Abstract
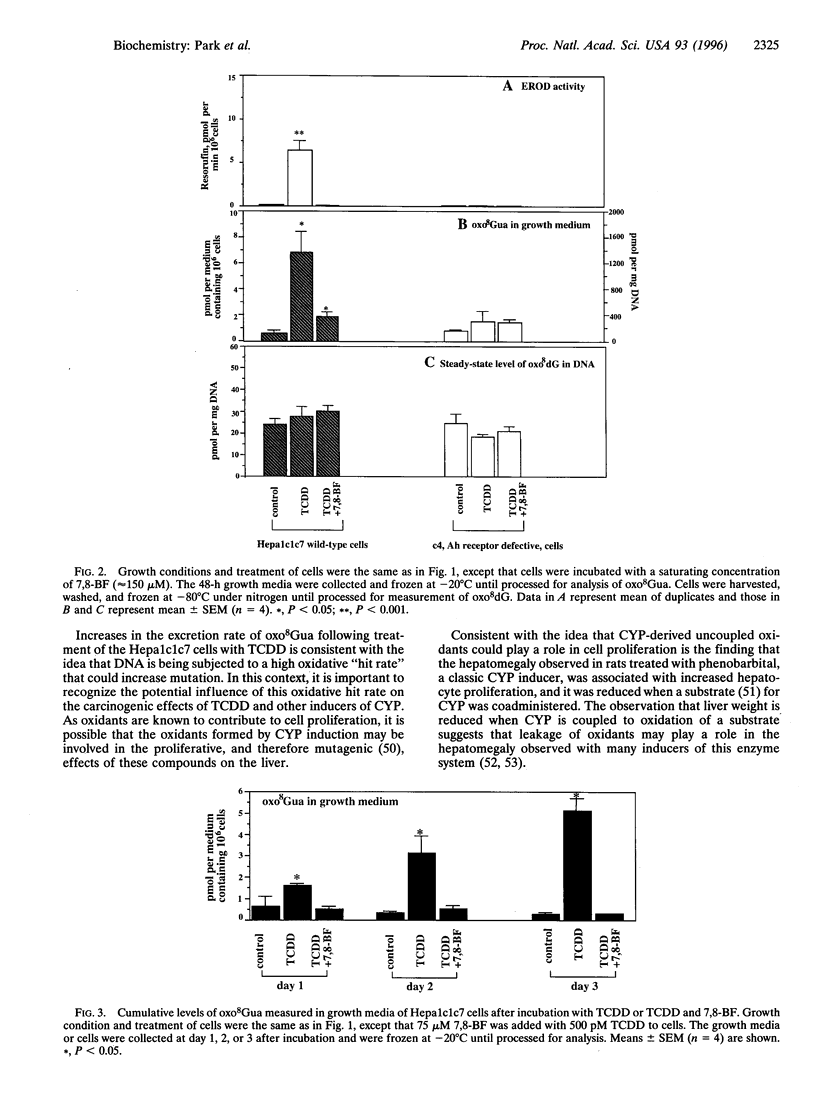
Induction of cytochrome P4501A1 (CYP1A1) in the hepatoma Hepa1c1c7 cell line results in an elevation in the excretion rate of 8-oxoguanine (oxo8Gua), a biomarker of oxidative DNA damage and the major repair product of 8-oxo-2'-deoxyguanosine (oxo8dG) residues in DNA. Treatment of this cell line with 2,3,7,8-tetrachloro-p-dibenzodioxin (TCDD), a nonmetabolized environmental contaminant, and indolo(3,2-b)carbazole (ICZ), a metabolite of a natural pesticide found in cruciferous vegetables, is shown to both induce CYP1A1 activity and elevate the excretion rate of oxo8Gua; 7,8-benzoflavone (7,8-BF or alpha-naphthoflavone), an inhibitor of CYP1A1 activity and an antagonist of the aryl hydrocarbon (Ah) receptor, reduced the excretion rate of oxo8Gua. The essential role of Ah-receptor, which mediates the induction of CYP1A1, is shown by the inability of TCDD to induce CYP1A1 and to increase excretion of oxo8Gua in Ah receptor-defective c4 mutant cells. While there was a significant 7.0-fold increase over 2 days in the excretion rate of oxo8Gua into the growth medium of TCDD-treated Hepa1c1c7 cells compared to control, no significant increase was detected in the steady-state level of oxo8dG in the DNA presumably due to efficient DNA repair. Thus, the induction of CYP1A1 appears to lead to a leak of oxygen radicals and consequent oxidative DNA damage that could lead to mutation and cancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S., Willett W. C. The causes and prevention of cancer. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessho T., Roy R., Yamamoto K., Kasai H., Nishimura S., Tano K., Mitra S. Repair of 8-hydroxyguanine in DNA by mammalian N-methylpurine-DNA glycosylase. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8901–8904. doi: 10.1073/pnas.90.19.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjeldanes L. F., Kim J. Y., Grose K. R., Bartholomew J. C., Bradfield C. A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach K. M., Poland A., Bradfield C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M. D., Orrenius S. The effect of albumin on the metabolism of ethoxyresorufin through O-deethylation and sulphate-conjugation using isolated rat hepatocytes. Biochem Pharmacol. 1978;27(11):1533–1538. doi: 10.1016/0006-2952(78)90481-1. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Oxidant stress and carcinogenesis. Eur J Clin Invest. 1991 Feb;21(1):1–5. doi: 10.1111/j.1365-2362.1991.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Chen Q., Fischer A., Reagan J. D., Yan L. J., Ames B. N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Denda A., Sai K. M., Tang Q., Tsujiuchi T., Tsutsumi M., Amanuma T., Murata Y., Nakae D., Maruyama H., Kurokawa Y. Induction of 8-hydroxydeoxyguanosine but not initiation of carcinogenesis by redox enzyme modulations with or without menadione in rat liver. Carcinogenesis. 1991 Apr;12(4):719–726. doi: 10.1093/carcin/12.4.719. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillner M., Bergman J., Cambillau C., Fernström B., Gustafsson J. A. Interactions of indoles with specific binding sites for 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Mol Pharmacol. 1985 Oct;28(4):357–363. [PubMed] [Google Scholar]
- Gonzalez F. J., Gelboin H. V. Human cytochromes P450: evolution and cDNA-directed expression. Environ Health Perspect. 1992 Nov;98:81–85. doi: 10.1289/ehp.929881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsky L. D., Koop D. R., Coon M. J. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J Biol Chem. 1984 Jun 10;259(11):6812–6817. [PubMed] [Google Scholar]
- Guengerich F. P. Reactions and significance of cytochrome P-450 enzymes. J Biol Chem. 1991 Jun 5;266(16):10019–10022. [PubMed] [Google Scholar]
