Background: Quelling and DNA damage induce small RNA from repetitive DNA regions.
Results: Genetic Screen identified RTT109 as a critical component of the siRNA production pathway in Neurospora.
Conclusion: RTT109 acts as a histone H3K56 acetyltransferase to mediate siRNA biogenesis through its role in homologous recombination.
Significance: This study identifies a new link between DNA damage response and small RNA production at the chromatin level.
Keywords: Homologous Recombination, Neurospora, RNA Interference (RNAi), RNA Silencing, siRNA
Abstract
Quelling and DNA damage-induced small RNA (qiRNA) production are RNA interference (RNAi)-related phenomenon from repetitive genomic loci in Neurospora. We have recently proposed that homologous recombination from repetitive DNA loci allows the RNAi pathway to recognize repetitive DNA to produce small RNA. However, the mechanistic detail of this pathway remains largely unclear. By systematically screening the Neurospora knock-out library, we identified RTT109 as a novel component required for small RNA production. RTT109 is a histone acetyltransferase for histone H3 lysine 56 (H3K56) and H3K56 acetylation is essential for the small RNA biogenesis pathway. Furthermore, we showed that RTT109 is required for homologous recombination and H3K56Ac is enriched around double strand break, which overlaps with RAD51 binding. Taken together, our results suggest that H3K56 acetylation is required for small RNA production through its role in homologous recombination.
Introduction
RNA interference (RNAi) is a post-transcriptional gene-silencing mechanism conserved from fungi to mammals (1–3). In most of the RNAi-related biogenesis pathways, the RNase III domain-containing enzyme Dicer cleaves double-stranded RNA/hairpin RNA to generate 21- to 25-nt small interfering RNA (siRNA) duplexes. The siRNAs are loaded onto an Argonaute (Ago) protein-containing complex called the RNA-induced silencing complex to mediate gene silencing of homologous RNAs. Despite its divergent roles in regulating gene expression, RNAi has been considered as an ancient genome defense mechanism that silences viral invasion and transposons (4–8). Consistent with this role, many organisms have developed a mechanism to produce small RNAs from repetitive DNA sequences that result from transposon replication or foreign DNA incorporation (9–11).
Neurospora crassa is known to have two types of siRNA pathways during the vegetative stage, quelling, and qiRNA2 production (10, 12). Quelling is triggered by the presence of multiple copies of transgenes and produces site-specific siRNAs from repetitive transgenic loci (1). We previously discovered a class of small RNAs that are induced after DNA damage agent treatment, known as qiRNA because of its interaction with Ago protein QDE-2 (12, 13). The majority of qiRNAs originate from ribosomal DNA cluster, which is the only highly repetitive DNA sequence in Neurospora (12). Although quelling occurs during normal growth condition and qiRNA is produced after DNA damage, their biogenesis is mechanistically similar. It has been proposed that the dual functional QDE-1 (quelling-deficient-1) first acts as a DNA-dependent RNA polymerase to produce aberrant RNA (aRNA) from repetitive loci and then covert it to double-stranded RNA precursor through its RNA-dependent RNA polymerase activity (14). RecQ DNA helicase QDE-3 and the single-stranded DNA binding complex replication protein A are also involved in this process (14–16). dsRNA is cleaved by Dicer proteins to produce siRNA, which is then loaded onto QDE-2 to mediate gene silencing (17). Although how siRNA is produced from dsRNA and acts are well understood, little is known about how aRNA and siRNA is specifically transcribed from repetitive loci.
Our recent study showed that both quelling and qiRNA production are caused by DNA damage/replication stress (18). DNA damage agent treatment can induce quelling-induced siRNA in the quelled strain. Similar to qiRNA, it was recently shown in plants and animals that DNA damage is a common trigger to induce small non-coding RNAs (19–21). We recently showed that homologous recombination (HR) pathway is required for quelling and qiRNA production. This lead to the proposal that HR serves a mechanism to recognize repetitive DNA and initiates aRNA transcription under DNA damage condition (18). However, the mechanistic details of this HR-based siRNA biogenesis pathway are not clear. A number of chromatin remodelers are also required for both HR and siRNA pathway in Neurospora (18), indicating that a favorable chromatin environment is essential for this siRNA biogenesis pathway (18).
In this study, by systematically screening the Neurospora knock-out mutants, we identify RTT109, a histone acetyltransferase for histone H3 on lysine 56, as a new component in the qiRNA production and quelling pathway. In addition, we demonstrate that RTT109 and its histone acetyltransferase activity are required for HR and the qiRNA production and quelling pathway.
EXPERIMENTAL PROCEDURES
Strains and Growth Conditions
A wild-type strain of Neurospora crassa (FGSC4200) was used in this study. The Neurospora knock-out mutant strains used in this study were obtained from the Fungal Genetic Stock Center (22). Strains carrying the I-SceI restriction site was first transformed with an al-1 gene containing an I-SceI site into the his-3 locus. Plasmids that express FLAG-tagged ISceI and Myc-RAD51, were co-transformed into Neurospora together with a benomyl resistance gene, BmlR. The expression of both FLAG-ISceI and Myc-RAD51 were confirmed by Western blot analysis after quinic acid (QA) induction. Liquid cultures were grown in minimal medium (1× Vogel's, 2% glucose). For liquid cultures containing QA, 10−3 m QA (pH 5.8), was added to the culture medium containing 1× Vogel's, 0.1% glucose, and 0.17% arginine. To induce qiRNA production, histidine (1 mg/ml) or the indicated concentrations of hydroxyurea (HU) were added, and cultures were collected 48 h later (12).
