Background: ST6Gal-1 sialyltransferase is a prominent circulatory glycosyltransferase whose function remained in question because an extracellular source of sugar donor substrate was unknown.
Results: Extracellular ST6Gal-1 catalysis uses platelet-supplied sugar donors.
Conclusion: Activated platelets release donor substrates to functionally drive extracellular glycosylation by soluble ST6Gal-1.
Significance: Remodeling by extracellular glycosyltransferases may be an important mechanism to generate physiologically important glycans.
Keywords: Cell Surface, Glycosylation, Plasma, Platelets, Serum, Sialyltransferase
Abstract
Sizable pools of freely circulating glycosyltransferases are in blood, but understanding their physiologic contributions has been hampered because functional sources of sugar donor substrates needed to drive extracellular glycosylation have not been identified. The blood-borne ST6Gal-1 produced and secreted by the liver is the most noted among the circulatory glycosyltransferases, and decorates marrow hematopoietic progenitor cells with α2,6-linked sialic acids and restricts blood cell production. Platelets, upon activation, secrete a plethora of bioactive molecules including pro- and anti-inflammatory mediators. Cargos of sugar donor substrates for glycosyltransferase activity have also been reported in platelets. Here, we implemented a cell-based system to interrogate platelets for their ability to deliver effectively the sugar donor substrate for extracellular ST6Gal-1 to function. We report that thrombin-activated platelets, at physiologic concentration and pH, can efficiently and effectively substitute for CMP-sialic acid in extracellular ST6Gal-1-mediated sialylation of target cell surfaces. Activated platelets can also supply the sialic acid donor to sialylate the synthetic acceptor, Gal(β1,4)GlcNAcα-o-benzyl, with the product Sia(α2,6)Gal(β1,4)GlcNAcα-o-benzyl structurally confirmed by LC/MS. Platelet-secreted donor substrate was recovered in the 100,000 × g sediment, strongly suggesting the association of this otherwise soluble substrate, putatively CMP-sialic acid, within platelet microparticles. Sequestration within microparticles may facilitate delivery of glycosylation substrate at effective dosages to sites of extracellular glycosylation while minimizing excessive dilution.
Introduction
The archetypical mammalian glycosyltransferase that constructs cell surface and secreted glycans is usually regarded as a resident of the endoplasmic reticulum-Golgi secretory apparatus, the intracellular site for nascent glycan assembly. However, sizable pools of extracellular glycosyltransferases exist, particularly in systemic circulation. Among the most studied of these blood-borne, soluble enzymes is the sialyltransferase ST6Gal-1 that constructs the α2,6-sialyl linkage to terminal Gal(β1,4)GlcNAc structures. Altered ST6Gal-1 levels in the blood have long been associated with a wide array of clinical conditions, such as the acute phase response (1–4), chronic inflammation (5), alcoholism (6, 7), and malignancies including breast (4, 8), liver (9, 10), and colon cancers (11, 12). Liver produces much of the blood-borne ST6Gal-1, where its expression and secretion are responsive to circulatory factors such as glucocorticoids and IL-6 (2, 13). Despite the potential of these extrinsic enzymes to remodel glycans on distal cell surfaces, circulatory glycosyltransferases have been generally ignored in recent years, and their true biologic roles have remained elusive. General acceptance for the possibility of extracellular glycosylation has been hampered by the belief that a source for extracellular sugar donor substrates does not exist, despite credible demonstrations in the 1970s that leakage of donor substrates such as CMP-sialic acid, particularly from dying cells, can be sufficient to fuel extracellular sialylation (14).
Recently, we obtained definitive evidence that the α2,6-sialyl structures on hematopoietic progenitor cell surfaces are constructed by the extracellular ST6Gal-1 in circulation, rather than the cell-autonomously expressed enzyme of the individual hematopoietic cells (15). In doing so, the circulatory ST6Gal-1 that principally originates from liver acts as a systemic regulator of hematopoiesis by delaying hematopoietic progenitor proliferation and differentiation. We also demonstrated that disrupted bone marrow cells could provide sufficient sialic acid donor substrate to drive extracellular ST6Gal-1 sialylation of the hematopoietic progenitors. However, significant cell death to provide sufficient sugar donors may be restricted to specialized situations, such as within necrotic tumors or during blood cell generation where apoptotic negative selection is an integral aspect of the process.
Wandall et al. (16) showed recently that cargos of sugar nucleotide substrates reside within platelets, which can be deployed upon platelet activation to support glycosylation of exogenously added tetramethylrhodamine-labeled acceptor substrates to levels detectable by MALDI-TOF. However, under physiologic conditions, it remained in question whether rapid dispersal of the sugar nucleotides upon release might render them too dilute to drive extracellular glycosylation of target cells to an appreciable extent. Here, we adopted a functional approach by implementing a cell-based target system to interrogate platelets for their ability to provide donor substrates for extracellular glycosylation. Coupled with LC/MS product analysis, we confirm that activated platelets are effective suppliers of sugar precursors to efficiently drive extracellular ST6Gal-1 sialylation under physiologic conditions. Taken together, the data strongly implicate platelets as important regulators in extrinsic ST6Gal-1 remodeling of target cell glycans by controlling access to the required sugar donor substrate for extracellular ST6Gal-1 catalysis.
