Abstract
Nuclear patch-clamp experiments can be performed with intact nuclei or with nuclei from which the outer nuclear membrane has been removed. This protocol presents procedures for harvesting different types of cultured cells, isolating nuclei, and exposing the inner nuclear membrane by agitating in the presence of sodium citrate. Particulars about obtaining and maintaining the cells of interest in culture are not described here. However, care should be taken not to allow the cells to grow beyond a density of 2–3 × 106 cells/mL because this may decrease both the cell viability and the success rate of detecting active inositol 1,4,5-trisphosphate receptor (InsP3R) channels in nuclear patches.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous material used in this protocol.
RECIPE: Please see the end of this article for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
Nuclei isolation solution (NIS):
-
NIS for patch-clamping the outer nuclear membrane (NIS-O)
For NIS-O, dissolve one tablet of complete protease inhibitor cocktail (Roche Applied Science 1 697 498) in 40 mL of sucrose buffer <R>. (Fractions of a tablet can be used if a smaller volume of NIS is required.) Add PMSF to give a final concentration of 0.05–0.2 mm, depending on the cell type (see Table 1). (We prepare a 100 mm PMSF stock solution in methanol. If the PMSF is dissolved in dimethyl sulfoxide, ensure that the final concentration of dimethyl sulfoxide in the NIS does not exceed 0.5% [v/v].)
-
NIS for patch-clamping the inner nuclear membrane (NIS-I)
Prepare NIS-I by supplementing NIS-O with appropriate amounts of sodium citrate. When nuclei are isolated in the presence of an appropriate amount of sodium citrate, on-nucleus membrane patches acquired exhibit no InsP3R channel activity with high (10 µm) InsP3 in the pipette solution and no InsP3 in the bath solution, but InsP3R channels are activated when InsP3 is added to the bath. The appropriate sodium citrate concentration must be determined by trial and error for each cell type. For primary Purkinje neurons, 1% (w/v) is sufficient (Marchenko et al. 2005), whereas 20% (w/v) is required for Sf9 cells (H Vais, unpubl.).
TABLE 1.
Optimal conditions for isolating nuclei from a variety of cell types
| Cell type | [PMSF] in NIS (µm)a |
Cell density in NIS (×106/mL) |
Homogenizerb | Number of strokesc |
Reference |
|---|---|---|---|---|---|
| Sf9 | 100 | 1.6 | Dounce | 4–6 | Ionescu et al. 2006 |
| HEK293-mRyR2d | 50 | 1.5–2 | Dounce | 4–6 | |
| S2 | 50 | 2–3 | Duall-1 | 7–9 | |
| DT40 | 50 | 2–3 | Duall-1 | 7–9 | Li et al. 2007 |
| N2a | 200 | 1.5–2 | Duall-2 | 14–16 | Kopil et al. 2011 |
| PC12 | 200 | 1.5–2 | Duall-2 | 12–14 | |
| SH-SY5Y | 200 | 1.5–2 | Duall-2 | 9–12 | |
| HEK293 | 200 | 1.5–2 | Duall-2 | 14–16 | |
| Human B lymphoblasts | 200 | 2–3 | Duall-2 | 10–12 | Cheung et al. 2010 |
| Mouse primary cortical neurons | 200 | 2–3 | Duall-2 | 8–10 | Cheung et al. 2010 |
| Mouse embryonic fibroblasts | 200 | 1.5–2 | Duall-2 | 12–15 | Cheung et al. 2010 |
These values are given only as a reference. The optimum [PMSF] varies depending on the culture conditions and cell type.
Duall-1 and Duall-2 refer to Duall homogenizers of weaker and stronger homogenizing strength, respectively. See Discussion for further explanation.
The number of strokes to use is very strongly dependent on the particular homogenizer used, as well as on the age of the cell culture.
HEK293 cells expressing recombinant RyR (Jiang et al. 2007).
Phosphate-buffered saline (PBS) (Ca2+ - and Mg2+-free)
Trypsin (if using adherent cells; see Step 1)
Equipment
Cell scraper (if using adherent cells; see Step 1)
Centrifuge (general-purpose, benchtop) (e.g., IEC Centra CL2 from Thermo Scientific)
Dounce homogenizer (all-glass; size 2 mL; use pestle B) (Kimble Chase 885300-0002) or Duall homogenizer (glass with PTFE pestle; size 1 mL) (Kimble Chase 885482-0020)
Horizontal shaker (for removal of outer nuclear membrane) (e.g., Polystat 12050-00 from Cole-Parmer)
Tubes (polystyrene; round-bottom; 5-mL; with water-tight cap) (e.g., Falcon 35-2003)
METHOD
Harvesting Cells
Cells in culture can be broadly divided into three categories, depending on the conditions in which they are cultured and how they grow. The method for harvesting these cells varies according to the cell type and appropriate procedures are outlined below. Cells from primary tissues can also be used for nuclear patch-clamp experiments, but to use the following protocol, such cells must first be isolated and then placed in culture using a tissue-specific method (a method for neurons is described in Cheung et al. 2010). Protocols for isolating nuclei directly from primary tissues are described elsewhere (Gerasimenko et al. 1995; Marchenko et al. 2005; Zima et al. 2007).
