and similarly ligated into pET15b-TEV. The coding region for the C-terminal WD40 repeat domain (aa 277–649) was generated by PCR from pCR4TOPOcagtA using the primers agtd (5′-gggctagcaaaaaagatagattcattgaatatggattatgg) and agth, and similarly cloned and ligated. Because this construct expressed poorly, amino acids 273–276 were introduced by replacing the MASE amino acid sequence from the linker after the TEV protease site sequence with GHRS by site-directed mutagenesis (5). The forward and reverse mutagenesis primers were, respectively, 5′-gtatttccagggccatgggcatagatcaaaaaaagatagattcattgaatatgg, and 5′-gaatctatctttttttgatctatgcccatggccctggaaatacaagttttctc. These plasmids were introduced into Escherichia coli using standard methods.
E. coli expressing His6-tagged versions of AgtA-FL, AgtA-CAT, and AgtA-WD40 were grown to an A600 of 0.5–0.6 in LB medium in the presence of 100 μg/ml of ampicillin. AgtA-WD40 was coexpressed with the protein chaperones GroEL and GroES (pGROELS was the gift of Xiaoqiang Wang, Nobel Foundation, OK). 1-Liter cultures were induced with 0.3 mm isopropyl 1-thio-β-d-galactopyranoside overnight at 22 °C, pelleted, and resuspended in 40 ml of 0.1 m Tris-HCl (pH 8.2), 5 mm benzamidine, 0.5 μg/ml of pepstatin A, 5 μg/ml of aprotinin, 5 μg/ml of leupeptin, 0.5 mm PMSF, and 1 mg/ml of lysozyme, lysed using a French Press at 18,000 p.s.i., and centrifuged at 100,000 × g for 1 h. The supernatant (S100) was applied to a 1-ml GE HiTrap Ni2+ column equilibrated in 20 mm Tris (pH 7.8), 0.5 m NaCl, and 5 mm imidazole. Protein was eluted using a 0.005–1.0 m gradient of imidazole in the same buffer. The His6-tagged proteins eluted as single peaks that were further purified on a HiLoad Superdex 200 size exclusion column in 50 mm Tris-HCl (pH 8.0), 0.2 mm EDTA, 5 mm β-mercaptoethanol.
Substrates
Fucα1-para-nitrophenol (F-pNP) was from Sigma, Fucα1,2Galβ1-octyl (FG-octyl) was the gift of Monica Palcic, Fucα1,2Galβ1,3GlcNAcα1-pNP (FGGn-pNP) was prepared as before (11), and FGGn-4(N-Ac)Hyp(4R,2S)-NH2 (FGGn-Hyp) and Ac-CIKNDFT(FGGn-)HypEEEEQIRK-NH2 (FGGn-peptide) were synthesized by Zoiesha Chinoy and Geert-Jan Boons (CCRC, University of Georgia).4 Skp1 isoforms were prepared recombinantly without epitope tags in E. coli (25), and purified essentially to homogeneity as described elsewhere (5).
Fbs1
A plasmid encoding guinea pig His6Fbs1 (also known as Ocp1) was provided by M. Henzl (University of Missouri). His6Fbs1 was purified essentially to homogeneity from E. coli as previously described (20).
AgtA Enzyme Assays
α-GalT activity was assayed as described previously (11). Reaction progress was monitored by the transfer of [3H]Gal from UDP-[6-3H]Gal to synthetic glycosides or Skp1. The standard reaction was prepared in 50 mm HEPES-NaOH (pH 7.2), 10 μm UDP-[3H]Gal, 50 mm NaCl, 2 mm MnCl2, and 5 mm DTT, in 20 μl for protein or peptide substrates and 50 μl for other substrates. Reactions were initiated by the addition of enzyme and incubated for 30 min at 22 °C. Conditions were varied as noted, and incubation times and enzyme levels were adjusted to ensure that measurements were carried out under initial velocity conditions.
UDP-[6-3H]Gal was diluted with non-radioactive UDP-αGal to achieve final concentrations. UDP-[6-3H]Gal was obtained from American Radiochemical Corporation (20 mCi/μmol) or Amersham Biosciences. The latter, a generous gift from Joel Shaper (Johns Hopkins School of Public Health), was originally synthesized in 1984 and stored according to the manufacturer's recommendation. UDP-[6-3H]Gal was repurified by chromatography over a Q-anion exchange column as described (21), and further purified by adsorption to a 150-mg Carbograph cartridge (Grace-Alltech) pre-equilibrated with 0.1% TFA (v/v). UDP-[6-3H]Gal was eluted with 30% acetonitrile in 0.1% TFA. Its properties were indistinguishable from that of recently prepared material in pilot enzyme assays.
Reactions using substrates F-pNP, FG-octyl, and FGGn-pNP were stopped by addition of 1 ml of ice-cold 1 mm sodium EDTA (pH 8.0). Reaction mixtures were applied to a 0.5-ml high-cap SepPak C18 cartridge (Grace-Alltech), washed six times with 5 ml of H2O, and eluted with 5 ml of MeOH. Samples were added to 8 ml of Biosafe II scintillation counting fluid (RPI) and radioactivity was quantitated using a Beckman L6500 scintillation counter. Blank activities determined from reactions lacking substrates were subtracted.
Reactions using Skp1 as a substrate were stopped by addition of 80 μl of ice-cold 10 mm sodium EDTA (pH 8.0), 20 μl of 100 mg/ml of BSA, and 1 ml of 10% (v/v) TCA to precipitate proteins. The precipitate was collected on a glass fiber filter under vacuum, and washed 4 times with 1.5 ml of ice-cold 10% TCA and 4 times with 1.5 ml of acetone. The filter was added to 15 ml of Biosafe NA scintillation fluid (RPI) and counted as above.
For activation or inhibition studies, purified Skp1 isoforms were added in advance. All data were analyzed by non-linear regression using GraphPad Prism.
PhyA and Gnt1 Enzyme Assays
Activities of the purified enzyme proteins with Skp1 acceptor protein preparations were assayed as described (7) in the presence of Fbs1. Briefly, Gnt1 reactions were monitored by TCA precipitation of Skp1 to determine incorporation of [3H]GlcNAc from UDP-[3H]GlcNAc. PhyA reactions were monitored using the same Gnt1 reaction based on its specificity for the HO-Skp1 product of the PhyA reaction. Gnt1 activity was assayed in cell extracts as described (4).
Mass Spectrometry of AgtA Reaction Products
Reactions containing Fuc-pNP, FG-octyl, and FGGn-pNP were carried to near completion, adsorbed to a SepPak C18, eluted in MeOH, dried, and analyzed by MALDI-TOF-MS on a Bruker Ultraflex II, in positive ion mode using dihydroxybenzoic acid as the matrix (19).
Size Exclusion Chromatography
Protein-protein interactions were monitored chromatographically on a Pharmacia 3.2/10 Superdex 200 column equilibrated in 50 mm HEPES-NaOH (pH 7.2), 50 mm NaCl (or 150 mm if indicated), 2 mm MnCl2, 5 mm DTT on a Pharmacia SMART System. Protein samples were pre-mixed in this same buffer for 30–60 min at 22 °C before analysis at a flow rate of 100 μl/min. Protein elution was monitored by A280 and Western blot analysis of column fractions.
Disuccinimidyl Suberate (DSS) Cross-linking
His6-AgtA FL and Skp1 were dialyzed exhaustively at 4 °C against 50 mm HEPES (pH 7.2), 2 mm DTT and co-incubated, each at 600 nm, in 50 mm HEPES (pH 7.2), 2 mm MnCl2, 2 mm DTT, 10 μm UDP-Gal, 0–250 mm NaCl for 1.5 h at 22 °C. DSS (Thermo) was dissolved in dry DMSO at 12.5 mm, diluted H2O to 1 mm, and introduced to the preincubated AgtA/Skp1 mixture at a final concentration of 250 μm. Cross-linking reactions were incubated for 10 min in the dark at 22 °C before quenching by 1 m Tris-HCl (pH 7.4) to a final concentration of 75 mm.
SDS-PAGE and Western Blotting
Samples were separated by SDS-PAGE and Western blotted using mAb 4E1 for Skp1, UOK87 for unmodified Skp1, UOK85 for HO-Skp1, and UOK72 or UOK101 for AgtA, as described (4). Alexa 680 fluorescence was imaged in an Odyssey Li-Cor scanner, and images were adjusted using the Levels control in Adobe Photoshop so that it, or a subregion as indicated, utilized the full brightness range available without saturation.