EXPERIMENTAL PROCEDURES
Animals, Cells, and Platelet Preparation
The Institute Animal Care and Use Committee of Roswell Park Cancer Institute have approved all animal studies presented. Mouse strains C57BL/6 (or wild-type, WT) and St6gal1-KO mice were used. The St6gal1-KO mouse has a globally inactivated ST6Gal-1 gene and was obtained originally from the Consortium of Functional Glycomics (17) and has been backcrossed into C57Bl/6 and characterized in this laboratory (5, 18). Platelet-rich plasma was obtained by retro-orbital bleed in with K2-EDTA or sodium citrate anticoagulant (Sigma-Aldrich) and centrifuged at 200 × g. Platelets were separated from plasma proteins by centrifugation at 900 × g (19), washed as described (16), and resuspended in 140 mm NaCl, 3 mm KCl, 0.5 mm MgCl2, 5 mm NaHCO3, 10 mm glucose, and 10 mm HEPES, pH 7.4 (resuspension buffer). Platelets were then activated by the addition of 0.2 units of human thrombin (Sigma-Aldrich) at 37 °C for 5 min. The concentration of platelets was determined via flow cytometry by using 5.5-μm SPHEROTM rainbow fluorescent particles (Spherotech) as a reference. Activated platelet supernatant S-001 was obtained by centrifugation at 1,000 × g for 5 min. The S-001 fraction, which consisted of the released platelet products free of the platelets, was further centrifuged at 100,000 × g for 60 min to generate the soluble (S-100) and sedimentable (P-100) fractions. The P-100 fraction was resuspended at equal volume at which it was obtained from. LK (lineagenegcKitpos) cells were isolated from bone marrow of St6gal1-KO animals. Bone marrow cells were collected from femurs of mice and resuspended in RBC lysis buffer, washed, resuspended in 1× PBS with 0.5% BSA or FBS, and 2 mm EDTA, and passed through a 100-μm cell strainer (BD Biosciences). The cells were centrifuged and resuspended in the same buffer (up to 2 × 108 cells/1 ml), and 50 μl/ml biotin-progenitor cell enrichment mixture was added to the cell suspension. Lineage depletion was accomplished by negative selection using magnetic microparticles according to the manufacturer's protocol (STEMCELL Technologies). LK cells were isolated from lineage-depleted pools using anti-cKit (CD117) microbeads (STEMCELL Technologies for LK cells). Soluble ST6Gal-1 used throughout this study was produced as described elsewhere (15).
Immunofluorescence and Flow Cytometric Analysis of Cells
For Fig. 1, KG1a cells were grown in Iscove's DMEM with 20% FBS supplemented with 1% penicillin-streptomycin-l-glutamine. For immunofluorescence experiments, cells were washed in 1× PBS and fixed in 10% neutral buffered formalin before being attached to glass slides via cytospin. Cells were treated with 0.4 milliunit/ml sialidase C (Sigma-Aldrich) in 1× PBS+ 1% FBS for 45 min at 37 °C. For flow cytometry experiments, cells were treated with sialidase prior to fixation with 1% neutral buffered formalin. Fixed cells were treated with activated platelets from WT and St6gal1-KO animals or their supernatant with or without recombinant ST6Gal-1 enzyme (225 microunits/ml and 30 microunits/ml for immunofluorescence and flow cytometry experiments, respectively) at 37 °C for 2 h in HEPES-buffered solution at pH 7.4. For immunofluorescence experiments, 1 × 109 platelets/ml, the normal physiologic mouse platelet density in blood, were used. For flow cytometry experiments, 4-fold fewer platelets were used to avoid platelet and cell aggregation. As a positive control of α2,6-sialylation, fixed cells were treated with ST6Gal-1 and 100 μm CMP-sialic acid (CMP-Sia). Cells were probed for degree of α2,6-sialylation with fluorescein-labeled Sambucus nigra lectin (SNA)3 (Vector Laboratories, 8 μg/ml) for 1 h at room temperature for immunofluorescence experiments or 15–25 min at 4 °C for flow cytometry. For immunofluorescence experiments, cells were co-stained with phycoerythrin-CD41 (BioLegend, CA; 1 μg/ml) followed by DAPI staining (BioLegend, CA; 0.5 μg/ml). Slides were mounted using FluorSave reagent (Calbiochem). Immunofluorescence imaging was performed by a Nikon Eclipse TE-2000E fluorescent microscope with a 60× objective and captured with a CoolSNAP HQ camera (Photometrix); shutter and image acquisition were controlled by MetaMorph software (MDS Analytical Technologies). Images presented are an average representation of staining intensity. Wide-field images (not shown) showed that cellular staining (typically >90% of cells) or lack thereof is uniform throughout. The unimodal distribution of the flow cytometry of the KG1a cells further supported the uniformity of SNA reactivity. Flow cytometry samples were obtained on a FACSCalibur.
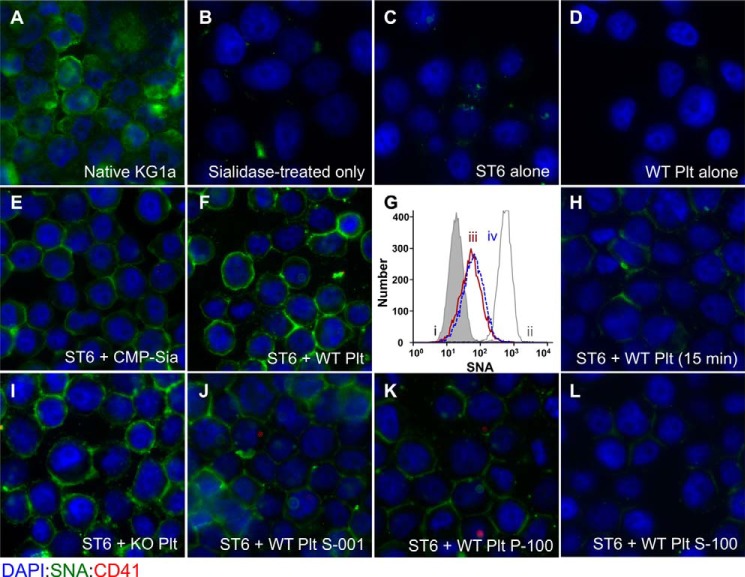
FIGURE 1.