Protein and RNA Analyses
Protein extraction, quantification, and Western blot analysis were performed as described previously (18, 23). Nuclear protein extracts were prepared as described previously (24). Equal amounts of total protein (40 μg) were loaded in each lane, and after electrophoresis, proteins were transferred onto PVDF membrane, and Western blot analysis was performed. Antibodies against H3K56Ac were purchased from Active Motif (catalog no. 39281). Antibodies against histone H3 and γΗ2Α were purchased from Abcam (ab1791 and ab15083, respectively). Immunoprecipitation followed by mass spectrometry was performed based on protocol described previously (23) to identify RTT109-interacting proteins. The resulting MS files were searched against NCBI-nr protein sequence databases for protein identification.
Total RNA extraction, enrichment of small sized RNA, and Northern blots were performed as described previously (17). RNA probes were made using the MAXIscript T7 kit (Ambion) from a T7 promoter on a PCR product template. The qiRNA probe specifically detects antisense small RNA from the 26 S rDNA region and aRNA probes were made to detect intergenic transcripts from the rDNA region as described previously (18).
Quelling Assay
Quelling assays were performed as described previously with minor modifications (25). The wild-type and the mutant strains used for quelling assays were grown for 7–10 days before harvesting the conidia in 1 m sorbitol. A mixture of 2 mg pBSKal-1 (carrying the al-1 fragment) and 0.5 mg of pBT6 (a benomyl-resistant gene-containing plasmid) was incubated with the conidial suspension for 4–5 h at 4 °C. The plasmids were then transformed into Neurospora by electroporation. The benomyl-resistant transformants were picked onto minimal slants and visually inspected to identify the completely quelled (white), partially quelled (yellow), or non-quelled (orange) strains.
Assay for DNA Damage Sensitivity
A spot test was used for measuring the sensitivity of different strains to various DNA mutagens. The conidia concentration of conidia suspensions was measured and dropped onto sorbose-containing agar plates with indicated serial dilutions. The plates were incubated for 3 days at room temperature. Camptothecin (CPT), histidine, or ethyl methanesulfonate was added into agar medium at a final concentration of 0.1 μg/ml, 6 μg/ml, and 0.2%, respectively. The description of the expression I-Scel in Neurospora will be described elsewhere.3
Homologous Recombination Assay
The HR assay was performed essentially as described (26). The Neurospora strains were grown for 7–10 days before harvesting the conidia in 1 m sorbitol. The EcoRI linearized bar-containing plasmid (pGS1–1KR, contains bar gene flanked by 1 kb of homologous sequence of mtr) was incubated with the conidial suspension on ice for 30 min. The fragment was transformed into Neurospora strains by electroporation. The transformed conidia were plated onto low nitrogen-containing top agar containing 0.4 mg/ml bialaphos. The bialaphos-resistant transformants were picked onto bialaphos-containing slants, and resistant transformants were further selected on p-fluorophenylalanine-containing slants. The HR rate was calculated as the ratio between the p-fluorophenylalanine-resistant colonies to the total bialaphos-resistant colonies.
Chromatin Immunoprecipitation (ChIP)
ChIP experiment was performed as described previously (18). A double strand break (DSB) was induced by culture in liquid medium containing QA for 2 days. The tissues were fixed in the culturing medium containing 1% formaldehyde for 15 min with shaking. After fixation and washing, cell lysates were subject to sonication with three cycles of 25 pulses with duty cycle 40 and output control 4. The samples of 2 mg of protein were precleared with 40 μl of slurry equilibrated with Gamma Sepharose beads for 2 h at 4 °C with rotation. The blocking beads were resuspended, and the lysate was transferred to a new tube. The monoclonal c-Myc antibody or antibody specific for H3K56ac was used to detect enrichment of Myc-RAD51 or H3K56Ac around DSB. The ChIP samples were diluted 1 to 2.5 before use as templates for quantitative PCR analysis. Tubulin gene was used as an internal control to normalize the ChIP-quantitative PCR data. Specific primers detecting the al-1 gene were qal-1F (5′-AAGGTGTTGGACGCTTTGGT-3′) and qal-1R (5′-GTACTTGACGCCCATCCTCTCT-3′). Primers detecting the am-1 gene were amf (5′-CGGTTACCGTGTCCAGTTCA-3′) and amR (5′-CTAGAGACGCCGAGTCAGCA-3′). Primers detecting the tubulin gene were q03 (5′-CTCCTCCTCCTCGTCAACACCA-3′) and q04 (5′-CTCAAGATGTCCTCCACCTTCG-3′).
