Abstract
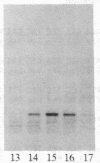
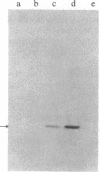
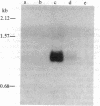
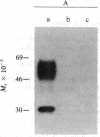
The synthesis and function of IgE are dependent on IgE-binding proteins, which include cell surface IgE receptors and IgE-binding lymphokines. To further our understanding of the IgE system, we have engaged in the molecular cloning of genes for some of these proteins. In studying the in vitro translation products of mRNA from rat basophilic leukemia (RBL) cells, we have identified a Mr 31,000 polypeptide that binds IgE and is also reactive with antibodies to proteins affinity-purified from RBL cells with IgE immunoadsorbent. For the molecular cloning, double-stranded cDNA was synthesized from sucrose gradient-fractionated RBL mRNA, inserted into plasmid pBR322, and used to transform Escherichia coli. By screening transformants with a hybridization-selection/in vitro translation procedure, we identified one clone containing cDNA that hybridized to mRNA coding for a Mr 31,000 IgE-binding protein. The DNA sequence of this cloned cDNA (571 base pairs) was determined and the amino acid sequence corresponding to part of the protein was deduced. In RNA blot analysis, the cDNA hybridized with a mRNA of 1100 nucleotides found in RBL cells but absent in cells not expressing IgE receptors. This cloned cDNA most likely codes for the Mr 31,000 IgE-binding protein identified in RBL cells, which appears to be related to the IgE-binding phenotype of the cells and which may have a significant role in the IgE-mediated activation of basophils and mast cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conrad D. H., Froese A. Characterization of the target cell receptor for IgE. II. Polyacrylamide gel analysis of the surface IgE receptor from normal rat mast cells and from rat basophilic leukemia cells. J Immunol. 1976 Feb;116(2):319–326. [PubMed] [Google Scholar]
- DUNN T. B., POTTER M. A transplantable mast-cell neoplasm in the mouse. J Natl Cancer Inst. 1957 Apr;18(4):587–601. [PubMed] [Google Scholar]
- Froese A. Structure and function of the receptor for IgE. Crit Rev Immunol. 1980 May;1(2):79–132. [PubMed] [Google Scholar]
- Goetze A., Kanellopoulos J., Rice D., Metzger H. Enzymatic cleavage products of the alpha subunit of the receptor for immunoglobulin E. Biochemistry. 1981 Oct 27;20(22):6341–6349. doi: 10.1021/bi00525a009. [DOI] [PubMed] [Google Scholar]
- Helm R. M., Conrad D. H., Froese A. Lentil-lectin affinity chromatography of surface glycoproteins and the receptor for IgE from rat basophilic leukemia cells. Int Arch Allergy Appl Immunol. 1979;58(1):90–98. doi: 10.1159/000232177. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Kulczycki A., Jr, Parker C. W. Phosphorylation of the IgE receptor from ionophore A23187 stimulated intact rat mast cells. Biochem Biophys Res Commun. 1981 Feb 12;98(3):815–822. doi: 10.1016/0006-291x(81)91184-0. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Parker C. W., Kulczycki A., Jr Selective phosphorylation of the IgE receptor in antigen-stimulated rat mast cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3050–3053. doi: 10.1073/pnas.80.10.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead B. L., Parker C. W., Kulczycki A., Jr The cell surface receptor for immunoglobulin E. Effect of tunicamycin on molecular properties of receptor from rat basophilic leukemia cells. J Biol Chem. 1981 Oct 25;256(20):10717–10723. [PubMed] [Google Scholar]
- Holowka D., Baird B. Lactoperoxidase-catalyzed iodination of the receptor for immunoglobulin E at the cytoplasmic side of the plasma membrane. J Biol Chem. 1984 Mar 25;259(6):3720–3728. [PubMed] [Google Scholar]
- Holowka D., Hartmann H., Kanellopoulos J., Metzger H. Association of the receptor for immunoglobulin E with an endogenous polypeptide on rat basophilic leukemia cells. J Recept Res. 1980;1(1):41–68. doi: 10.3109/10799898009039254. [DOI] [PubMed] [Google Scholar]
- Holowka D., Metzger H. Further characterization of the beta-component of the receptor for immunoglobulin E. Mol Immunol. 1982 Feb;19(2):219–227. doi: 10.1016/0161-5890(82)90334-0. [DOI] [PubMed] [Google Scholar]