Activated platelet exudates can substitute for CMP-sialic acid in extrinsic ST6Gal-1-mediated sialylation of target cell surfaces. Fixed native KG1a cells were immobilized onto glass slides, sialidase-treated as described under “Experimental Procedures,” and used as target cells to interrogate extrinsic ST6Gal-1-mediated sialylation of the cell surface. Shown are immunofluorescence images of the target cells after staining with DAPI (blue), SNA (green), and the platelet cell surface marker, CD41 (red). A and B, KG1a cells before and after sialidase treatment, respectively. Unless otherwise specifically noted, sialidase-treated target cells were incubated for 120 min as follows: C, ST6Gal-1 alone; D, wild-type activated platelets (WT-Plt) alone; E, ST6Gal-1 and CMP-sialic acid; and F, ST6Gal-1 and WT-Plt. G shows flow cytometric SNA fluorescence intensities of the target cells that were not immobilized onto slides, as follows: sialidase-treated cells (gray filled in line), sialidase-treated cells upon further treatment with ST6Gal-1 with CMP-Sia (gray line), and ST6Gal-1 with WT- or ST6gal1-KO-Plts (red and blue peaks labeled as ii, iii, and iv, respectively). H, ST6Gal-1 with WT-Plt for only 15 min. I, ST6Gal-1 and St6gal1-KO platelets. J–L, ST6Gal-1 with WT-Plt 1,000 × g supernatant, 100,000 × g pellet, and 100,000 × g supernatant, respectively. All incubations with ST6Gal-1 and platelet fractions, unless stated otherwise specifically, were for 120 min at 37 °C in HEPES buffer at pH 7.4. 225 microunits/ml ST6Gal-1 was used for all except the flow cytometric experiment in G, where 30 microunits/ml was used. CMP-sialic acid was at 100 μm (E). Where platelets or platelet products were used, the equivalent starting amount was 109 platelets/ml except for the flow cytometric experiment (G) where 2.5 × 108 platelets/ml was used.
Isolated LK cells were incubated at 37 °C with 1 × 108 St6gal-1KO platelets in the presence or absence of recombinant ST6Gal-1 enzyme (25 microunits/ml) in 250 μl of RPMI for 2 h. Additionally, LK cells were incubated with recombinant ST6Gal-1 and 100 μm CMP-Sia as positive control. For self-sialylation experiments, 5 × 106 platelets from St6gal1-KO mice were incubated with or without recombinant ST6Gal-1 enzyme for 1 h. Cells were probed for degree of α2,6-sialylation with fluorescein-labeled SNA as noted above.
For all ST6Gal-1 enzymatic studies, 1 unit is the enzymatic activity generating 1 μmol of Sia(α2,6)Gal(β1,4)GlcNAc-o-Bn in 1 min (37 °C, pH 6.5) from Gal(β1,4)GlcNAc-o-Bn. SNA, used throughout this study, has a stringent specificity for α2,6-linked sialic acids (20, 21) and was further validated against an extensive panel of >600 glycans. This information is publically available at The Consortium For Functional Glycomics website.
Analysis of Glycosylation via LC/MS
Type-II N-acetyl-d-lactosamine (Gal(β1,4)GlcNAcα-O-Bn) was obtained from Toronto Research Chemicals (Toronto, Canada). The sialyltransferase assay (20 μl) contained 5.0 mm acceptor supplemented with recombinant ST6Gal-1, 5.0 mm CMP-Sia or 5 × 108 platelets/ml buffered with 50 mm sodium cacodylate at pH 7.4. Assays were incubated at 37 °C for 1.5 h. O-Benzyl acceptors were isolated from unreacted sugar nucleotides and other byproducts by C18-reverse phase chromatography (Waters) (22, 23). Isolated O-benzyl acceptors were then permethylated via spin column (Harvard Apparatus) using DMSO (dimethyl sulfoxide), sodium hydroxide, and iodomethane as described (24). Permethylated products were purified via liquid-liquid extraction with dichloromethane and 0.5 m aqueous sodium chloride. The dried products were reconstituted in 2:1 water/methanol, and approximately half of the total sample mass was injected on the column. LC/MS experiments were performed using an LTQ (Thermo Fisher) equipped with an electrospray source interfaced with a Thermo Surveyor MS pump and autosampler. Separation was performed with a BDS Hypersil C18 column (2.1 × 150, 3 μm) and a gradient of 25 μm sodium acetate and acetonitrile at a 200 μl/min flow rate. The sodium acetate modifier was added to provide sodium ions for the MS analysis rather than to effect any separation. MS data were acquired in profile mode. Identification of products was accomplished by collecting fractions eluting from the LC column followed by direct infusion and MSn analysis (25). Offline analysis was required due to the limited LC/MS timescale. Once peak identification was accomplished, subsequent analyses were based on retention time and intact mass of the eluting products. Chromatograms shown are extracted ion chromatograms (range 955–956 for sialylated products) with 7-point boxcar-type smoothing.