AgtA Antiserum
His6AgtA-FL and His6AgtA-CAT were used to immunize separate female New Zealand White rabbits as described previously for other rabbit Abs (4). The exsanguination of UOK72 (His6AgtA-FL) and UOK101 (His6AgtA-CAT) were used at a 1:1000 dilution in Western blotting.
RESULTS
Importance of the WD40 Repeat Domain for AgtA Enzyme Activity
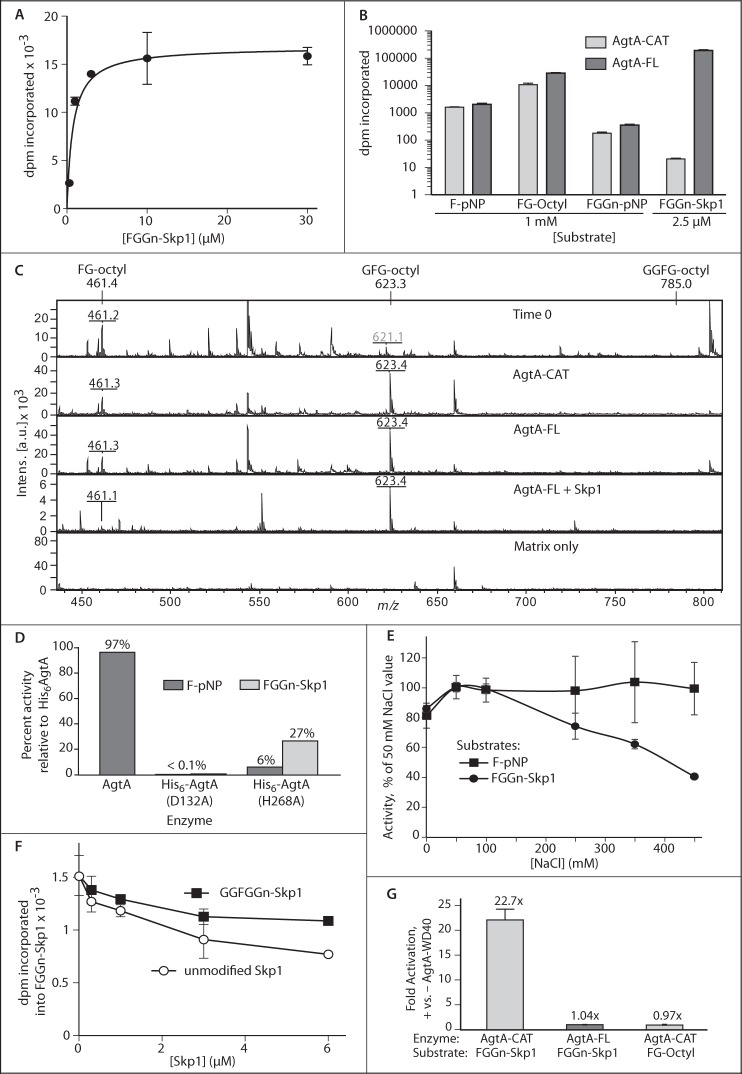
His6-tagged full-length AgtA (AgtA-FL) was purified essentially to homogeneity from E. coli (Fig. 1C) and kinetically analyzed using a highly purified recombinant preparation of the natural substrate, Fucα1,2Galβ1,3GlcNAcα1-Skp1 (FGGn-Skp1). The reaction exhibited Michaelis-Menten characteristics with an apparent Km of 0.76 μm (Fig. 2A) and an apparent kcat of 0.11 s−1. The apparent Km compares with a value of 6 μm previously exhibited by a partially purified native preparation from Dictyostelium (11). Further characterization revealed two component reactions (Fig. 1A): addition of αGal-1 to the 3-position of Fuc (4), and addition of αGal-2 to αGal-1 at an undetermined position. It is not known which step is rate-limiting for the overall reaction, and the impact of Skp1 dimerization (20) is also unknown.
FIGURE 2.
Domain dependence of AgtA α-GalT activity. A, His6AgtA-FL was incubated with a series of FGGn-Skp1 concentrations in the presence of 40 μm UDP-[3H]Gal and 50 mm NaCl for 30 min. Incorporation of [3H]Gal was quantified by TCA precipitation. The curve was derived by non-linear regression according to the Michaelis-Menten model. B, His6AgtA-FL or His6AgtA-CAT was incubated in the presence of 10 μm UDP-[3H]Gal, 50 mm NaCl, and the indicated acceptor substrate for 30 min. Incorporation was quantitated by Sep-Pak C18 capture (pNP and octyl aglycons) or TCA precipitation (Skp1). His6AgtA-CAT protein concentration was 10-fold higher than that of His6AgtA-FL to yield similar activity toward F-pNP. Results are reported as the mean of triplicate reactions ± S.D. C, MALDI-TOF-MS of the reaction of His6AgtA-FL or His6AgtA-CAT with FG-octyl. Expected and experimental m/z values ((M + Na+)+) for FG-octyl and the potential products GFG-octyl and GGFG-octyl are indicated. Other ions represent contaminants, which are common in the low mass range for MALDI-TOF-MS experiments. D, activities of AgtA (tag removed), His6AgtA(D132A), and His6AgtA(H268A), relative to full-length His6AgtA, toward substrates F-pNP and FGGn-Skp1 were determined as in panel B. E, salt dependence of α-GalT activity of AgtA-FL toward F-pNP or FGGn-Skp1 was assayed as in B. Average values ± S.E. of triplicate reactions are reported. F, effect of non-substrate isoforms of Skp1 on AgtA activity, measured as transfer of [3H] from UDP-[3H]Gal to 0.25 μm FGGn-Skp1. Error bars represent ± S.D. for assays conducted in triplicate, which are representative of 3 independent trials. G, α-GalT reactions with 0.15 μm FGGn-Skp1 or 840 μm FG-octyl were supplemented with 0.31 μm His6AgtA-WD40, and the results are reported as fold-activation relative to activity in its absence. Data are reported as representative mean ± S.D.
To assess the importance of the C-terminal WD40 repeat domain for catalytic activity, the N-terminal catalytic domain (AgtA-CAT) and the His6-tagged C-terminal domain (AgtA-WD40) were separately expressed in E. coli (Fig. 1B) and purified (Fig. 1C). In initial assays conducted with small sugar acceptor substrates, AgtA-CAT exhibited substantial activity comparable with that of full-length AgtA-FL, and AgtA-WD40 had no activity. To compare initial reaction velocities, acceptor substrates were tested near their Km values as previously determined by studies of partially purified AgtA from Dictyostelium (11), and enzyme protein levels were adjusted to yield similar incorporation for the simplest substrate, Fuc-pNP (F-pNP). Both AgtA-FL and AgtA-CAT exhibited substantially greater activity toward Fucα1,2Galβ1-octyl (FG-octyl), and substantially lower activity toward Fucα1,2Galβ1,3GlcNAcα1-pNP (FGGn-pNP) (Fig. 2B). This trend was similar to that of partially purified native AgtA (11), indicating that this unusual pattern of relative activity, in which the longer and more native glycan is less active, was a property of the interaction between the substrates and the catalytic domain itself. MALDI-TOF-MS analysis of the products from reactions carried to near completion revealed that only a single Gal residue was added by any of the enzyme preparations to the small glycan substrates (Fig. 2C), confirming previous observations (11).
AgtA-FL was almost 10-fold more active toward a 400-fold lower concentration of FGGn-Skp1 compared with FG-octyl (Fig. 2B). In contrast, AgtA-CAT was severely deficient in modification of the natural FGGn-Skp1 substrate, exhibiting <0.2% activity of AgtA-FL. This low activity was, however, measurable at prolonged times and greater AgtA-CAT concentration. Based on extrapolation of measurements conducted at different substrate concentrations, the activity of AgtA-CAT toward the Fuc-moiety was comparable irrespective of whether it was attached to Skp1 or pNP (data not shown). Removal of the His6 tag by TEV-protease did not affect activity, and a mutant version in which the DXD motif was modified by site-directed mutagenesis, His6AgtA(D132A), was inactive (Fig. 2D). The importance of His-268, which is perfectly conserved in all CAZy GT77 family members to date, was examined by replacement with Ala. AgtA(H268A) exhibited 27% activity toward Skp1 but little activity toward F-pNP (Fig. 2D), and so is not absolutely required for catalysis.