-
1Harvest cells of interest using one of the following procedures.
- For Cells Growing Strongly Attached to the Bottom of the Culture Flask
- While they are still attached to the substrate, wash the cells twice with ice-cold PBS to remove dead cells. Remove the PBS.
- Detach the cells using a suitable concentration of trypsin for an appropriate length of time (e.g., use the same conditions as those used for passaging the cells).
- Wash the cells with ice-cold PBS and centrifuge at 300g for 5 min. Repeat, and after the second centrifugation, resuspend the cell pellet in an appropriate volume of ice-cold NIS for patch-clamping the outer or inner nuclear membrane (NIS-O or NIS-I, respectively; see Step 2).
- For Cells Growing Weakly Attached to the Bottom of the Culture Flask
- While they are still attached to the substrate, wash the cells twice with ice-cold PBS to remove dead cells. Remove the PBS.
- Add an appropriate volume of ice-cold NIS-O or NIS-I (see Step 2) and mechanically detach the cells by scraping.Sf9 cells are an example of cells in this category. When ~8 × 106 Sf9 cells grown in suspension are transferred to a stationary T-25 culture flask, the cells settle, and after 1 h they become weakly attached to the bottom of the flask. These cells can be detached from the substrate without damage by scraping.
- For Cells Growing in Suspension
- Wash the cells with ice-cold PBS and centrifuge at 300g for 5 min. Repeat, and after the second centrifugation, resuspend the cell pellet in an appropriate volume of ice-cold NIS-O or NIS-I (see Step 2).
-
2Suspend the cells in NIS at the right density for optimal levels of nucleus isolation (see Table 1). Keep the cell suspensions on ice and use within 4 h.It is critically important to suspend the cells of interest in NIS containing a protease inhibitor cocktail and PMSF before they are mechanically disrupted. Otherwise, the success rate of detecting InsP3R channels in nuclear patches will be severely reduced. For cell types not shown in Table 1, the optimal density must be determined by trial and error. Do not use cell suspension that was prepared more than 4 h before. Prepare fresh suspension instead.
Isolating Nuclei
-
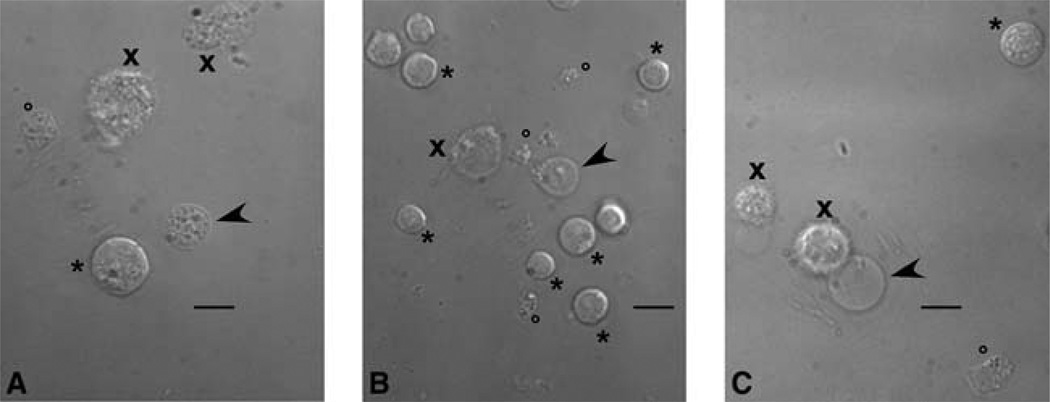
3Transfer 1 mL of cell suspension to an ice-cold homogenizer (either a 2-mL Dounce or a 1-mL Duall homogenizer). Subject the cell suspension to a number of up-and-down strokes of the pestle (see Table 1). Do not rotate the pestle during the strokes because this introduces shearing stress that distorts the nuclei.This process yields a homogenate that contains 2–5 “good” nuclei per 100 cells. Good nuclei are those that are released from cells with their outer nuclear membrane intact and so give a high rate of success in nuclear patch-clamp experiments (see Fig. 1). See Discussion.