RESULTS AND DISCUSSION
Platelets Can Substitute for CMP-Sialic Acid in ST6Gal-1 Sialylation of Target Cells
Wandall et al. (16) recently demonstrated that circulating platelets have cargos of sugar nucleotides that can be released upon activation. Likewise, dying cells also release their intracellular content of sugar nucleotides (14). These extracellular sugar nucleotides have the potential to fuel extracellular glycan construction by the extrinsic glycosyltransferases. However, the extracellular milieu is a complex, dynamic environment where released substrates can be diluted rapidly. Thus classic considerations of sugar nucleotide concentration in circulation may be a meaningless way to judge whether sufficient sugar donors are available to drive substantive extrinsic enzyme catalysis, which might occur transiently and only at highly localized sites. We therefore implemented a simple cell-based platform to measure functional extracellular glycosylation at physiologic conditions. The platform, consisting of target KG1a cells that have been fixed and immobilized onto glass slides, allows rapid interrogation of cellular sources for extrinsic enzymes and sugar donor substrates. The interrogation of mouse platelets as suppliers of sialic acid donor substrates is summarized in Fig. 1. Native KG1a cells, a human bone marrow-derived myeloid progenitor-like cell line, is normally richly decorated with cell surface α2,6-linked sialic acids, as visualized by the bright green cell surface binding of fluorescein-labeled SNA (Fig. 1A) that was completely removed upon treatment with sialidase C (Fig. 1B). The sialidase-treated, immobilized cells were used to interrogate functional extracellular sialylation by ST6Gal-1. Treatment of the immobilized cells either with ST6Gal-1 alone or with thrombin-activated platelets alone was insufficient to restore SNA binding (Fig. 1, C and D, respectively), but SNA reactivity was restored by ST6Gal-1 with added CMP-Sia (Fig. 1E). As demonstrated clearly in Fig. 1F, thrombin-activated platelets can efficiently substitute CMP-Sia in bestowing cell SNA reactivity in the presence of ST6Gal-1, where typically >90% of the target cells became SNA-positive using either CMP-Sia or activated platelets. 109/ml platelet density was used, which is the density of platelets normally in circulation in the mouse, and the incubations were carried out at pH 7.4 at 37 °C, mimicking physiological conditions.
A more quantitative approach to measure fluorescein-SNA reactivity on target cells is shown in Fig. 1G, whereby flow cytometry of fixed but not immobilized target cells was performed. In the presence of 8-fold fewer ST6Gal-1, 2.5 × 108 activated platelets/ml could restore surface SNA reactivity to target cells. This was true for either platelets from WT mice (line ii) or St6gal1-KO platelets. SNA reactivities of the sialidase-treated target cells (shaded gray line) and the positive control with KG1a cells treated with CMP-Sia and ST6Gal-1 (gray line) have been included as references.
A number of lines of evidence challenged the possibility that the reacquired SNA reactivity was due to the passive transfer of α2,6-sialylated platelet glycans onto the cells. First, acquisition of target cell SNA reactivity was time-dependent, and incubation with ST6Gal-1 and activated platelets for 15 min rather than 120 min resulted only in minimal SNA reactivity (Fig. 1H). This strongly suggests that the process was not absorption of prefabricated sialylated platelet glycoproteins to the target cell surface because an absorption mechanism is expected to have significantly more rapid kinetics. Secondly, St6gal1-KO platelets, which do not have prefabricated α2,6-linked sialyl glycans, were equally effective as wild-type platelets in driving ST6Gal-1 sialylation (Fig. 1G, line iii, and Fig. 1I). Finally, the use of phycoerythrin-conjugated CD41, a platelet cell surface marker, did not reveal significant nonspecific transfer of platelet fragments to the target cells after the washing steps (Fig. 1, A–L). Together, these observations demonstrate that platelets, at physiologic densities and pH, can efficiently supply the sialic acid donor co-substrate to functionally drive extracellular glycosylation by extrinsic ST6Gal-1.
Direct platelet contact with the target cells was not necessary for ST6Gal-1 sialylation of the targets. To demonstrate this, activated platelets were sedimented by 1,000 × g centrifugation, leaving only the exudates of platelets in the supernatant (S-001). Fig. 1J shows that the wild-type platelet S-001 fraction was equally effective in driving target cell sialylation. Activated platelets released a wide range of bioactive molecules participating in hemostasis and thrombosis, inflammation, angiogenesis, and immunity (26). Some of these molecules are released as freely soluble factors, whereas others are released in association with microparticles. To determine whether the platelet sialic acid donor was released as a freely soluble factor or in some way associated with vesicles, e.g. platelet microparticles, 100,000 × g sedimentation was performed on the S-001 fraction to generate S-100 and P-100 fractions that represent the soluble and microparticle-associated materials, respectively. The required sialylation substrate for ST6Gal-1 was enriched in the P-100 fraction (Fig. 1K) but not in the S-100 fraction (Fig. 1L). The data above strongly suggest that platelet sialic acid donor substrates are delivered by microparticles, but it remains to be determined in which type of granules are these sugar donor substrates sequestered. Presumably, the platelet-supplied sialic acid donor substrate is CMP-Sia, which Wandall et al. (16) have already reported to be present in human platelets in 104 pmol/mg concentration. However, the possibility of additional platelet co-factors that facilitate extracellular sialylation at physiologic conditions cannot be discounted at this time.