The αGalT activity of a partially purified preparation of AgtA from Dictyostelium toward the disaccharide was previously shown to be sensitive to salt above 50 mm (11). Reinvestigation using purified recombinant proteins showed that intrinsic activity toward FGGn-Skp1 is progressively inhibited by increasing NaCl, reaching 60% at 450 mm NaCl, whereas F-pNP was unaffected (Fig. 2E). The difference in salt sensitivity toward small molecule substrates, between partially and fully purified AgtA, suggests the existence of a modifying factor in cells but was not investigated further. Because only the reaction with FGGn-Skp1 depends on the WD40 repeat domain, its function might be selectively sensitive to salt.
The WD40 repeat domain commonly mediates protein-protein interactions, and may recognize a second site on Skp1 to facilitate substrate recognition. This model predicts that unmodified Skp1, which lacks the trisaccharide required to interact with the active site, can compete with FGGn-Skp1 because of the second site interaction. Unmodified Skp1 reduced activity in a manner consistent with competitive inhibition, with 50% inhibition occurring at 25-fold excess over FGGn-Skp1 (Fig. 2E). Fully modified GGFGGn-Skp1, the product of the AgtA reaction, was also inhibitory but in a manner that plateaued near 30% inhibition at the same concentration. Thus the proposed second site that interacts with AgtA appears to be present in all Skp1 isoforms, but is less active in GGFGGn-Skp1, consistent with a need to separate from the enzyme to complete the catalytic cycle.
To more directly examine the importance of the WD40 domain, the effect of a recombinant preparation (Fig. 1C) was examined when introduced into AgtA reactions. AgtA-WD40 modestly stimulated the activity of AgtA-CAT toward FGGn-Skp1 under standard conditions (50 mm NaCl), but not of AgtA-CAT toward FG-octyl, or AgtA-FL toward FGGn-Skp1 (Fig. 2G). The finding that the untethered WD40 repeat domain potentiates activity of the free catalytic domain for only the Skp1 substrate implies a direct role in Skp1 recognition, although more complex interpretations cannot be excluded. The WD40 domain may mediate the hypothesized salt-sensitive second-site interaction to facilitate Skp1 recognition in the cell.
Skp1 Activation of AgtA Enzyme Activity
The two-site hypothesis stipulates that the catalytic and WD40 repeat domains interact with separate sites on Skp1. To test for functional synergy of these proposed interactions, the effect of unmodified Skp1, lacking the acceptor Fuc that interacts with the active site, on catalytic modification of free F-pNP was examined. Remarkably, Skp1 exerted substantial concentration-dependent activation of F-pNP modification (Fig. 3A) such that, at saturation, activity of AgtA-FL toward F-pNP was stimulated 50-fold with activity approaching that toward the native substrate FGGn-Skp1 when analyzed at concentrations near their respective Km values. A comparison of acceptor substrates showed that 50 nm Skp1 activated the galactosylation of F-pNP, FG-octyl, and FGGn-pNP by 30-, 16-, and 17-fold, respectively (Fig. 3, A and B). A kinetic analysis of the dependence of the activated reaction on the F-pNP concentration showed a Km of 6 mm, similar to the previously reported value of 4 mm in the absence of Skp1 (11), indicating that Skp1 improved the reaction velocity rather than the affinity toward F-pNP. MALDI-TOF-MS analysis of a scaled-up activated reaction showed that still only a single Gal residue was added to F-pNP or FG-octyl (Fig. 2C). Skp1 activation exhibited hyperbolic saturation expected of a single binding event with an apparent KD of 60 nm. Consistent with the involvement of the WD40 repeat domain, Skp1 did not activate AgtA-CAT activity toward F-pNP (Fig. 3B). Furthermore, activation was suppressed at high salt (Fig. 3C), suggesting that activation was mediated by the same salt-sensitive, WD40-dependent second-site interaction that supports high activity toward FGGn-Skp1. HO-Skp1 and Gn-Skp1 were similarly effective at activating catalysis (Fig. 3A). In contrast, the product of the reaction, GGFGGn-Skp1, had no effect (Fig. 3B), suggesting that terminal galactosylation altered the second-site interaction, or the effect of the interaction on AgtA activation.
FIGURE 3.
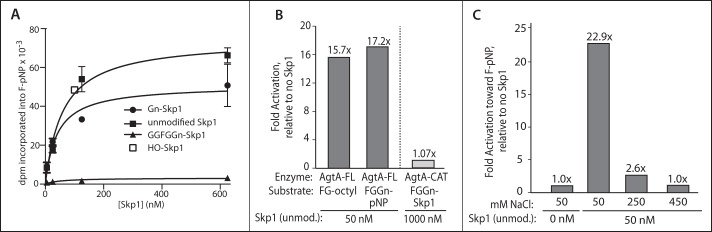
Skp1 activation of AgtA. A, activity was measured based on the transfer of [3H] from UDP-[3H]Gal to 3.7 mm F-pNP by His6AgtA-FL, in the presence of the indicated concentrations of non-substrate Skp1 isoforms. The curve was fitted by non-linear regression modeled as a single site binding interaction. Data are reported as representative mean ± S.D. B, fold-activation of AgtA-FL and AgtA-CAT activity toward FG-octyl, FGGn-pNP, or FGGn-Skp1 in the presence of the indicated concentration of unmodified Skp1. C, reactions were prepared as in A with the indicated concentrations of unmodified Skp1 and NaCl. Total activation levels of triplicate reactions relative to AgtA-FL alone are plotted.
AgtA Is a Novel Skp1-binding Protein
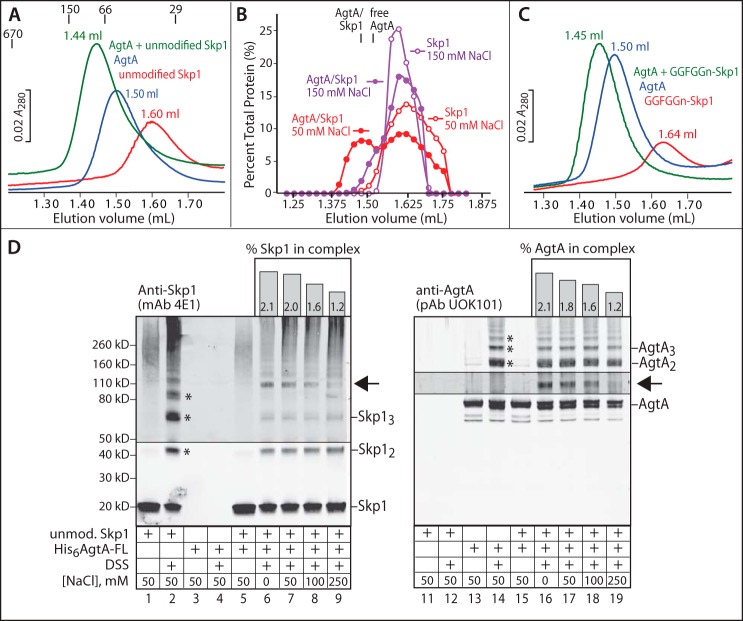
Physical interactions between AgtA and Skp1 were investigated as a potential basis for the AgtA activation. Initial studies were conducted at pH 7.2 in a buffer supplemented with 50 mm NaCl, which correspond to optimal αGalT activity and to conditions reported for the Dictyostelium cytoplasm (22–24). Analysis of a 1:1 molar mixture of AgtA-FL and unmodified Skp1 by size exclusion chromatography revealed a novel peak that eluted earlier than AgtA-FL or Skp1 alone (Fig. 4A), at an elution position consistent with formation of a 1:1 complex at an estimated peak concentration of 2 μm. However, the assessment of stoichiometry is uncertain because AgtA-FL (Mr 77,440) eluted after the Mr 66,000 standard, which suggests retardation because of the interaction with resin, and Skp1 (Mr 18,710) eluted before the Mr 29,000 standard, which is consistent with homodimerization (20, 25). Western blotting of the column fractions showed the expected shift of AgtA and Skp1, and densitometric analyses showed that ∼40% of Skp1 interacted with AgtA based on elution in earlier fractions (Fig. 4B). In contrast, a much smaller fraction of Skp1 was associated with AgtA in 150 mm NaCl (Fig. 4B). This salt-sensitive association likely represents the second-site interaction that mediates Skp1 recognition and catalytic activation described above.
FIGURE 4.