RESULTS
A Genetic Screen Identified RTT109 to Be Essential for the DNA Damage-induced qiRNA Production and Quelling
We sought to characterize the mechanism of the DNA damage-induced qiRNA production by carrying a systematic genetic screen using the Neurospora knock-out library (18). Previously, we demonstrated that DNA damage agents, such as histidine, HU, or ethyl methanesulfonate, are able to induce the production of qiRNA and QDE-2 protein expression in Neurospora crassa (12, 27). In addition, DNA damage-induced QDE-2 expression is abolished in the mutants that are defective in quelling and qiRNA pathways (12). To identify new components of the quelling and qiRNA pathways, we reasoned that mutants that are deficient in the DNA damage-induced QDE-2 expression would also be defective in the quelling and qiRNA pathways. Therefore, we screened >4000 Neurospora knock-out mutants using QDE-2 Western blot-based analysis to identify mutants with impaired histidine-induced QDE-2 expression. In addition to the homologous recombination components and chromatin remodelers that are characterized previously (18), we also found that the knock-out strain of NCU09825 was deficient in the histidine-induced QDE-2 expression (Fig. 1A). Sequence analysis showed that the predicted protein encoded by NCU09825 shares a strong sequence homology across the entire open reading frame with the RTT109 protein in budding yeast.
FIGURE 1.
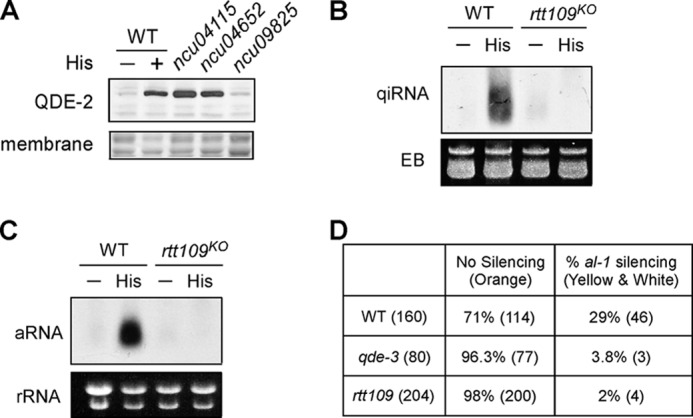
RTT109 is required for qiRNA and quelling pathway. A, QDE-2 Western blot analysis of a panel of Neurospora knock-out strains, including rtt109 (NCU09825), from the unbiased screen. All of the strains were grown in histidine (1 mg/ml) for 2 days. B and C, Northern blot analysis of qiRNA and aRNA products from the wild-type strain and rtt109 mutant. Cultures were treated with 1 mg/ml histidine for 2 days. Ethidium bromide (EB)-stained membranes were used as loading controls. D, quelling efficiency of the indicated strains. The numbers in the parentheses indicate the number of strains examined after the quelling assays.
The yeast RTT109 has been demonstrated to be a bona fide histone acetyltransferase for histone H3 on lysine 56 (H3K56) both in vivo and in vitro (28–30). Its histone acetyltransferase activity is largely dependent on its association with either of two histone chaperones, VPS75 and ASF1 (28, 29, 31). Interestingly, even though RTT109 and its sequence homologs are structurally similar to its functional counterpart in flies and humans, p300/CBP, they share no sequence homology (32, 33). H3K56Ac catalyzed by RTT109 plays an important role in DNA damage response/repair as mutants that are deficient in H3K56Ac are sensitive to genotoxic stress (29, 34, 35). This sensitivity is at least partly due to its role in the deposition of newly synthesized histones and histone replacement during DNA replication and repair (36, 37). However, little is known about the function of RTT109 beside its role in DNA damage response.
To investigate the phenotype of RTT109 in the qiRNA and quelling pathway, we first examined the damage-induced qiRNA production in the rtt109KO mutant. Fig. 1B shows that histidine treatment induced qiRNA (21–25 nt) production in the wild type strain (12, 18), but such a response was abolished in the rtt109 mutant. Then, we examined the expression of the rDNA-specific aRNA (∼0.3–1 kb) (12), the precursor of qiRNA, and found that the histidine-induced aRNA production was also abolished in the rtt109 mutant (Fig. 1C), indicating that RTT109 is required for qiRNA synthesis in a step that is upstream of aRNA production.
Previous studies show that quelling pathway and damage-induced qiRNA pathway are mechanistically similar (10, 14, 18). Thus, we asked whether RTT109 is also required for the quelling pathway. To test this, we performed quelling assay after transformation of cells with an al-1 transgene. As shown in Fig. 1D, 29% of the wild-type transformants exhibited quelling, as indicated by the change of conidia color from orange to yellow or white. In contrast, quelling efficiency of the rtt109KO strain is only 2%, which is similar to that in the qde-3 strain, indicating that similar to QDE-3, RTT109 is also required for quelling. Taken together, these results suggest that RTT109 is a novel component involved in qiRNA and quelling-induced siRNA biogenesis pathway.
H3K56 Acetylation Is Essential for the Quelling-induced Small RNA Production
To examine whether RTT109 is a histone acetyltransferase for H3K56 in Neurospora, Western blot against H3K56Ac was performed in both wild-type and rtt109 strains. Fig. 2A shows that H3K56Ac was abolished in the rtt109 mutant. This global loss of H3K56Ac was restored when a construct that expresses the c-Myc-tagged wild-type RTT109 was transformed into the rtt109KO strain. On the other hand, point mutations of the conserved catalytic aspartate residues (29) to alanines (D145A and DD304305AA) completely abolished H3K56Ac. These results indicate that RTT109 is an essential histone acetyltransferase for H3K56Ac in Neurospora.
FIGURE 2.