In a separate study, we showed that the surface α2,6-sialyl structures of hematopoietic progenitor cells were generated exclusively by extrinsic ST6Gal-1 rather than the endogenously expressed enzyme, and the action of the extrinsic sialyltransferase resulted in hematopoietic arrest (15). Here, we demonstrate that activated platelets can functionally support extrinsic ST6Gal-1 sialylation of hematopoietic progenitor cells. Hematopoietic stem and progenitor cells, operationally represented by Linneg cKitpos cells (LK), served as sialylation targets. LK cells from the globally ST6Gal-1 defective mouse, St6gal1-KO, were used because these cells do not have the inherent ability to autonomously generate α2,6-sialyl structures. In Fig. 2A, LK cells were fixed, sialidase-treated, and immobilized identically to the previously used KG1a target cells (Fig. 2A, panel a). The LK cells acquire cell surface SNA reactivity upon incubation with extrinsic ST6Gal-1 and activated platelets (Fig. 2A, panel b). St6gal1-KO platelets and the P-100 microparticle-enriched fraction were also competent in driving sialylation of the LK cells by extrinsic ST6Gal-1 (data not shown).
FIGURE 2.
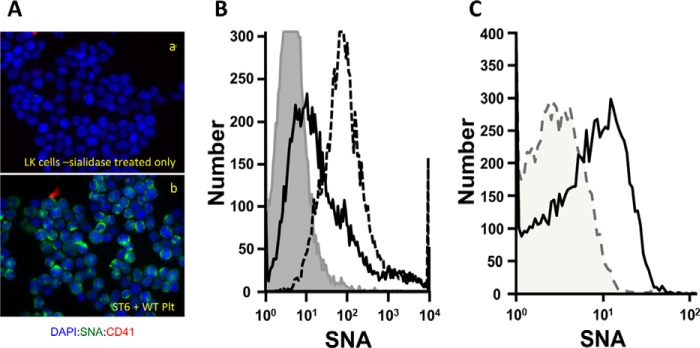
Cell surface remodeling of primary hematopoietic cells by extrinsic ST6Gal-1 and activated platelets. A, platelet-assisted ST6Gal-1 remodeling of sialidase-treated primary hematopoietic progenitor cells. Hematopoietic progenitors represented by the LK cells from the St6gal1-KO marrow were sialidase-treated, immobilized on glass slides as per the preparation of KG1a cells in Fig. 1, and visualized after staining with DAPI (blue), SNA (green), and the platelet cell surface marker, CD41 (red). Shown are the sialidase-treated LK cells without (panel a) and after incubation with ST6Gal-1 (25 microunits/ml) and thrombin-activated platelets (1 × 108/ml) at 37 °C in RPMI medium for 120 min (panel b). B, extrinsic sialylation of St6gal1-KO LK cells does not require sialidase pretreatment. Native LK cells from the St6gal1-KO marrow were incubated in suspension for 120 min at 37 °C in RPMI medium in the presence of ST6Gal-1 (25 microunits/ml), with 1 × 108/ml thrombin-activated St6gal1-KO platelets (solid line), with 100 μm CMP-sialic acid (dashed line), or with ST6Gal-1 alone (shaded area). C, St6gal1-KO platelets self-sialylate with added ST6Gal-1. 5 × 106 activated St6gal1-KO platelets were incubated for 60 min at 37 °C in RPMI in the presence (solid line) or absence (dashed line) of added ST6Gal-1 (25 microunits/ml).
Living, rather than fixed LK cells are also efficiently sialylated by extrinsic ST6Gal-1 in the presence of activated platelets, as shown by the flow cytometric analysis in Fig. 2B. Moreover, pretreatment of the LK cells with sialidase was not required. For this study, LK cells from St6gal1-KO marrow that cannot express endogenous ST6Gal-1 and are natively α2,6-sialic acid-deficient were used. St6gal1-KO platelets, which do not have endogenous α2,6-sialyl glycans, were also used to minimize the possibility of nonspecific absorption of prefabricated platelet glycans by the live LK cells. The extent of sialylation using platelets was not as high as that using 100 μm CMP-Sia (dashed line), but the reduced sialylation is likely because the amount of platelets used was 10-fold reduced from that used for the fixed and immobilized cells (1 × 108/ml, as compared with 109/ml used in Fig. 2A, panel b). A higher concentration of platelets could not be used for the living cells due to technical difficulties encountered relating to excessive platelet-cell aggregation at the higher density under these incubation conditions. Finally, to definitively demonstrate that platelets can supply the sialic acid donor, St6gal1-KO platelets incubated alone with ST6Gal-1 also acquire SNA reactivity (Fig. 2B). Because there were only two components in this experiment, platelets and ST6Gal-1, the only possible source for sialic acid was the platelets. For these live cell studies, physiologically relevant conditions, including RPMI medium at pH 7.4, were used. We used 108/ml platelets, whereas platelet concentration in normal systemic circulation is 10-fold higher, at 109/ml, and no doubt significantly higher in thrombotic events where activated platelets aggregate. We used 25 microunits/ml ST6Gal-1, which is within the level normally encountered in human blood (4.5–13.5 microunits/ml in healthy volunteers at baseline, and it can elevate severalfold during systemic inflammation).4 Altogether, the data demonstrate that platelets contain a sufficient cargo of sialic acid donor substrate that can functionally and effectively drive physiologic extracellular sialylation of target glycans.