- Guo N., Conaway C. C., Hussain N. S., Fiala E. S. Sex and organ differences in oxidative DNA and RNA damage due to treatment of Sprague-Dawley rats with acetoxime or 2-nitropropane. Carcinogenesis. 1990 Sep;11(9):1659–1662. doi: 10.1093/carcin/11.9.1659. [DOI] [PubMed] [Google Scholar]
- Hankinson O. Single-step selection of clones of a mouse hepatoma line deficient in aryl hydrocarbon hydroxylase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):373–376. doi: 10.1073/pnas.76.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Johansson I. Mechanisms of hydroxyl radical formation and ethanol oxidation by ethanol-inducible and other forms of rabbit liver microsomal cytochromes P-450. J Biol Chem. 1984 May 25;259(10):6447–6458. [PubMed] [Google Scholar]
- Kasai H., Chung M. H., Jones D. S., Inoue H., Ishikawa H., Kamiya H., Ohtsuka E., Nishimura S. 8-Hydroxyguanine, a DNA adduct formed by oxygen radicals: its implication on oxygen radical-involved mutagenesis/carcinogenesis. J Toxicol Sci. 1991 Feb;16 (Suppl 1):95–105. doi: 10.2131/jts.16.supplementi_95. [DOI] [PubMed] [Google Scholar]
- Kasai H., Chung M. H., Yamamoto F., Ohtsuka E., Laval J., Grollman A. P., Nishimura S. Formation, inhibition of formation, and repair of oxidative 8-hydroxyguanine DNA damage. Basic Life Sci. 1993;61:257–262. doi: 10.1007/978-1-4615-2984-2_23. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Okada Y., Nishimura S., Rao M. S., Reddy J. K. Formation of 8-hydroxydeoxyguanosine in liver DNA of rats following long-term exposure to a peroxisome proliferator. Cancer Res. 1989 May 15;49(10):2603–2605. [PubMed] [Google Scholar]
- Kleman M. I., Overvik E., Mason G. G., Gustafsson J. A. In vitro activation of the dioxin receptor to a DNA-binding form by food-borne heterocyclic amines. Carcinogenesis. 1992 Sep;13(9):1619–1624. doi: 10.1093/carcin/13.9.1619. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Kukiełka E., Cederbaum A. I. NADPH- and NADH-dependent oxygen radical generation by rat liver nuclei in the presence of redox cycling agents and iron. Arch Biochem Biophys. 1990 Dec;283(2):326–333. doi: 10.1016/0003-9861(90)90650-n. [DOI] [PubMed] [Google Scholar]
- Kukiełka E., Cederbaum A. I. Oxidation of ethylene glycol to formaldehyde by rat liver microsomes. Role of cytochrome P-450 and reactive oxygen species. Drug Metab Dispos. 1991 Nov-Dec;19(6):1108–1115. [PubMed] [Google Scholar]
- Kuthan H., Ullrich V. Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur J Biochem. 1982 Sep 1;126(3):583–588. doi: 10.1111/j.1432-1033.1982.tb06820.x. [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Lee H. S., Park M. K., Hwang E. S., Park E. M., Kasai H., Chung M. H. Identification of 8-hydroxyguanine glycosylase activity in mammalian tissues using 8-hydroxyguanine specific monoclonal antibody. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1545–1551. doi: 10.1006/bbrc.1993.2427. [DOI] [PubMed] [Google Scholar]
- Legraverend C., Hannah R. R., Eisen H. J., Owens I. S., Nebert D. W., Hankinson O. Regulatory gene product of the Ah locus. Characterization of receptor mutants among mouse hepatoma clones. J Biol Chem. 1982 Jun 10;257(11):6402–6407. [PubMed] [Google Scholar]
- Loub W. D., Wattenberg L. W., Davis D. W. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst. 1975 Apr;54(4):985–988. [PubMed] [Google Scholar]
- Lucier G. W., Tritscher A., Goldsworthy T., Foley J., Clark G., Goldstein J., Maronpot R. Ovarian hormones enhance 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated increases in cell proliferation and preneoplastic foci in a two-stage model for rat hepatocarcinogenesis. Cancer Res. 1991 Mar 1;51(5):1391–1397. [PubMed] [Google Scholar]
- Nordblom G. D., Coon M. J. Hydrogen peroxide formation and stoichiometry of hydroxylation reactions catalyzed by highly purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1977 Apr 30;180(2):343–347. doi: 10.1016/0003-9861(77)90047-9. [DOI] [PubMed] [Google Scholar]