Physical interaction of AgtA and Skp1. A, 0.1 nmol of His6AgtA-FL, unmodified Skp1, or His6AgtA-FL preincubated with unmodified Skp1 were chromatographed on a Superdex 200 column in 50 mm NaCl, and monitored based on A280. Peak elution volumes are denoted. Elution positions for porcine thyroglobulin (Mr 670,000), alcohol dehydrogenase (150,000), bovine serum albumin (66,000), and carbonic anhydrase (29,000) are denoted. B, densitometric analysis of Western blots of Skp1 elution positions from A and an identical experiment in 150 mm NaCl. The elution positions of free AgtA-FL and the AgtA·Skp1 complex, which were not affected by the NaCl concentration, are denoted. C, gel filtration analysis of the interaction of AgtA-FL with fully modified Skp1 (GGFGGn-Skp1) as in A. Profiles shown in panels A–C are representative of 2–3 independent trials. D, parallel Western blots of cross-linking reactions performed on AgtA-FL (600 nm), unmodified Skp1 (600 nm), or a mixture with each at 600 nm. Samples were reacted with 0.25 mm DSS for 10 min at the indicated NaCl concentrations. Lower order multimers of Skp1 and AgtA are denoted with asterisks, and the position of a novel AgtA- and Skp1-dependent band is denoted with an arrow. Imaging of this region of the blot was reoptimized with respect to this band. The percentage of Skp1 and AgtA found in this band is plotted as a function of NaCl concentration above the Western blot lanes. Data shown are representative of 3 independent trials.
Similar findings were obtained with the substrate FGGn-Skp1, in the presence or absence of 10 μm UDP-Gal (data not shown). Surprisingly, the product of the AgtA reaction, GGFGGn-Skp1, bound AgtA-FL as well as Skp1 under these conditions (Fig. 4C). Thus in vitro, AgtA is a novel Skp1-binding protein irrespective of its status as a substrate for α-galactosylation. The nature of the binding interaction is predicted to differ, however, as GGFGGn-Skp1 does not activate AgtA (Fig. 3A). Notably, GGFGGn-Skp1 elutes reproducibly later than Skp1 and FGGn-Skp1 (compare Fig. 4, A and C), at both 50 and 150 mm NaCl. Although the reason for the difference in elution time is not known, it is possible that it reflects a conformational difference that does not allow the catalytic activation shown in Fig. 3.
The nature of the complex was examined further by cross-linking studies with DSS, a bifunctional amine-reactive reagent incorporating an 8-carbon (11.4-Å) spacer. Using conditions optimized for DSS concentration and reaction time, SDS-PAGE/Western blotting showed that Skp1 and AgtA could be trapped as dimers and higher order multimers in 50 mm NaCl (Fig. 4D, lanes 2 and 14, asterisks). In comparison, the Skp1/AgtA mixture exhibited less oligomerization of Skp1, and a unique novel band with an apparent Mr of 108,000 was detected by both anti-Skp1 and anti-AgtA antibodies (arrow, in lanes 7 and 17). The intensity of this band was salt-sensitive (lanes 6–9 and 16–19, and histograms above), suggesting that it corresponds to the complex detected by gel filtration. The cross-linked complex appeared to be less salt-sensitive than was the α-galactosylation of FGGn-Skp1 (Fig. 2C), the activation of AgtA catalytic activity (Fig. 3), and the gel filtration complex (Fig. 4B), but this is likely because cross-linking is irreversible and thus cumulative over time. Although the fractions of total Skp1 and AgtA that could be trapped by cross-linking was low, it is likely that the majority of Skp1 was complexed with AgtA prior to denaturing SDS-PAGE. This is proposed based on the smaller fraction of Skp1 oligomers that could be cross-linked in the presence of AgtA because of competitive interaction. Interestingly, interacting with Skp1 did not appear to impact AgtA oligomerization. The apparent Mr of the novel band (108,000) lies between that of the 1:1 Skp1·AgtA complex (96,200) and a 2:1 complex (114,910). The observation that complex formation reduces the level of Skp1 dimer that can be cross-linked suggests that Skp1 is largely monomeric and residing in a 1:1 complex whose Mr cannot be accurately determined by SDS-PAGE.
Effects on Other Skp1 Interactions
Skp1 interacts with each enzyme of the modification pathway in its role as acceptor substrate. The influence of AgtA binding on these functions was investigated by introducing AgtA into the assays at 50 mm NaCl. At a 5-fold excess over Skp1, AgtA-FL inhibited reactions of the Skp1 prolyl 4-hydroxylase (PhyA) and the αGlcNAc-transferase (Gnt1) with their specific Skp1 isoform substrates by 75 and 50%, respectively (Fig. 5A). The inactive mutant AgtA(D132A) was nearly as inhibitory as the active enzyme, suggesting that the effect did not involve binding via its active site.
FIGURE 5.
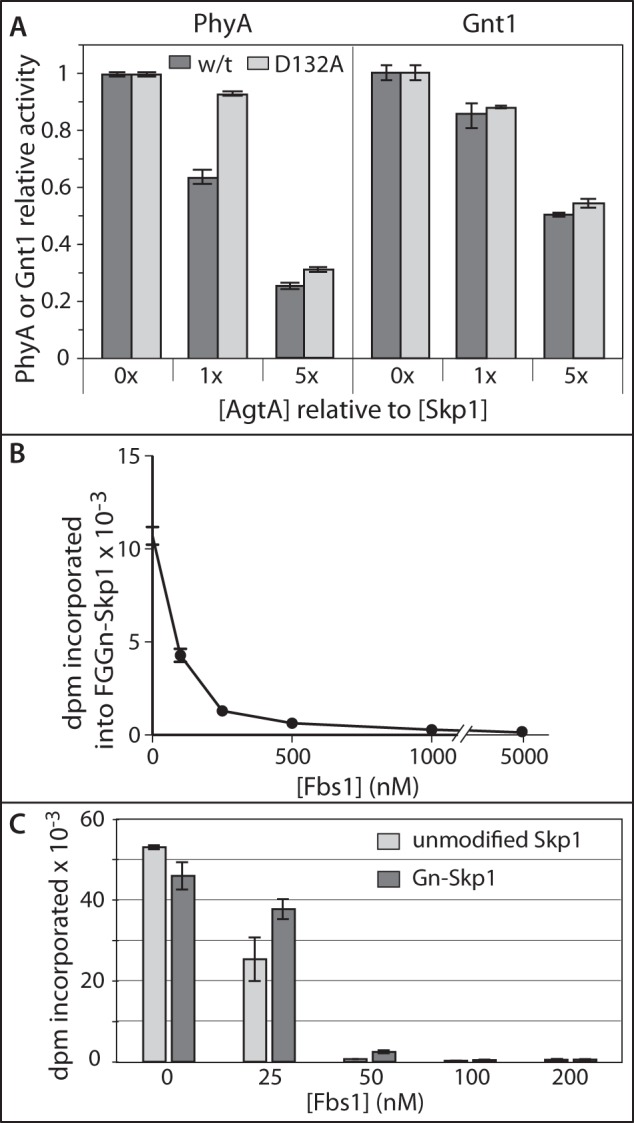
Relationship between AgtA and other Skp1 interactors. A, effect of AgtA on Skp1 PhyA and Gnt1 reactions. Reactions containing 0.1 μm unmodified Skp1 (left panel) or HO-Skp1 (right panel) were supplemented with 0, 0.1, or 0.5 μm His6AgtA-FL or His6AgtA(D132A) as indicated. B, effect of Fbs1 on AgtA-mediated α-galactosylation of Skp1. FGGn-Skp1 (0.5 μm) was preincubated with 0–50 μm His6Fbs1, and its α-galactosylation was tested as described in the legend to Fig. 2A. C, effect of Fbs1 on Skp1 activation of AgtA activity. AgtA modification of 1 mm F-pNP was measured in the presence of 100 nm unmodified (light gray) or α-GlcNAcylated (dark gray) Skp1 (as in Fig. 3A), 10 μm UDP-[3H]Gal, and the indicated concentration of Fbs1, for 30 min. Data are presented as mean ± S.D. for assays conducted in triplicate, and are representative of at least 2 independent trials.