LC/MS Confirmation That Platelets Drive ST6Gal-1 Sialylation of Artificial Acceptor Substrates
To definitively confirm that platelets supply the sialic acids for extracellular ST6Gal-1 sialylation, the synthetic acceptor substrate, Gal(β1,4)GlcNAc-o-Bn, was used. Conversion of the acceptor into the products Sia(α2,3/6)Gal(β1,4)GlcNAc-o-Bn yields the ion m/z 955 by MS, which when subjected to LC analysis yielded two peaks with retention times of 27 and 28 min, corresponding to the α2,6- and α2,3-linked sialyl-trisaccharides, respectively (Fig. 3A). The sialyl-trisaccharide products were not generated by either ST6Gal-1 or platelets alone (Fig. 3, B and C, respectively). In the presence of both platelets and ST6Gal-1, an LC/MS peak consistent with the α2,6-sialyl-trisaccharide product was identified (Fig. 3E), which eluted with an identical retention time as the sialyl-trisaccharide product produced when ST6Gal-1 and CMP-Sia were used (Fig. 3D). It is important to note that 5 mm CMP-Sia was used to saturate the reaction to ensure a clean reference product signal (Fig. 3D). If CMP-Sia was more limiting, a correspondingly reduced peak intensity resembling Fig. 3E would be expected.
FIGURE 3.
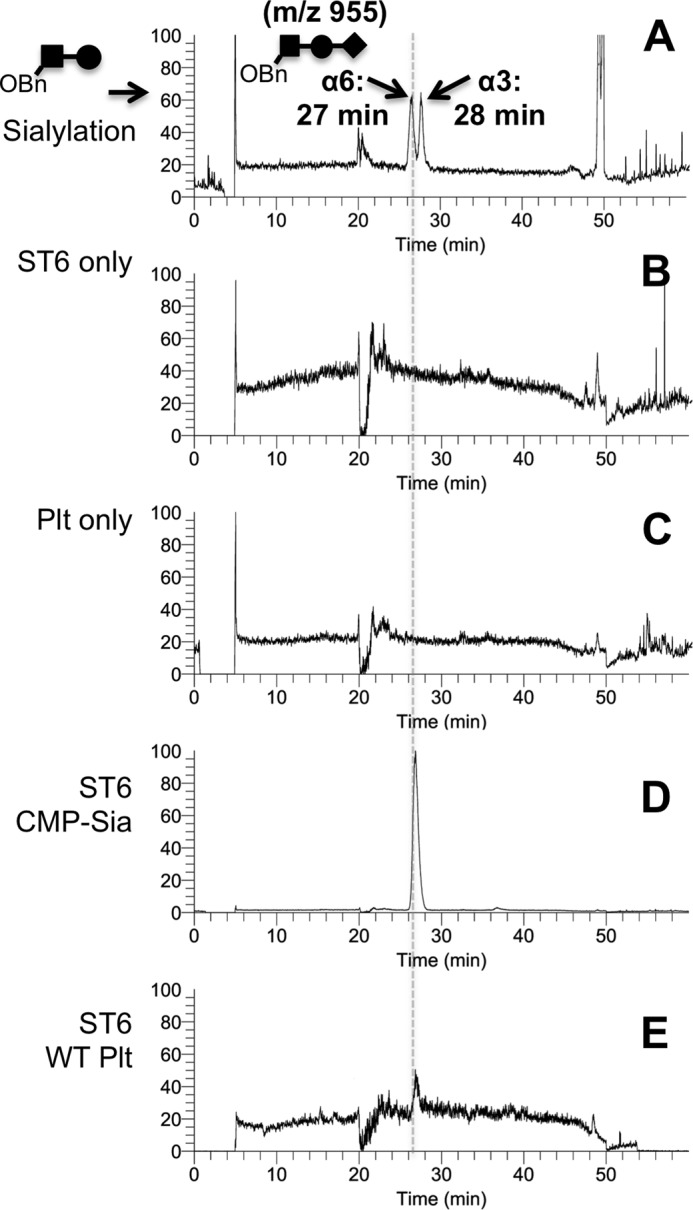
Activated platelets can replace CMP-sialic acid in the sialylation of Gal(β1,4)GlcNAcα-o-Bn. The conversion of the synthetic acceptor substrate to Sia(α2,6)Gal(β1,4)GlcNAcαo-Bn was monitored by LC/MS, as illustrated using authentic standards in A, where the m/z 955 MS ion that is diagnostic of the trisaccharide yields resolution of α2,6- and α2,3-trisaccharide products on LC, as described under “Experimental Procedures.” B–E, the synthetic acceptor substrate was incubated as follows: B, ST6Gal-1 alone; C, wild-type platelets alone; D, ST6Gal-1 and CMP-sialic acid (5 mm); and E, ST6Gal-1 with wild-type activated platelets (5 × 108/ml). Incubations were performed in 50 mm sodium cacodylate at pH 7.4 for 90 min.