- Nordmann R., Ribière C., Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12(3):219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- Ohde G., Schuppler J., Schulte-Hermann R., Keiger H. Proliferation of rat liver cells in preneoplastic nodules after stimulation of liver growth by xenobiotic inducers. Arch Toxicol Suppl. 1979;(2):451–455. doi: 10.1007/978-3-642-67265-1_55. [DOI] [PubMed] [Google Scholar]
- Park E. M., Shigenaga M. K., Degan P., Korn T. S., Kitzler J. W., Wehr C. M., Kolachana P., Ames B. N. Assay of excised oxidative DNA lesions: isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3375–3379. doi: 10.1073/pnas.89.8.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Poland A., Palen D., Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982 Nov 18;300(5889):271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Reyes H., Reisz-Porszasz S., Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992 May 22;256(5060):1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Sato K., Ito K., Kohara H., Yamaguchi Y., Adachi K., Endo H. Negative regulation of catalase gene expression in hepatoma cells. Mol Cell Biol. 1992 Jun;12(6):2525–2533. doi: 10.1128/mcb.12.6.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hermann R., Leberl C., Ruberg I. Stimulation of cell proliferation in rat liver by alpha-hexachlorocyclohexane or partial hepatectomy and end points during G1 of the inhibitory action of beta-diethyl-aminoethylphenyldiallyl acetate-HC1 (CFT 1201), beta-diethylaminoethyldiphenylpropyl acetate. HC1 (SKF 525-A) and actinomycin D. Biochim Biophys Acta. 1976 Nov 1;447(4):413–424. doi: 10.1016/0005-2787(76)90079-4. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Aboujaoude E. N., Chen Q., Ames B. N. Assays of oxidative DNA damage biomarkers 8-oxo-2'-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1994;234:16–33. doi: 10.1016/0076-6879(94)34073-0. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Gimeno C. J., Ames B. N. Urinary 8-hydroxy-2'-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9697–9701. doi: 10.1073/pnas.86.24.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga M. K., Park J. W., Cundy K. C., Gimeno C. J., Ames B. N. In vivo oxidative DNA damage: measurement of 8-hydroxy-2'-deoxyguanosine in DNA and urine by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1990;186:521–530. doi: 10.1016/0076-6879(90)86146-m. [DOI] [PubMed] [Google Scholar]
- Stohs S. J. Oxidative stress induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Free Radic Biol Med. 1990;9(1):79–90. doi: 10.1016/0891-5849(90)90052-k. [DOI] [PubMed] [Google Scholar]
- Tai H. L., McReynolds J. H., Goldstein J. A., Eugster H. P., Sengstag C., Alworth W. L., Olson J. R. Cytochrome P4501A1 mediates the metabolism of 2,3,7,8-tetrachlorodibenzofuran in the rat and human. Toxicol Appl Pharmacol. 1993 Nov;123(1):34–42. doi: 10.1006/taap.1993.1218. [DOI] [PubMed] [Google Scholar]
- Tchou J., Grollman A. P. Repair of DNA containing the oxidatively-damaged base, 8-oxoguanine. Mutat Res. 1993 May;299(3-4):277–287. doi: 10.1016/0165-1218(93)90104-l. [DOI] [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindberg N., Ingelman-Sundberg M. Cytochrome P-450 and oxygen toxicity. Oxygen-dependent induction of ethanol-inducible cytochrome P-450 (IIE1) in rat liver and lung. Biochemistry. 1989 May 16;28(10):4499–4504. doi: 10.1021/bi00436a056. [DOI] [PubMed] [Google Scholar]
- Tukey R. H., Negishi M., Nebert D. W. Quantitation of hepatic cytochrome P1-450 mRNA with the use of a cloned DNA probe. Effects of various P-450 inducers in C57BL/6N and DBA/2N mice. Mol Pharmacol. 1982 Nov;22(3):779–786. [PubMed] [Google Scholar]
- Whitlock J. P., Jr Mechanistic aspects of dioxin action. Chem Res Toxicol. 1993 Nov-Dec;6(6):754–763. doi: 10.1021/tx00036a003. [DOI] [PubMed] [Google Scholar]