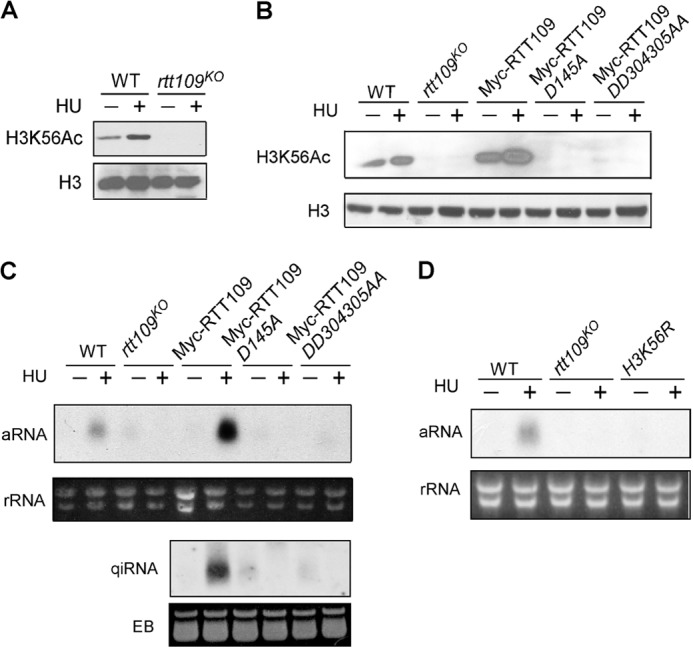
Histone acetyltransferase activity of RTT109 is required for qiRNA production. A, H3K56Ac Western blot of the wild-type and rtt109KO strains. Cultures were grown in media with/without 1 mg/ml HU for 2 days. H3 Western blot was used as a loading control. B, Western blot analysis showing the level of H3K56Ac in the indicated strains. C and D, Northern blot analysis showing the levels of the rDNA-specific aRNA and qiRNA in the indicated strains. Cultures were grown in media with/without 1 mg/ml HU treatment for 2 days. EB, ethidium bromide.
To examine whether the histone acetyltransferase activity of RTT109 is required for siRNA biogenesis in Neurospora, we examined the production of rDNA-specific aRNA. As shown in Fig. 2C, whereas the wild-type RTT109 was able to rescue the HU-induced aRNA and qiRNA production, the RTT109 D145A and DD304305AA mutants were deficient in this response. This result indicates that RTT109 acts as a histone acetyltransferase in the qiRNA production pathway.
HU treatment resulted in an increase of H3K56ac level (Fig. 2, A and B). Fig. 2C also shows that the production of aRNA in Myc-RTT109 strain was higher compared with the wild-type strain. This is correlated with the higher level of H3K56Ac in the Myc-RTT109 strain (Fig. 2B). To further confirm H3K56Ac is required for qiRNA production, we generated an H3K56R knock-in mutant strain in which lysine 56 of H3 was mutated to arginine. The H3K56R homokaryon strains was obtained by microconidia purification and confirmed by DNA sequencing. As expected, the H3K56R strain is deficient in HU-induced aRNA production (Fig. 2D), further indicating the importance of H3K56Ac in the qiRNA production pathway.
To better understand how RTT109 functions, we sought to identify RTT109 interacting proteins by immunoprecipitation of the Myc-tagged RTT109 from Neurospora and followed by mass spectrometry (Fig. 3A). One of RTT109-interacting proteins was identified to be the Neurospora homolog of the yeast VPS75, which has been shown to be in complex with RTT109 in yeast and is important for H3K56Ac both in vivo and in vitro (31, 38). It was previously reported that another histone chaperon ASF1 is also essential for H3K56Ac (28, 31, 35). To examine whether VPS75 and ASF1 act together with RTT109, we examined the H3K56Ac levels in the Neurospora vps75 and asf1 knock-out mutants. We found that both ASF1 and VPS75 are required for H3K56Ac (Fig. 3B). Furthermore, the production of HU-induced aRNA and qiRNA was also dramatically reduced in the asf1 and vps75 mutants (Fig. 3, C and D). These results further support that the importance of H3K56 acetylation in the qiRNA biogenesis pathway.
FIGURE 3.
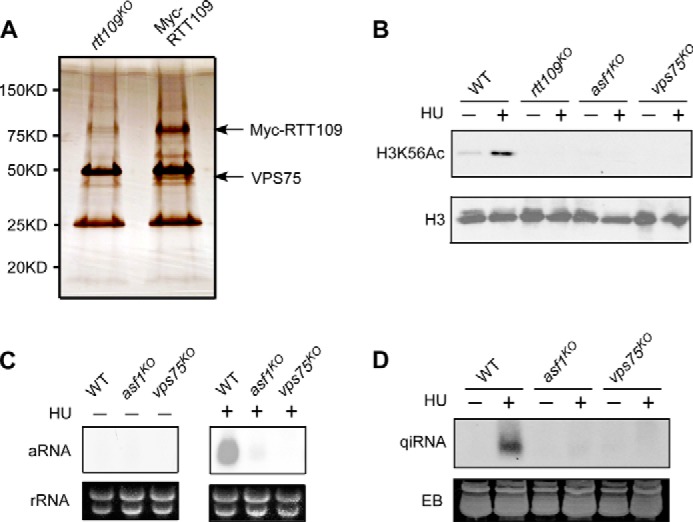
VPS75 and ASF1 are required for qiRNA production. A, silver-stained SDS-PAGE gel showing the affinity purified Myc-RTT109 products. Cell extracts of the rtt109KO and rtt109KO strain expressing Myc-RTT109 were used for purification. B, Western blot showing the levels of H3K56Ac in the indicated strains. Cultures were grown with/without 1 mg/ml HU for 2 days. C and D, Northern blot analysis of aRNA and qiRNA production in the indicated strains. EB, ethidium bromide.