Platelets, which are derived from fragmentation of megakaryocytes in the bone marrow, are best known for their role in the maintenance of hemostasis, as in the formation of blood clots in vessel injury. Numerous granules are packed within platelets containing bioactive molecules important not only for hemostasis and wound repair, such as fibrinogen and von Willebrand factor, but also important modifiers of immunity, such as inflammatory and anti-inflammatory chemokines, as well as adhesive mitogenic and angiogenic factors (27). In fact, a proteomic analysis indicated that platelets have the ability to manufacture and secrete more than 300 different proteins following activation (28). Physical interaction of platelets with other blood cells and vascular endothelium through complex interactions involving selectins and integrins further promotes the roles of platelets in diverse aspects of diseases including atherosclerosis (29), sepsis (30, 31), rheumatoid arthritis (32, 33), and cancer (34, 35). Wandall et al. (16) demonstrated the presence of sugar nucleotides in platelets and proposed that they can be used to glycosylate extracellular acceptors. Here, we demonstrate unambiguously that, in physiologic relevant conditions including pH, enzyme, and platelet concentrations, activated platelets can efficiently supply the sugar donor substrate for extracellular ST6Gal-1-mediated remodeling of target cells. Altogether, we propose a heretofore previously unrecognized capability of platelets, which is to supply a sugar donor substrate critical to drive glycosylation by a circulatory, extracellular glycosyltransferase. Glycans occupy the critical interface between each cell and its outside environment. Extrinsic glycosyltransferases, such as the circulatory ST6Gal-1, can alter cell behavior by remodeling the glycan interface (15). Because circulatory ST6Gal-1 is freely available, platelets may serve as a pivotal moderator of glycosylation by controlling the release of the necessary substrates.
This work was supported, in whole or in part, by National Institutes of Health Grants P01HL107146 and R01AI056082 (to J. T. Y. L.).
This article was selected as a Paper of the Week.
J. Lau, unpublished observation.
- SNA
- S. nigra L. agglutinin
- Bn
- benzyl
- Plt
- platelets.
REFERENCES
- 1. Kaplan H. A., Woloski B. M., Hellman M., Jamieson J. C. (1983) Studies on the effect of inflammation on rat liver and serum sialyltransferase: evidence that inflammation causes release of Galβ1→4GlcNAc α2→6 sialyltransferase from liver. J. Biol. Chem. 258, 11505–11509 [PubMed] [Google Scholar]
- 2. Dalziel M., Lemaire S., Ewing J., Kobayashi L., Lau J. T. Y. (1999) Hepatic acute phase induction of murine β-galactoside α2,6-sialyltransferase (ST6Gal I) is IL-6 dependent and mediated by elevation of exon H-containing class of transcripts. Glycobiology 9, 1003–1008 [DOI] [PubMed] [Google Scholar]
- 3. Jamieson J. C., Lammers G., Janzen R., Woloski B. M. (1987) The acute phase response to inflammation: the role of monokines in changes in liver glycoproteins and enzymes of glycoprotein metabolism. Comp. Biochem. Physiol. B. 87, 11–15 [DOI] [PubMed] [Google Scholar]
- 4. Bernacki R. J., Kim U. (1977) Concomitant elevations in serum sialytransferase activity and sialic acid content in rats with metastasizing mammary tumors. Science 195, 577–580 [DOI] [PubMed] [Google Scholar]
- 5. Nasirikenari M., Chandrasekaran E. V., Matta K. L., Segal B. H., Bogner P. N., Lugade A. A., Thanavala Y., Lee J. J., Lau J. T. (2010) Altered eosinophil profile in mice with ST6Gal-1 deficiency: an additional role for ST6Gal-1 generated by the P1 promoter in regulating allergic inflammation. J. Leukoc. Biol. 87, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sillanaukee P., Pönniö M., Seppä K. (1999) Sialic acid: new potential marker of alcohol abuse. Alcohol Clin Exp Res. 23, 1039–1043 [DOI] [PubMed] [Google Scholar]
- 7. Gong M., Garige M., Hirsch K., Lakshman M. R. (2007) Liver Galβ1,4GlcNAc α2,6-sialyltransferase is down-regulated in human alcoholics: possible cause for the appearance of asialoconjugates. Metabolism 56, 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ip C., Dao T. (1978) Alterations in serum glycosyltransferases and 5′-nucleotidase in breast cancer patients. Cancer Res. 38, 723–728 [PubMed] [Google Scholar]
- 9. Kim Y. S., Perdomo J., Whitehead J. S., Curtis K. J. (1972) Glycosyltransferases in human blood: II. Study of serum galactosyltransferase and N-acetylgalactosaminyltransferase in patients with liver diseases. J. Clin. Invest. 51, 2033–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poon T. C., Chiu C. H., Lai P. B., Mok T. S., Zee B., Chan A. T., Sung J. J., Johnson P. J. (2005) Correlation and prognostic significance of β-galactoside α-2,6-sialyltransferase and serum monosialylated α-fetoprotein in hepatocellular carcinoma. World J Gastroenterol. 11, 6701–6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiser M. M., Podolsky D. K., Iselbacher K. J. (1976) Cancer-associated isoenzyme of serum galactosyltransferase. Proc. Natl. Acad. Sci. U.S.A. 73, 1319–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gessner P., Riedl S., Quentmaier A., Kemmner W. (1993) Enhanced activity of CMP-neuAc:Gal β1–4GlcNAc:α2,6-sialyltransferase in metastasizing human colorectal tumor tissue and serum of tumor patients. Cancer Lett. 75, 143–149 [DOI] [PubMed] [Google Scholar]