In the cell, Skp1 is typically associated with FBPs. To examine the impact of this on the interaction of Skp1 with AgtA, the FBP Fbs1 (also known as OCP1 or Fbg1) from guinea pig, which unlike most FBPs is soluble in the absence of Skp1 (20), was chosen for analysis. His6-Fbs1 was purified essentially to homogeneity from E. coli and tested for its influence on AgtA-mediated α-galactosylation of FGGn-Skp1. As shown in Fig. 5B, Fbs1 exhibited concentration-dependent inhibition of AgtA activity that was nearly complete at a 1:1 stoichiometry of Fbs1 to Skp1, and almost 90% inhibition occurred at 0.5 stoichiometry. This suggests that not only does formation of the Skp1·Fbs1 complex block processing of Skp1 by AgtA, the interaction with Fbs1 is tight with high affinity, consistent with the previously reported KD value of 25 nm (20). In addition, Skp1 may exist as a dimer that is inactivated by the Fbs1 monomer as suggested by the substoichiometric nature of the inhibition (20), but the possibility that the calculated extinction coefficient used to determine protein concentrations is inaccurate was not excluded.
The effect of Fbs1 on Skp1 activation of AgtA modification of F-pNP is shown in Fig. 5C. Increasing concentrations strongly inhibited activation by either Skp1 or Gn-Skp1. As observed for inhibition of the AgtA reaction with FGGn-Skp1 (Fig. 5B), inhibition of activation by Fbs1 was strong and appeared to be substoichiometric. The similar effects of Fbs1 on both the substrate activity of Skp1 and activation by Skp1 of AgtA is consistent with a model in which they are both mediated by the same binding event, which may overlap with the site of interaction of Skp1 with the Fbs1 F-box.
A Nonenzymatic Role of AgtA in Dictyostelium Development
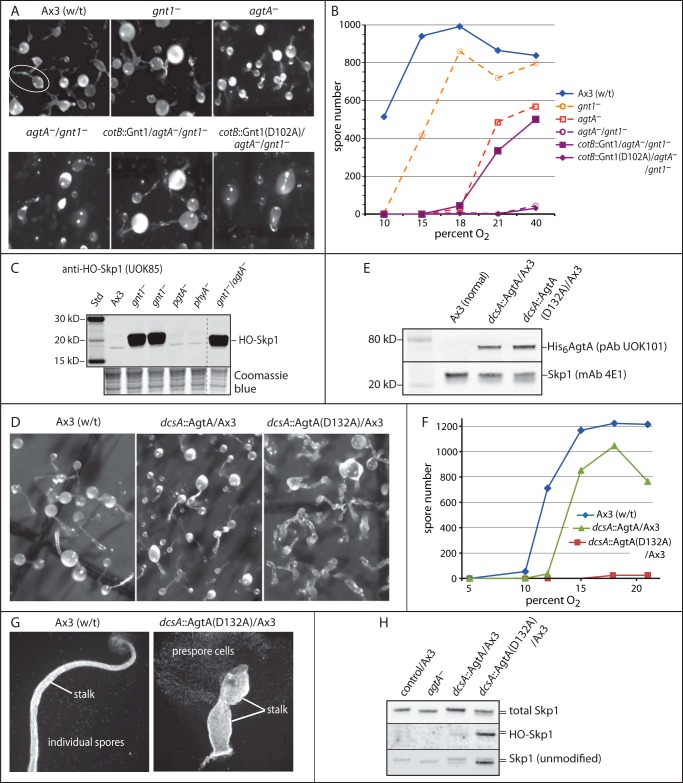
Based on previous studies of the agtA-disruption strain (6), AgtA contributes to the ability of cells to sense O2 concentrations of ∼8–10% to allow culmination, i.e. the reorganization and differentiation of the migrating slug into a fruiting body consisting of spores supported on a cellular stalk. In the absence of AgtA, culmination can occur but requires a higher level of O2 (18–21%), almost as much as that required by phyA− strains. Interestingly, partial functionality (culmination at ∼12–15% O2) is achieved by hydroxylation of Skp1 ± αGlcNAc addition, as revealed by disrupting gnt1 or pgtA (4). We reasoned that if AgtA contributes a function in addition to its role in α-galactosylation, it might be revealed in a genetic background in which the substrate for AgtA is not generated. Thus we created an agtA−/gnt1− double knock-out, by disrupting gnt1 in an agtA− background. The double mutant lacked detectable Gnt1 activity in extracts, accumulated HO-Skp1, like gnt1− cells (Fig. 6C), and failed to culminate at even the highest O2 level tested, 70%. An example at 21% O2 is shown in Fig. 6A, where both gnt1− and agtA− cells formed normal appearing fruiting bodies (top row), as expected because O2 was above their thresholds, but the double mutant (bottom left) remained in the slug-like stage. Failure to differentiate into spores is documented in Fig. 6B. Because the path to generating the double mutant involved disrupting gnt1 in a previously validated agtA− strain, specificity of the disruption was addressed by a rescue experiment in which gnt1 was restored by cotB-promoter dependent expression of Gnt1 or catalytically inactive Gnt1 in prespore cells. As shown in Fig. 6, A and B, normal development was restored by active but not inactive Gnt1, demonstrating that the original change was specific to gnt1, and confirming (4) that the αGlcNAcT activity of Gnt1 is important for its function in culmination. The results imply that AgtA is required for culmination when Skp1 is not glycosylated by a mechanism that does not involve its catalytic activity, which might involve the novel Skp1 binding activity documented in vitro above.
FIGURE 6.
Non-enzymatic activity of AgtA in vivo. A, representative images of development of wild-type (w/t) and mutant strains after 42 h at 21% O2. Normal and single knock-out strains are in the top row. The white oval encircles a typical fruiting body showing the spore-containing sorus linked to the stalk. The double agtA−/gnt1− mutant strain HW520 and strains complemented with normal or enzymatically inactive Gnt1(D102A) expressed under control of the prespore cell specific cotB promoter are in the bottom row. B, quantification of culmination, based on spore number, as a function of O2 in the atmosphere. C, Western blot analysis for HO-Skp1 in S100 extracts from growing cells of the agtA−/gnt1− double mutant strain compared with other mutant strains. The dashed line shows where other lanes from the same blot were omitted. The lower panel shows a region of the blotted gel after Coomassie Blue staining, as a loading control. D, representative images of development for 42 h at 21% O2 of strains constitutively overexpressing His6AgtA or enzymatically inactive His6AgtA(D132A) under control of the semi-constitutive dcsA promoter. E, a Western blot of growing cell S100 extracts from selected overexpression clones was probed with anti-AgtA, and with anti-Skp1 as a loading control. F, spore number as a function of the O2 concentration from strains shown in D. Data in B and D are representative of ≥3 independent trials where each strain was analyzed in parallel. G, examples of individual fruiting bodies from normal and AgtA(D132A)oe strains from panel D, imaged using fluorescent Calcofluor staining for the cellulosic cell walls of stalk and spore cells. Ax3(w/t) shows a typical slender stalk and spores that disperse during preparation. The AgtA(D132A)oe strain culminates to form a poorly organized bloated stalk, and the prespore cells remain contiguous because of failure to differentiate into spores. H, Western blots of S100 cell extracts from agtA−, His6AgtAoe, and His6AgtA(D132A)oe cells (in an Ax3 background) grown at 21% O2 were probed with anti-Skp1 antibodies that recognize total Skp1 (mAb 4E1), unmodified Skp1 (UOK87), or HO-Skp1 (UOK85). The slower migration of fully modified Skp1 relative to unmodified or hydroxylated Skp1 is denoted by the double bar.
In a second approach, the effect of semi-constitutive overexpression of native and catalytically inactive mutant AgtA was examined in agtA− and normal (Ax3) cells. As previously reported (6), AgtA-overexpression (AgtAoe) rescued culmination and spore formation of agtA− cells at lower O2 levels (15%). Overexpression of AgtA in normal cells had a slightly deleterious effect by raising the O2 requirement (Fig. 6F). A much more pronounced effect was observed when mutant AgtA(D132A) was overexpressed in normal cells, documented in Fig. 6E, which resulted in complete inhibition of spore formation (Fig. 6F). However, the block was not at the culmination step, as the slugs executed aberrant culmination characterized by irregular stalk-like structures supporting cells that failed to terminally differentiate into spores (Fig. 6D). Close inspection of the stalks revealed a bulbous and bloated appearance (Fig. 6G) that was easily disrupted by shearing under the microscope slide. Consistent with these observations, AgtAoe in the agtA− background partially rescued the O2-dependent spore formation to a level similar to that of AgtAoe in the Ax3 background, whereas AgtA(D132A) had little or no activity (not shown). Thus the normal organization of stalk cells was severely disrupted by the catalytically inactive protein. No effect on the rate of cell proliferation in axenic medium was observed, which is typical of the other Skp1 modification mutants.