RTT109 Is Required for Homologous Recombination
To understand how RTT109 involves in the qiRNA and quelling pathway. We examined the physiological function of RTT109 in Neurospora. It was previously reported in yeast that the rtt109 mutant is sensitive to DNA damage (28–30), and the basal level of RAD52 foci is increased in the rtt109 mutant (29). Consistent with these findings, we found that the Neurospora rtt109 and H3K56R mutants are also very sensitive to DNA damage agent treatment (Fig. 4A), including camptothecin, histidine, and ethyl methanesulfonate, indicating a role for H3K56Ac in the DNA damage response or repair.
FIGURE 4.
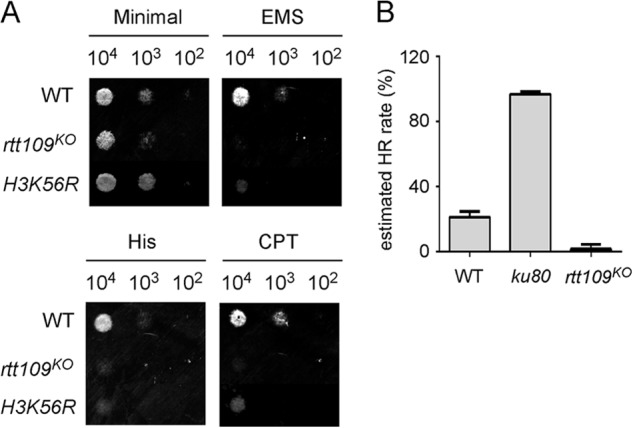
RTT109 is required for homologous recombination. A, a spot test showing the rtt109 and H3K56R mutants are sensitive to DNA damage agents, including CPT, histidine, and ethyl methanesulfonate (EMS). The numbers of conidia used in the spot test are indicated. B, homologous recombination assays showing the HR rates of the indicated strains (n = 3). Error bars indicate S.D.
Two major mechanisms are involved in repairing damaged DNA, the HR and the non-homologous end joining pathways (39). Because we previously showed that HR is required for both quelling- and damage-induced siRNA production (18), we hypothesized that H3K56Ac catalyzed by RTT109 is involved in HR. To test this hypothesis, we examined homologous recombination rate at the methyltryptophan resistance (mtr) locus in different Neurospora strains by transforming cells with a construct containing the bialaphos-resistance gene (bar) that can disrupt the mtr gene by homologous recombination (26). The targeting of bar gene into the mtr gene by HR will result in transformants that are resistant to both bialaphos and the amino acid analog p-fluorophenylalanine. As shown in Fig. 4B, the wild-type strain has a recombination rate of ∼20%, which is a typical HR rate in Neurospora. As expected, the HR rate was nearly 100% in a ku80 knock-out mutant in which the non-homologous end joining pathway is abolished and HR becomes the dominant repair pathway. As expected, the HR rate was dramatically reduced in the rtt109 mutant. This result indicates that RTT109 and H3K56Ac play a critical role in the HR process.
To understand how H3K56Ac responds to DNA damage agent treatment, we treated the wild-type Neurospora cells with different concentration of HU for 2 days. We observed a global induction H3K56Ac after HU treatment (Fig. 5A). However, the level of H3K56Ac did not further increase at high concentrations of HU treatment. Because qiRNA production will be abolished under high concentration of HU treatment where DNA replication is completely stopped (18), this result suggests that H3K56Ac is necessary but not sufficient to result in qiRNA production.
FIGURE 5.
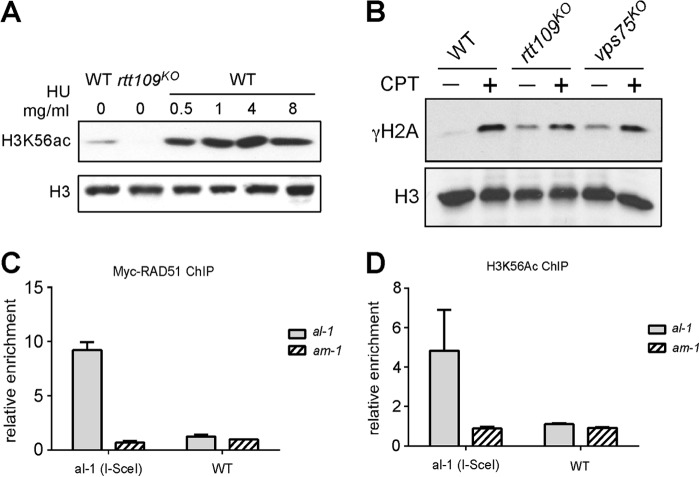
H3K56Ac and Myc-RAD51 is enriched around DSB. A, Western blot analysis showing the level of H3K56Ac in the rtt109 and wild-type strains grown in indicated concentration of HU. B, Western blot analysis showing the level of γΗ2Α in the indicated strains. γΗ2Α was examined after cultures were treated with 0.1 μg/ml CPT treatment for 2 days. C, ChIP-quantitative PCR analysis showing the enrichment of MycRAD51 around the I-SceI-induced DSB, which is flanked by the al-1 sequence. A primer set detecting the am-1 gene is used as a negative control D, ChIP assays showing the H3K56Ac enrichment around the DSB following the same procedure in C (n = 3). Error bars indicate S.D.