- 13. Wang X. C., O'Hanlon T. P., Lau J. Y. (1989) Regulation of β-galactoside α2,6-sialyltransferase gene expression by dexamethasone. J. Biol. Chem. 264, 1854–1859 [PubMed] [Google Scholar]
- 14. Hoflack B., Cacan R., Montreuil J., Verbert A. (1979) Detection of ectosialyltransferase activity using whole cells. Biochim. Biophys. Acta 568, 348–356 [DOI] [PubMed] [Google Scholar]
- 15. Nasirikenari M., Veillon L., Collins C. C., Azadi P., Lau J. T. Y. (2014) Remodeling of marrow hematopoietic stem and progenitor cells by non-self ST6Gal-1 sialytransferase. J. Biol. Chem. 289, 7178–7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wandall H. H., Rumjantseva V., Sørensen A. L. T., Patel-Hett S., Josefsson E. C., Bennett E. P., Italiano J. E., Jr., Clausen H., Hartwig J. H., Hoffmeister K. M. (2012) The origin and function of platelet glycosyltransferases. Blood 120, 626–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin L. T., Marth J. D., Varki A., Varki N. M. (2002) Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J. Biol. Chem. 277, 32930–32938 [DOI] [PubMed] [Google Scholar]
- 18. Nasirikenari M., Segal B. H., Ostberg J. R., Urbasic A., Lau J. T. (2006) Altered granulopoietic profile and exaggerated acute neutrophilic inflammation in mice with targeted deficiency in the sialyltransferase ST6Gal I. Blood 108, 3397–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffmeister K. M., Felbinger T. W., Falet H., Denis C. V., Bergmeier W., Mayadas T. N., von Andrian U. H., Wagner D. D., Stossel T. P., Hartwig J. H. (2003) The clearance mechanism of chilled blood platelets. Cell 112, 87–97 [DOI] [PubMed] [Google Scholar]
- 20. Toma V., Zuber C., Winter H. C., Goldstein I. J., Roth J. (2001) Application of a lectin from the mushroom Polysporus squamosus for the histochemical detection of the NeuAcα2,6Galβ1,4Glc/GlcNAc sequence of N-linked oligosaccharides: a comparison with the Sambucus nigra lectin. Histochem Cell Biol. 116, 183–193 [DOI] [PubMed] [Google Scholar]
- 21. Shibuya N., Goldstein I. J., Broekaert W. F., Nsimba-Lubaki M., Peeters B., Peumans W. J. (1987) The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(α2–6)Gal/GalNAc sequence. J. Biol. Chem. 262, 1596–1601 [PubMed] [Google Scholar]
- 22. Appenheimer M. M., Huang R.-Y., Chandrasekaran E. V., Dalziel M., Hu Y. P., Soloway P. D., Wuensch S. A., Matta K. L., Lau J. T. Y. (2003) Biologic contribution of P1 promoter-mediated expression of ST6Gal I sialyltransferase. Glycobiology 13, 591–600 [DOI] [PubMed] [Google Scholar]
- 23. Ujita M., McAuliffe J., Schwientek T., Almeida R., Hindsgaul O., Clausen H., Fukuda M. (1998) Synthesis of poly-N-acetyllactosamine in core 2 branched O-glycans: the requirement of novel β-1,4-galactosyltransferase IV and β-1,3-N-acetylglucosaminyltransferase. J. Biol. Chem. 273, 34843–34849 [DOI] [PubMed] [Google Scholar]
- 24. Kang P., Mechref Y., Klouckova I., Novotny M. V. (2005) Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun. Mass Spectrom. 19, 3421–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashline D. J., Hanneman A. J., Zhang H., Reinhold V. N. (2014) Structural documentation of glycan epitopes: Sequential mass spectrometry and spectral matching. J. Am. Soc. Mass. Spectrom. 25, 444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Italiano J. E., Jr., Mairuhu A. T. A., Flaumenhaft R. (2010) Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 17, 578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Semple J. W., Italiano J. E., Jr., Freedman J. (2011) Platelets and the immune continuum. Nat. Rev. Immunol. 11, 264–274 [DOI] [PubMed] [Google Scholar]
- 28. Coppinger J. A., Cagney G., Toomey S., Kislinger T., Belton O., McRedmond J. P., Cahill D. J., Emili A., Fitzgerald D. J., Maguire P. B. (2004) Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 103, 2096–2104 [DOI] [PubMed] [Google Scholar]
- 29. Huo Y., Ley K. F. (2004) Role of platelets in the development of atherosclerosis. Trends Cardiovasc. Med. 14, 18–22 [DOI] [PubMed] [Google Scholar]
- 30. Secor D., Li F., Ellis C. G., Sharpe M. D., Gross P. L., Wilson J. X., Tyml K. (2010) Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med. 36, 1928–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogura H., Kawasaki T., Tanaka H., Koh T., Tanaka R., Ozeki Y., Hosotsubo H., Kuwagata Y., Shimazu T., Sugimoto H. (2001) Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma. 50, 801–809 [DOI] [PubMed] [Google Scholar]
- 32. Schmitt-Sody M., Metz P., Gottschalk O., Birkenmaier C., Zysk S., Veihelmann A., Jansson V. (2007) Platelet P-selectin is significantly involved in leukocyte-endothelial cell interaction in murine antigen-induced arthritis. Platelets 18, 365–372 [DOI] [PubMed] [Google Scholar]
- 33. Knijff-Dutmer E. A. J., Koerts J., Nieuwland R., Kalsbeek-Batenburg E. M., Van de Laar M. A. F. J. (2002) Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 46, 1498–1503 [DOI] [PubMed] [Google Scholar]
- 34. Gay L. J., Felding-Habermann B. (2011) Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11, 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bambace N. M., Holmes C. E. (2011) The platelet contribution to cancer progression. J. Thromb. Haemost. 9, 237–249 [DOI] [PubMed] [Google Scholar]