Analysis of Skp1 modification in these strains showed that AgtA(D132A)oe led to the accumulation of Skp1 and HO-Skp1 in cells (Fig. 6H), consistent with the in vitro effects (Fig. 5A). However, the structural and differentiation defects, which occurred at even 70% O2, were distinct from those seen in phyA− and gnt1− cells that accumulate high levels of less modified Skp1. These findings indicate that AgtA(D132A) is dominant negative in a manner consistent with a non-enzymatic Skp1 binding function that because of high protein levels sequesters Skp1 from its normal functions.
DISCUSSION
The cellular glycome is defined by the non-templated actions of GTs and glycosidases and, therefore, the varied mechanisms of their selectivity and regulation are of great interest. AgtA exhibits features that are, based on current knowledge, unusual among glycosyltransferases, including second-site recognition of the polypeptide carrier of the trisaccharide substrate, and catalytic activation via polypeptide recognition. Domain analysis indicates that these novel properties depend on an interplay between the two parts of AgtA, in which recognition of the Skp1 polypeptide by the C-terminal WD40 repeat domain activates the catalytic domain. Remarkably, polypeptide recognition includes all isoforms of Skp1, allowing for AgtA to influence Skp1 even before it becomes a glycosylation substrate. In turn, α-galactosylation of the Skp1 polypeptide alters recognition by AgtA, possibly allowing for termination of the non-enzymatic influence on Skp1 concomitant with acquisition of new functions associated with the final pentasaccharide. These findings, interpreted below, suggest a model in which the α-galactosyltransferase activity of AgtA modulates its role as a Skp1-binding protein and potentially an FBP exchange factor (Fig. 7).
FIGURE 7.
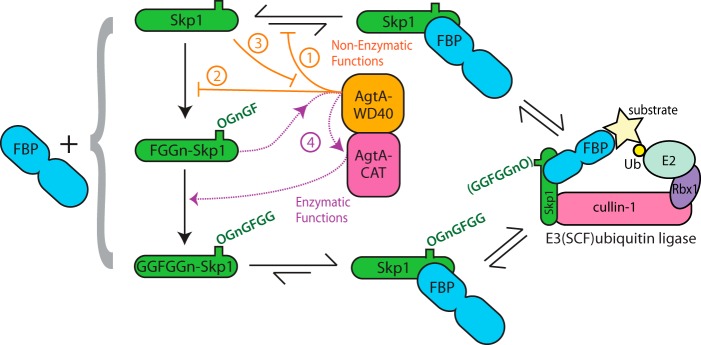
Proposed impact of AgtA on Skp1 interactions. Progressive Skp1 glycosylation is shown at the left from top to bottom. Each Skp1 isoform can bind to an F-box protein (FBP, depicted at left), but evidence (25) indicates that FBPs prefer glycosylated Skp1s, which are reflected by the equilibria shown at the top and bottom. The Skp1·FBP complex ultimately enters the E3SCF·ubiquitin ligase complex shown at the right. AgtA, in the center, is both a Skp1-binding protein and the enzyme that α-galactosylates FGGn-Skp1 to yield GGFGGn-Skp1. We propose that AgtA, via its WD40 repeat domain (in orange), competes with FBP binding to Skp1 (step 1), and competes with recognition of Skp1 by PhyA and Gnt1 (step 2), thereby inhibiting Skp1 function and processing in cells. This interaction in turn titrates AgtA from its later catalytic role (step 3). FBP binding also interferes with Skp1 hydroxylation by PhyA (7). Once Skp1 is modified to the trisaccharide state as FGGn-Skp1, its second-site, salt-sensitive recognition by the WD40 repeat domain of AgtA activates the catalytic activity of AgtA (purple) resulting in switch-like α-galactosylation of Skp1 (step 4).
A Role for the WD40 Repeat Domain in Substrate Recognition
The importance of the WD40 repeat domain in Skp1 recognition is indicated by its necessity for AgtA to efficiently galactosylate FGGn-Skp1 at low, physiological concentrations (Fig. 2A). In fact, even the free WD40 repeat domain can promote activity of the catalytic domain toward FGGn-Skp1 (Fig. 2G). Because the WD40 repeat domain is not required to modify the free glycan, we propose that it binds to Skp1 at a site separate from the interaction of the active site with the glycan acceptor (Fig. 7, step 4). The existence of the second site is supported by detection of a stable complex between AgtA-FL and Skp1 under native conditions by gel filtration chromatography in the absence or presence of the co-substrate UDP-Gal (Fig. 4, A and B), and a complex that could be trapped by DSS cross-linking (Fig. 4D). The binding interaction detected by gel filtration and cross-linking likely mediates Skp1 substrate recognition and AgtA activation because of their similar salt sensitivities. This might assist in substrate recognition in the cell, as proposed for the Dictyostelium myosin heavy chain kinases MhckA and MhckB whose WD40 repeat domains target their myosin substrate (26). Nevertheless, AgtA-FL also forms a stable complex with the reaction product GGFGGn-Skp1 (Fig. 4C), which raises the question of how the product dissociates to permit a new catalytic cycle.
Second-site recognition mechanisms that contribute to acceptor substrate specificity have been described in several membrane-bound GTs including βGalNAcT3 and -T4 (27), polysialyltransferase-I (18), polypeptide αGalNAc transferases (16), and lysosomal enzyme GlcNAc-phosphotransferase (28). The mechanism of second-site recognition in these enzymes appears also to involve distinct domains, or subunits, and βGalNAcT3 and -T4 binding to their polypeptide substrates is sensitive to higher ionic strength (29) as for AgtA. The mechanism of product release for these enzymes is poorly understood and is usually assumed to involve a simple equilibrium process. The model that second-site recognition facilitates targeting of Skp1 seems sensible for an enzyme whose substrates are not concentrated within secretory pathway vesicles as for most glycosylation pathways, but a need for such as mechanism can be questioned considering that Skp1 is thought to be the only glycoprotein in the cytoplasmic compartment that bears the acceptor Fuc residue (2). Furthermore, the earlier GTs in the pathway, Gnt1 and PgtA, are also highly specific for Skp1 but lack obvious domains like that in AgtA to mediate this specificity (6, 19). Thus the WD40 repeat domain may have other functions.
A Role for the WD40 Repeat Domain in Catalytic Activation
Skp1 binding also supports a remarkable and substantial (30–50-fold) activation of catalytic activity toward all free glycan substrates examined (Fig. 3A). Because activation required the WD40 repeat domain of AgtA, and was salt-sensitive, it is likely initiated by the same binding interaction that promotes Skp1 recognition. Because activation was due to an increase in Vmax rather than a change in Km, the mechanism likely involves a process separate from active site recognition. This mechanism likely helps to explain why FGGn-Skp1 is so much better of a substrate for AgtA than are the free glycan and glycan-peptide substrates. Taking everything together, we infer that a major function of the second site interaction with Skp1 is not only to assist in seeking out free FGGn-Skp1, but also to ensure its rapid processing once encountered (Fig. 7, step 4).
If the Skp1 concentration-dependent activation is modeled as a simple binding interaction, the apparent KD is 50–70 nm. This value is well below the apparent Km of AgtA toward Skp1, 0.75 μm (Fig. 2A). However, the apparent KD value was measured for non-substrate isoforms of Skp1. Because the reaction product GGFGGn-Skp1 still binds Skp1 (Fig. 4C) but has no stimulatory activity (Fig. 3A), this isoform likely binds in a distinct fashion with a KD that could not be measured by substrate activation. We speculate that the substrate isoform FGGn-Skp1 also binds differently by a mechanism that is better described by the apparent Km of the reaction. However, a more complex reaction mechanism cannot be excluded in the absence of structural studies to evaluate the physical interaction.
AgtA Is a Skp1-binding Protein
The binding of AgtA-FL to substrate precursor isoforms of Skp1 (Fig. 4B) appears to be irrelevant to catalysis, because dissociation appears to be required for prior hydroxylation and αGlcNAcylation (Fig. 5A). This is reminiscent of the effect of the FBP FbxA, whose binding to Skp1 also inhibits its modification by PhyA (7). This effect potentially occurs in vivo because overexpression of AgtA led to an accumulation of unmodified Skp1 in cells (Fig. 6H). Even higher levels accumulated using catalytically inactive AgtA(D132A), and HO-Skp1 was also detected. Thus AgtA is a novel Skp1-binding protein that may buffer against the actions of PhyA and Gnt1 toward Skp1 (Fig. 7, step 2).