Next, we examined how H3K56Ac functions in the process of homologous recombination. H3K56Ac has been proposed to function in nucleosome assembly/disassembly (36, 37). A recent study revealed that H3K56ac can direct replacement of H2A.Z with H2A by altering the substrate specificity of a chromatin remodeling complex (40). Because γΗ2Α formation around the DSB site is the first step of DNA damage response/repair (41). We first examined whether H3K56Ac will affect γΗ2Α formation. As expected, γH2A in the wild-type strain was barely detectable without DNA damage and was induced after treatment by CPT, a topoisomerase I inhibitor (Fig. 5B). The basal levels of γΗ2Α were higher in the rtt109 and vps75 mutants than that in the wild-type strain, indicating a higher basal level of DNA damage or replication stress in these mutants. However, γΗ2Α can still be further induced in the presence of CPT in the rtt109 and vps75 mutants (Fig. 5B). This result suggests that H3K56Ac is required in the homologous recombination process in a step that is downstream of γΗ2Α formation.
Then, we examined that whether H3K56Ac is enriched around DSB during homologous recombination. We used a Neurospora strain with an I-SceI cutting site flanked by al-1 sequence at the his-3 locus.3 In this strain, FLAG-tagged ISceI and Myc-RAD51 was co-expressed under the control of QA-inducible promoter. Thus, a DSB will be created by FLAG-ISceI at the I-SceI restriction site flanked by al-1 sequences at the his-3 locus in the presence of QA. We examined the enrichment of Myc-RAD51 around the DSB by ChIP assay. As shown in Fig. 5C, a significant enrichment of Myc-RAD51 was observed around al-1 DSB site (∼10-fold). This result confirmed the production of DSB followed by homologous recombination repair after I-SceI induction. Similarly, we also detected a significant enrichment of H3K56Ac around the al-1 DSB flanking region but not at a control (am-1) locus. The correlation between the enrichment of H3K56Ac and Myc-RAD51 around the DSB site further suggests that H3K56Ac facilitates HR process.
DISCCUSION
Quelling-induced small RNAs and DNA damage induced qiRNAs are produced in Neurospora during the vegetative stage from repetitive DNA loci (4, 5). We previously showed that homologous recombination process are essential for the production of these small RNAs and is likely the mechanism to distinguish the repetitive sequences from the rest of the genome. All of the key components in HR are required for siRNA pathway, including RAD51, RAD52, RAD54, and replication protein A (14, 15, 18). Consistent with this model, several chromatin remodelers that are required for the small RNA production are also required for HR (18). These results suggest that HR among the repetitive DNA sequences allow the formation of appropriate chromatin structures that are specifically recognized by the RNAi pathway to produce aRNA and siRNA. In this study, we identified the H3K56 acetyltransferase, RTT109, as a new component of the quelling and qiRNA pathway. Further supporting the critical role of HR in repetitive DNA-induced small RNA production, we showed that RTT109 is required for homologous recombination in Neurospoa. Our results not only identified a histone acetyltransferase in a small RNA biogenesis pathway but also suggest that H3K56ac is required for the homologous recombination process in eukaryotic organisms.
Previous studies in yeast have shown that the histone chaperones VPS75 and/or ASF1 stimulate the H3K56ac activity of RTT109 (28–31). Consistent with these studies (29, 31), we found that RTT109 interacts with VPS75 in Neurospora (Fig. 3A), and both ASF1 and VPS75 are critical for H3K56Ac in Neurospora. In addition, qiRNA production is also impaired in the asf1 and vps75 mutants, further supporting the role of H3K56Ac in siRNA biogenesis.
Defects in the H3K56ac result in increased sensitivity to genotoxic stress that cause DNA damage during replication (28, 29, 34). It has been proposed that H3K56Ac participates in the replication- and repair-coupled nucleosome assembly (36, 37). Similarly, we also showed that the rtt109 mutant is sensitive to DNA damage. Furthermore, we showed that the rtt109 mutant is deficient in HR, suggesting that the DNA damage sensitivity of the rtt109 mutant is due to its impaired HR-dependent DNA repair pathway. Furthermore, the global level of H3K56Ac level is increased after DNA damage treatment (Fig. 5A). Importantly, we showed that H3K56ac occurs at a defined DSB locus that is correlated with RAd51 enrichment (Fig. 5, C and D). It has been shown that H3K56ac promotes rapid nucleosomes replacement or facilitates the deposition of histone in the context of transcription, DNA replication, and repair (36, 37, 40, 42). Because several chromatin remodelers have been shown to play a role in HR (18), it is possible that H3K56Ac may also participate in the chromatin remodeling process to create a favorable chromatin environment for HR to proceed. However, the exact function of H3K56Ac in HR remains unclear. We have tried to obtain a strain that has a defined DSB in the rtt109 mutant background to examine whether RTT109 is required for RAD51 loading onto the single-stranded DNA after DSB. However, we were not successful after repeated attempts, probably because the rtt109 mutant is very sensitive to DNA damage (Fig. 4A).