- Huff T. F., Uede T., Ishizaka K. Formation of rat IgE-binding factors by rat-mouse T cell hybridomas. J Immunol. 1982 Aug;129(2):509–514. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Mechanisms of reaginic hypersensitivity and IgE antibody response. Immunol Rev. 1978;41:109–148. doi: 10.1111/j.1600-065x.1978.tb01462.x. [DOI] [PubMed] [Google Scholar]
- Ishizaka K. Regulation of IgE synthesis. Annu Rev Immunol. 1984;2:159–182. doi: 10.1146/annurev.iy.02.040184.001111. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos J. M., Liu T. Y., Poy G., Metzger H. Composition and subunit structure of the cell receptor for immunoglobulin E. J Biol Chem. 1980 Oct 10;255(19):9060–9066. [PubMed] [Google Scholar]
- Kanellopoulos J., Rossi G., Metzger H. Preparative isolation of the cell receptor for immunoglobulin E. J Biol Chem. 1979 Aug 25;254(16):7691–7697. [PubMed] [Google Scholar]
- Kulczycki A., Jr, Isersky C., Metzger H. The interaction of IgE with rat basophilic leukemia cells. I. Evidence for specific binding of IgE. J Exp Med. 1974 Mar 1;139(3):600–616. doi: 10.1084/jem.139.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulczycki A., Jr, McNearney T. A., Parker C. W. The rat basophilic leukemia cell receptor for IgE. I. Characterization as a glycoprotein. J Immunol. 1976 Aug;117(2):661–665. [PubMed] [Google Scholar]
- Kulczycki A., Jr, Parker C. W. The cell surface receptor for immunoglobulin E. I. The use of repetitive affinity chromatography for the purification of a mammalian receptor. J Biol Chem. 1979 May 10;254(9):3187–3193. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Albrandt K., Sutcliffe J. G., Katz D. H. Cloning and nucleotide sequence of mouse immunoglobulin epsilon chain cDNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7852–7856. doi: 10.1073/pnas.79.24.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Liu F. T., Orida N. Synthesis of surface immunoglobulin E receptor in Xenopus oocytes by translation of mRNA from rat basophilic leukemia cells. J Biol Chem. 1984 Sep 10;259(17):10649–10652. [PubMed] [Google Scholar]
- Mayrhofer G., Bazin H., Gowans J. L. Nature of cells binding anti-IgE in rats immunized with Nippostrongylus brasiliensis: IgE synthesis in regional nodes and concentration in mucosal mast cells. Eur J Immunol. 1976 Aug;6(8):537–545. doi: 10.1002/eji.1830060803. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Metzger H. The receptor on mast cells and related cells with high affinity for IgE. Contemp Top Mol Immunol. 1983;9:115–145. doi: 10.1007/978-1-4684-4517-6_4. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoud A. R., Ruddy S., Conrad D. H. Functional and partial chemical characterization of the carbohydrate moieties of the IgE receptor on rat basophilic leukemia cells and rat mast cells. J Immunol. 1981 Apr;126(4):1624–1629. [PubMed] [Google Scholar]
- Perez-Montfort R., Kinet J. P., Metzger H. A previously unrecognized subunit of the receptor for immunoglobulin E. Biochemistry. 1983 Dec 6;22(25):5722–5728. doi: 10.1021/bi00294a007. [DOI] [PubMed] [Google Scholar]
- Rivnay B., Wank S. A., Poy G., Metzger H. Phospholipids stabilize the interaction between the alpha and beta subunits of the solubilized receptor for immunoglobulin E. Biochemistry. 1982 Dec 21;21(26):6922–6927. doi: 10.1021/bi00269a047. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Sharrow S. O., Jones J. F., Siraganian R. P. Fc (IgG) receptors on rat basophilic leukemia cells. J Immunol. 1981 Jan;126(1):138–145. [PubMed] [Google Scholar]
- Spiegelberg H. L. Structure and function of Fc receptors for IgE on lymphocytes, monocytes, and macrophages. Adv Immunol. 1984;35:61–88. doi: 10.1016/s0065-2776(08)60574-x. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Taki T., Hachimine K., Sadasivan R. Biochemical properties of biologically active Fc gamma receptors of human B lymphocytes. Mol Immunol. 1981 Jan;18(1):55–65. doi: 10.1016/0161-5890(81)90048-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]