The binding affinity of Skp1 is sufficient to inhibit the reaction of AgtA with FGGn-Skp1 (Figs. 2F and 7, step 2). The product GGFGGn-Skp1 was less inhibitory, corresponding to altered gel filtration behavior (Fig. 4C) and suggesting an altered binding mode that may be related to product release.
Competition between AgtA and an FBP was examined using guinea pig Fbs1, which forms a tight binding complex with Dictyostelium Skp1 based on gel filtration (25). Fbs1 efficiently blocked activation of AgtA by Skp1 (Fig. 5C), which could be explained by formation of a competing Fbs1·Skp1 complex. Indeed, Fbs1 has a lower KD for mammalian Skp1, 25 nm (20), than does AgtA (as measured indirectly) for Dictyostelium Skp1 (∼60 nm). If AgtA and FBPs compete for binding Skp1 in cells, then they could dampen each other's activities and jointly slow the hydroxylation and glycosylation of Skp1 in cells (Fig. 7, steps 1 and 3).
Importance of the Skp1 Binding Function of AgtA in Cells
Genetic studies suggest that the AgtA binding activities observed in vitro impact Skp1 in cells. Disruption of agtA has an anomalously severe effect on culmination in which O2 dependence rises to a level almost as high as that required for phyA-null cells to culminate (6). This contrasts with the intermediate level of O2 required for gnt1- or pgtA-mutant cells, and the low level of O2 required for wild-type cells that can assemble the pentasaccharide. An even more severe effect was observed when agtA was deleted in a gnt1-null background (Fig. 6, A and B), in which culmination could not be rescued at any O2 level tested (up to 70%). Because the AgtA catalytic activity is irrelevant in the absence of a substrate that cannot be assembled without Gnt1, the failure to culminate suggests a need for the Skp1 binding activity of AgtA that is exacerbated by the inability to escape the effect of AgtA by completion of glycosylation. Indeed, the importance of glycosylation per se is emphasized by the ability of highly (23-fold) overexpressed AgtA-CAT to complement the absence of AgtA (5, 6). In complementary fashion, the binding activity of AgtA lessens the requirement for O2, and may be a mediator of the O2-sensing mechanism that rescues culmination of phyA− cells at high O2 levels (19). Absence of the binding activity of AgtA may alter the kinetics of assembly of new SCF complexes containing different FBPs during developmental transitions (Fig. 7).
Overexpression of catalytically inactive AgtA(D132A) in a wild-type background was also deleterious with a selective and dramatic effect on late culmination. Proliferation and development up to the slug stage were morphologically normal and culmination was initiated at the normal time. However, the stalk that formed was flaccid with a larger volume (Fig. 6G) suggesting that a larger fraction of cells contributed to its formation, and the fewer cells that rose to the apex failed to sporulate (Fig. 6, D and F). This defect was not rescued at even the high O2 levels that normally override Skp1 modification defects. The failure to sporulate under high O2 is reminiscent of the inability of gnt1− or pgtA− cells to sporulate in O2-limited submerged development (30). It is unlikely that the effect of AgtA(D132A) overexpression is due solely to hypomodified Skp1 that accumulates (Fig. 6H), because glycosylation mutants that accumulate these isoforms can form almost normal fruiting bodies at high O2 (19, 4). The O2-independent effect of AgtA(D132A) overexpression is most easily explained by the advantage that a high concentration affords the protein in competing with F-box interactions, which is expected to impede timely removal of critical regulatory proteins during culmination and terminal cell differentiation. Overexpression of active AgtA had, in contrast, little effect, suggesting that the ability to α-galactosylate Skp1 ultimately terminates the inhibition rendered by AgtA-binding. Because Skp1 is the only enzymatic substrate that has been detected in biochemical complementation studies (6) and agtA disruption suppresses disruption of one of the two Skp1 genes (8), the aberrant culmination likely involves Skp1 as a biochemical target despite the possibility that AgtA WD40 repeats bind other proteins as well.
Possible Impact of AgtA on SCF Complex Assembly
The importance of the Skp1 binding function of AgtA is consistent with previous evidence that AgtA and Skp1 are expressed at similar levels in vivo and to co-purify over DEAE- and phenyl-Sepharose chromatography columns (11), but future studies are needed to address the stability of AgtA binding to Skp1 in cells and potential interactions with other proteins. The optimal buffer for in vitro binding and catalysis contained 50 mm NaCl, and its osmolarity of 156 mosm lies within the physiological range of the Dictyostelium cytoplasm, which has been estimated at 85 to 200 mosm (23, 24). The dependence of binding on ionic strength raises the possibility that changes in osmolarity in response to varied environments may regulate the association of AgtA and Skp1.
Dictyostelium development involves global changes in protein profiles, which doubtless involve regulated protein turnover (e.g. Ref. 31). E3SCFUb-ligases (1) likely contribute to turnover as prescribed by developmentally programmed changes (32) in the expression of more than 50 predicted FBPs.5 Potentially, AgtA promotes equilibration of interactions between Skp1 and newly synthesized FBPs by serving as an FBP exchange factor analogous to how Cand1 acts like an exchange factor to control Skp1/Cul1 interactions (33–35). Cand1, which binds to Cul1, helps dissociate the Skp1/FBP dimer from Cul1, but promotes SCF function in vivo, via a mechanism of mutually exclusive binding of Cand1 and Skp1 to Cul1. As evidenced by the effects of too much or too little binding competent AgtA in cells (Fig. 6), mutually exclusive binding of AgtA and an FBP to Skp1 may facilitate the switching and activation of FBPs during developmental transitions in a manner that is kinetically modulated by hydroxylation and glycosylation in response to environmental factors such as O2 and internal metabolic factors such as sugar availability (Fig. 7). Structural studies to elucidate the molecular contacts that underlie these interactions are needed to further evaluate this model.
Acknowledgments
We are grateful to Xiaoqiang Wang and Qing Chang from the Noble Foundation (Ardmore, OK) for their assistance in preparing AgtA and providing pGroELS, Joel Shaper (Johns Hopkins University) for UDP-[3H]Gal, and Michael Henzl (University of Missouri) for providing the Fbs1 plasmid.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-GM084383 and R01-GM037539 and Oklahoma Center for Advancement of Science and Technology Grant HR10-0181.
Z. Chinoy, C. M. West, and G. J. Boons, unpublished studies.
Y. Xu and C. W. West, unpublished studies.
- SCF
- Skp1·Cullin-1·FBP·Rbx1 subcomplex of the Cullin-1/RING ligase class of E3 ubiquitin ligases
- α-GalT
- α-galactosyltransferase
- AgtA-FL
- full-length His6AgtA
- AgtA-CAT
- His6-tagged N-terminal catalytic domain of AgtA
- AgtA-WD40
- His6-tagged C-terminal WD40-repeat domain of AgtA
- DSS
- disuccinimidyl suberate
- FBP
- F-box protein
- F-pNP
- Fucα1-para-nitrophenol
- FG-octyl
- Fucα1,2Galβ1-octyl
- FGGn-pNP
- Fucα1,2Galβ1,3GlcNAcα1-pNP
- GT
- glycosyltransferase
- TCA
- trichloroacetic acid
- PhyA
- prolyl 4-hydroxylase
- TEV
- tobacco etch virus.