Since our discovery of qiRNA, it is recently shown that DNA damage can also induce small non-coding RNAs in both plants and animals (19–21). Thus, a common pathway may be responsible for the DNA damage-induced small RNA production in fungi, plants, and animals. Because H3K56Ac is involved in both DNA damage repair and small RNA biogenesis pathways, our study uncovers a mechanistic link at the chromatin level between DNA damage response and small RNA production.
Acknowledgments
We thank Shwu-Shin Chang, Zhihong Xue, Cuihong Chen, and Guojun Wu for assistance in the genetic screen. We thank Dr. Jessie Fernandez and members of the Liu lab for critical comments and Haiyan Yuan and Annie Ye for excellent technical assistance. We thank Dr. Hirokazu Inoue for providing the bar-containing plasmid for HR assay. We thank the Fungal Genetic Stock Center and the Neurospora Functional Genomics Project for providing the Neurospora knock-out library.
This work was supported by grants from the National Institutes of Health (GM084283) and the Welch Foundation (I-1560) (to Y. L.).
Q. Yang and Y. Liu, unpublished data.
- qiRNA
- quelling and DNA damage-induced small RNA
- aRNA
- aberrant RNA
- HR
- homologous recombination
- QA
- quinic acid
- HU
- hydroxyurea
- CPT
- camptothecin
- DSB
- double strand break
- rDNA
- ribosomal DNA.
REFERENCES
- 1. Catalanotto C., Nolan T., Cogoni C. (2006) Homology effects in Neurospora crassa. FEMS Microbiol. Lett. 254, 182–189 [DOI] [PubMed] [Google Scholar]
- 2. Bühler M., Moazed D. (2007) Transcription and RNAi in heterochromatic gene silencing. Nat. Struct. Mol. Biol. 14, 1041–1048 [DOI] [PubMed] [Google Scholar]
- 3. Ghildiyal M., Zamore P. D. (2009) Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 10, 94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li L., Chang S. S., Liu Y. (2010) RNA interference pathways in filamentous fungi. Cell. Mol. Life Sci. 67, 3849–3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang S. S., Zhang Z., Liu Y. (2012) RNA interference pathways in fungi: mechanisms and functions. Annu. Rev. Microbiol. 66, 305–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sijen T., Plasterk R. H. (2003) Transposon silencing in the Caenorhabditis elegans germ line by natural RNAi. Nature 426, 310–314 [DOI] [PubMed] [Google Scholar]
- 7. Siomi M. C., Saito K., Siomi H. (2008) How selfish retrotransposons are silenced in Drosophila germline and somatic cells. FEBS Lett. 582, 2473–2478 [DOI] [PubMed] [Google Scholar]
- 8. Wang X., Hsueh Y. P., Li W., Floyd A., Skalsky R., Heitman J. (2010) Sex-induced silencing defends the genome of Cryptococcus neoformans via RNAi. Genes Dev. 24, 2566–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Napoli C., Lemieux C., Jorgensen R. (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Romano N., Macino G. (1992) Quelling: transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol. Microbiol. 6, 3343–3353 [DOI] [PubMed] [Google Scholar]
- 11. Hsieh J., Fire A. (2000) Recognition and silencing of repeated DNA. Annual review of genetics 34, 187–204 [DOI] [PubMed] [Google Scholar]
- 12. Lee H. C., Chang S. S., Choudhary S., Aalto A. P., Maiti M., Bamford D. H., Liu Y. (2009) qiRNA is a new type of small interfering RNA induced by DNA damage. Nature 459, 274–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cecere G., Cogoni C. (2009) Quelling targets the rDNA locus and functions in rDNA copy number control. BMC Microbiol. 9, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee H. C., Aalto A. P., Yang Q., Chang S. S., Huang G., Fisher D., Cha J., Poranen M. M., Bamford D. H., Liu Y. (2010) The DNA/RNA-dependent RNA polymerase QDE-1 generates aberrant RNA and dsRNA for RNAi in a process requiring replication protein A and a DNA helicase. PLoS Biol. 8, e1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cogoni C., Macino G. (1999) Posttranscriptional gene silencing in Neurospora by a RecQ DNA helicase. Science 286, 2342–2344 [DOI] [PubMed] [Google Scholar]
- 16. Nolan T., Cecere G., Mancone C., Alonzi T., Tripodi M., Catalanotto C., Cogoni C. (2008) The RNA-dependent RNA polymerase essential for post-transcriptional gene silencing in Neurospora crassa interacts with replication protein A. Nucleic Acids Res. 36, 532–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maiti M., Lee H. C., Liu Y. (2007) QIP, a putative exonuclease, interacts with the Neurospora Argonaute protein and facilitates conversion of duplex siRNA into single strands. Genes Dev. 21, 590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Z., Chang S. S., Zhang Z., Xue Z., Zhang H., Li S., Liu Y. (2013) Homologous recombination as a mechanism to recognize repetitive DNA sequences in an RNAi pathway. Genes Dev. 27, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Francia S., Michelini F., Saxena A., Tang D., de Hoon M., Anelli V., Mione M., Carninci P., d'Adda di Fagagna F. (2012) Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature 488, 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michalik K. M., Böttcher R., Förstemann K. (2012) A small RNA response at DNA ends in Drosophila. Nucleic Acids Res. 40, 9596–9603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei W., Ba Z., Gao M., Wu Y., Ma Y., Amiard S., White C. I., Rendtlew Danielsen J. M., Yang Y. G., Qi Y. (2012) A role for small RNAs in DNA double-strand break repair. Cell 149, 101–112 [DOI] [PubMed] [Google Scholar]