REFERENCES
- 1. Willems A. R., Schwab M., Tyers M. (2004) A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim. Biophys. Acta 1695, 133–170 [DOI] [PubMed] [Google Scholar]
- 2. West C. M., Wang Z. A., van der Wel H. (2010) A cytoplasmic prolyl hydroxylation and glycosylation pathway modifies Skp1 and regulates O2-dependent development in Dictyostelium. Biochim. Biophys. Acta 1800, 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu Y., Brown K. M., Wang Z. A., van der Wel H., Teygong C., Zhang D., Blader I. J., West C. M. (2012) The Skp1 protein from Toxoplasma is modified by a cytoplasmic prolyl 4-hydroxylase associated with oxygen sensing in the social amoeba Dictyostelium. J. Biol. Chem. 287, 25098–25110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang D., van der Wel H., Johnson J. M., West C. M. (2012) The Skp1 prolyl 4-hydroxylase of Dictyostelium contributes glycosylation-independent and -dependent effects on O2-dependent development without affecting Skp1 stability. J. Biol. Chem. 287, 2006–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ercan A., Panico M., Sutton-Smith M., Dell A., Morris H. R., Matta K. L., Gay D. F., West C. M. (2006) Molecular characterization of a novel UDP-galactose:fucoside α3-galactosyltransferase that modifies Skp1 in the cytoplasm of Dictyostelium. J. Biol. Chem. 281, 12713–12721 [DOI] [PubMed] [Google Scholar]
- 6. Wang Z. A., van der Wel H., Vohra Y., Buskas T., Boons G. J., West C. M. (2009) Role of a cytoplasmic dual-function glycosyltransferase in O2 regulation of development in Dictyostelium. J. Biol. Chem. 284, 28896–28904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Wel H., Johnson J. M., Xu Y., Karunaratne C. V., Wilson K. D., Vohra Y., Boons G. J., Taylor C. M., Bendiak B., West C. M. (2011) Requirements for Skp1 processing by cytosolic prolyl 4(trans)-hydroxylase and α-N-acetylglucosaminyltransferase enzymes involved in O2 signaling in Dictyostelium. Biochemistry 50, 1700–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z. A., Singh D., van der Wel H., West C. M. (2011) Prolyl hydroxylation- and glycosylation-dependent functions of Skp1 in O2-regulated development of Dictyostelium. Dev. Biol. 349, 283–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu C., Min J. (2011) Structure and function of WD40 domain proteins. Protein Cell 2, 202–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu L., Gaitatzes C., Neer E., Smith T. F. (2000) Thirty-plus functional families from a single motif. Protein Sci. 9, 2470–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ketcham C., Wang F., Fisher S. Z., Ercan A., van der Wel H., Locke R. D., Sirajud-Doulah K., Matta K. L., West C. M. (2004) Specificity of a soluble UDP-galactose: fucoside α1,3-galactosyltransferase that modifies the cytoplasmic glycoprotein Skp1 in Dictyostelium. J. Biol. Chem. 279, 29050–29059 [DOI] [PubMed] [Google Scholar]
- 12. Stirnimann C. U., Petsalaki E., Russell R. B., Müller C. W. (2010) WD40 proteins propel cellular networks. Trends Biochem. Sci. 35, 565–574 [DOI] [PubMed] [Google Scholar]
- 13. Colley K. J. (1997) Golgi localization of glycosyltransferases: more questions than answers. Glycobiology 7, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ihara H., Ikeda Y., Toma S., Wang X., Suzuki T., Gu J., Miyoshi E., Tsukihara T., Honke K., Matsumoto A., Nakagawa A., Taniguchi N. (2007) Crystal structure of mammalian α1,6-fucosyltransferase, FUT8. Glycobiology 17, 455–466 [DOI] [PubMed] [Google Scholar]
- 15. Iyer S. P., Hart G. W. (2003) Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J. Biol. Chem. 278, 24608–24616 [DOI] [PubMed] [Google Scholar]
- 16. Pedersen J. W., Bennett E. P., Schjoldager K. T., Meldal M., Holmér A. P., Blixt O., Cló E., Levery S. B., Clausen H., Wandall H. H. (2011) Lectin domains of polypeptide GalNAc transferases exhibit glycopeptide binding specificity. J. Biol. Chem. 286, 32684–32696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qasba P. K., Ramakrishnan B. (2007) Letter to the Glyco-Forum: catalytic domains of glycosyltransferases with “add-on” domains. Glycobiology 17, 7G-9G [DOI] [PubMed] [Google Scholar]
- 18. Zapater J. L., Colley K. J. (2012) Sequences prior to conserved catalytic motifs of polysialyltransferase ST8Sia IV are required for substrate recognition. J. Biol. Chem. 287, 6441–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. West C. M., van der Wel H., Wang Z. A. (2007) Prolyl 4-hydroxylase-1 mediates O2 signaling during development of Dictyostelium. Development 134, 3349–3358 [DOI] [PubMed] [Google Scholar]
- 20. Tan A., Tanner J. J., Henzl M. T. (2008) Energetics of OCP1-OCP2 complex formation. Biophys. Chem. 134, 64–71 [DOI] [PubMed] [Google Scholar]
- 21. Fudem-Goldin B., Voulalas P., Orr G. A. (1988) Synthesis and rapid purification of UDP-[6-3H]galactose. J. Biochem. Biophys. Methods 17, 199–202 [DOI] [PubMed] [Google Scholar]
- 22. Furukawa R., Wampler J. E., Fechheimer M. (1990) Cytoplasmic pH of Dictyostelium discoideum amebae during early development: identification of two cell subpopulations before the aggregation stage. J. Cell Biol. 110, 1947–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steck T. L., Chiaraviglio L., Meredith S. (1997) Osmotic homeostasis in Dictyostelium discoideum: excretion of amino acids and ingested solutes. J. Euk. Microbiol. 44, 503–510 [DOI] [PubMed] [Google Scholar]
- 24. Oyama M., Kubota K. (1997) H+ secretion induced by hypertonic stress in the cellular slime mold Dictyostelium discoideum. J. Biochem. 122, 64–70 [DOI] [PubMed] [Google Scholar]
- 25. Sheikh M. O., Schafer C. M., Powell J. T., Rodgers K. K., Mooers B. H. M., West C. M. (2014) Glycosylation of Skp1 affects its conformation and promotes binding to a model F-box protein. Biochemistry, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steimle P. A., Naismith T., Licate L., Egelhoff T. T. (2001) WD-repeat domains target Dictyostelium myosin heavy chain kinases by binding directly to myosin filaments. J. Biol. Chem. 276, 6853–6860 [DOI] [PubMed] [Google Scholar]
- 27. Fiete D., Beranek M., Baenziger J. U. (2012) Peptide-specific transfer of N-acetylgalactosamine to O-linked glycans by the glycosyltransferases β1,4-N-acetylgalactosaminyl transferase 3 (β4GalNAc-T3) and β4GalNAc-T4. J. Biol. Chem. 287, 29204–29212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qian Y., Flanagan-Steet H., van Meel E., Steet R., Kornfeld S. A. (2013) The DMAP interaction domain of UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase is a substrate recognition module. Proc. Natl. Acad. Sci. U.S.A. 110, 10246–10251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fiete D., Beranek M., Baenziger J. U. (2012) Molecular basis for protein-specific transfer of N-acetylgalactosamine to N-linked glycans by the glycosyltransferases β1,4-N-acetylgalactosaminyl transferase 3 (β4GalNAc-T3) and β4GalNAc-T4. J. Biol. Chem. 287, 29194–29203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu Y., Wang Z. A., Green R. S., West C. M. (2012) Role of the Skp1 prolyl-hydroxylation/glycosylation pathway in oxygen dependent submerged development of Dictyostelium. BMC Dev. Biol. 12, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mohanty S., Lee S., Yadava N., Dealy M. J., Johnson R. S., Firtel R. A. (2001) Regulated protein degradation controls PKA function and cell-type differentiation in Dictyostelium. Genes Dev. 15, 1435–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parikh A., Miranda E. R., Katoh-Kurasawa M., Fuller D., Rot G., Zagar L., Curk T., Sucgang R., Chen R., Zupan B., Loomis W. F., Kuspa A., Shaulsky G. (2010) Conserved developmental transcriptomes in evolutionarily divergent species. Genome Biol. 11, R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pierce N. W., Lee J. E., Liu X., Sweredoski M. J., Graham R. L., Larimore E. A., Rome M., Zheng N., Clurman B. E., Hess S., Shan S. O., Deshaies R. J. (2013) Cand1 promotes assembly of new SCF complexes through dynamic exchange of F-box proteins. Cell 153, 206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zemla A., Thomas Y., Kedziora S., Knebel A., Wood N. T., Rabut G., Kurz T. (2013) CSN- and CAND1-dependent remodelling of the budding yeast SCF complex. Nat. Commun. 4, 1641. [DOI] [PubMed] [Google Scholar]
- 35. Wu S., Zhu W., Nhan T., Toth J. I., Petroski M. D., Wolf D. A. (2013) CAND1 controls in vivo dynamics of the cullin 1-RING ubiquitin ligase repertoire. Nat. Commun. 4, 1642. [DOI] [PMC free article] [PubMed] [Google Scholar]