- 22. Colot H. V., Park G., Turner G. E., Ringelberg C., Crew C. M., Litvinkova L., Weiss R. L., Borkovich K. A., Dunlap J. C. (2006) A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. U.S.A. 103, 10352–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng P., He Q., He Q., Wang L., Liu Y. (2005) Regulation of the Neurospora circadian clock by an RNA helicase. Genes Dev. 19, 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo C., Loros J. J., Dunlap J. C. (1998) Nuclear localization is required for function of the essential clock protein FRQ. EMBO J. 17, 1228–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cogoni C., Irelan J. T., Schumacher M., Schmidhauser T. J., Selker E. U., Macino G. (1996) Transgene silencing of the al-1 gene in vegetative cells of Neurospora is mediated by a cytoplasmic effector and does not depend on DNA-DNA interactions or DNA methylation. EMBO J. 15, 3153–3163 [PMC free article] [PubMed] [Google Scholar]
- 26. Ishibashi K., Suzuki K., Ando Y., Takakura C., Inoue H. (2006) Nonhomologous chromosomal integration of foreign DNA is completely dependent on MUS-53 (human Lig4 homolog) in Neurospora. Proc. Natl. Acad. Sci. U.S.A. 103, 14871–14876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choudhary S., Lee H. C., Maiti M., He Q., Cheng P., Liu Q., Liu Y. (2007) A double-stranded-RNA response program important for RNA interference efficiency. Mol. Cell Biol. 27, 3995–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Driscoll R., Hudson A., Jackson S. P. (2007) Yeast Rtt109 promotes genome stability by acetylating histone H3 on lysine 56. Science 315, 649–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han J., Zhou H., Horazdovsky B., Zhang K., Xu R. M., Zhang Z. (2007) Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science 315, 653–655 [DOI] [PubMed] [Google Scholar]
- 30. Schneider J., Bajwa P., Johnson F. C., Bhaumik S. R., Shilatifard A. (2006) Rtt109 is required for proper H3K56 acetylation: a chromatin mark associated with the elongating RNA polymerase II. J. Biol. Chem. 281, 37270–37274 [DOI] [PubMed] [Google Scholar]
- 31. Tsubota T., Berndsen C. E., Erkmann J. A., Smith C. L., Yang L., Freitas M. A., Denu J. M., Kaufman P. D. (2007) Histone H3-K56 acetylation is catalyzed by histone chaperone-dependent complexes. Mol. Cell 25, 703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang Y., Holbert M. A., Wurtele H., Meeth K., Rocha W., Gharib M., Jiang E., Thibault P., Verreault A., Cole P. A., Marmorstein R. (2008) Fungal Rtt109 histone acetyltransferase is an unexpected structural homolog of metazoan p300/CBP. Nat. Struct. Mol. Biol. 15, 738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Das C., Lucia M. S., Hansen K. C., Tyler J. K. (2009) CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature 459, 113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masumoto H., Hawke D., Kobayashi R., Verreault A. (2005) A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 436, 294–298 [DOI] [PubMed] [Google Scholar]
- 35. Han J., Zhou H., Li Z., Xu R. M., Zhang Z. (2007) Acetylation of lysine 56 of histone H3 catalyzed by RTT109 and regulated by ASF1 is required for replisome integrity. J. Biol. Chem. 282, 28587–28596 [DOI] [PubMed] [Google Scholar]
- 36. Chen C. C., Carson J. J., Feser J., Tamburini B., Zabaronick S., Linger J., Tyler J. K. (2008) Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell 134, 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Q., Zhou H., Wurtele H., Davies B., Horazdovsky B., Verreault A., Zhang Z. (2008) Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell 134, 244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fillingham J., Recht J., Silva A. C., Suter B., Emili A., Stagljar I., Krogan N. J., Allis C. D., Keogh M. C., Greenblatt J. F. (2008) Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109. Mol. Cell Biol. 28, 4342–4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ninomiya Y., Suzuki K., Ishii C., Inoue H. (2004) Highly efficient gene replacements in Neurospora strains deficient for nonhomologous end-joining. Proc. Natl. Acad. Sci. U.S.A. 101, 12248–12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watanabe S., Radman-Livaja M., Rando O. J., Peterson C. L. (2013) A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science 340, 195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Attikum H., Gasser S. M. (2009) Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 19, 207–217 [DOI] [PubMed] [Google Scholar]
- 42. Kaplan T., Liu C. L., Erkmann J. A., Holik J., Grunstein M., Kaufman P. D., Friedman N., Rando O. J. (2008) Cell cycle- and chaperone-mediated regulation of H3K56ac incorporation in yeast. PLoS Genet. 4, e1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]