FIGURE 1.
Micrographs showing nuclei isolated from (A) Sf9 cells, (B) DT40 cells stably expressing recombinant rat type 3 InsP3R, (C) PC12 cells. All micrographs were taken with the same magnification. Scale bars, 10 µm. Arrowheads indicate good nuclei suitable for patch-clamping. Tips of the arrowheads point to clean areas of the isolated nuclei where the tip of the patch-clamp pipette should be positioned. Crosses (×) indicate disrupted/damaged cells. Asterisks (*) indicate intact cells. Circles (o) indicate cell debris.
Patch-Clamping
-
4Prepare the nuclei for patch-clamping as follows.
- For the Outer Nuclear Membrane
- Transfer the homogenate (in NIS-O) to a microcentrifuge tube and keep on ice. Use the preparation within 90–120 min.
- For the Inner Nuclear Membrane
- Remove the outer nuclear membrane by gently shaking the homogenate (in NIS-I containing an appropriate sodium citrate concentration) for an appropriate length of time at 4°C. Determine the conditions by trial and error for nuclei from different cells.With an optimal sodium citrate concentration and optimal agitation time, it should be possible to obtain 2–5 good nuclei with inner nuclear membrane exposed per 100 cells. For Sf9 nuclei, we found that outer nuclear membrane removal can be optimally achieved by shaking 4 mL of homogenate in NIS-I (obtained by repeating Step 3 four times) in a 6-mL plastic-capped tube placed horizontally at slow speed (50 cycles/min) for 1 h.
- Transfer the homogenate (in NIS-I) to a microcentrifuge tube and keep on ice. Use the preparation within 90–120 min.
-
5
See Nuclear Patch-Clamp Electrophysiology of Ca2+ Channels (Mak et al. 2013a).
DISCUSSION
Different cell types vary significantly in terms of how easy it is to obtain nuclei suitable for a nuclear patch-clamp experiment. Among the cells we have used, Sf9 cells require the mildest treatment and a Dounce homogenizer is recommended. All other cells can be homogenized with a Duall homogenizer, which is a stronger homogenizer and so fewer strokes are needed for effective treatment. Even so, it is necessary to try several homogenizers of the same model to find the right one for each cell type because there are significant differences in the “homogenizing strength” even among homogenizers of the same model. The variability is greater for the Duall homogenizer. The homogenizers tested should be labeled according to their “homogenizing strength.” Such a strategy is particularly useful for providing more choice when different cell types are used. In Table 1, labels Duall-1 and Duall-2 are used to emphasize the difference in homogenizing strength we found appropriate for the various cell types we have used.
Once the homogenizer best suited for a particular type of cells is found, the optimum number of strokes required for obtaining good nuclei has to be established. This depends not only on the cell type of interest and the homogenizer, but also on the force applied to move the pestle up and down through the homogenate. Each person has to go through his/her own learning process. A general principle is to start by using fewer strokes than those indicated in Table 1 and gradually increase them until a satisfactory yield of good nuclei (2–5 per 100 cells) is achieved. Beyond this, although more strokes can yield more exposed nuclei, the amount of cellular debris generated, which can contaminate the tip of the patch-clamp micropipette, will also increase substantially. More importantly overhomogenization increases both the rate of failure in obtaining giga-ohm seals and the rate of obtaining unstable nuclear membrane patches, suggesting that the outer membrane is damaged.
RELATED INFORMATION
For a discussion of the application of nuclear patch-clamp experiments to the study of InsP3R channels in the outer and inner nuclear membrane and their role in [Ca2+]i signaling, see Patch- Clamp Electrophysiology of Intracellular Ca2+ Channels (Mak et al. 2013b).
RECIPE
Sucrose Buffer (SB)
| Reagent | Final concentration |
|---|---|
| KCl | 150 mm |
| Sucrose | 250 mm |
| Tris–HCl | 10 mm |
| β-Mercaptoethanol | 1.4 mm |
Adjust the pH of the solution to 7.3 with 1 m KOH before adding β-mercaptoethanol. Prepare the solution freshly every 3–4 d. Store at 4°C.
REFERENCES
- Cheung KH, Mei L, Mak D-OD, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- Ionescu L, Cheung KH, Vais H, Mak D-OD, White C, Foskett JK. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: Implications for quantal Ca2+ release. J Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopil CM, Vais H, Cheung KH, Siebert AP, Mak D-OD, Foskett JK, Neumar RW. Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor (InsP3R1) has InsP3-independent gating and disrupts intracellular Ca2+ homeostasis. J Biol Chem. 2011;286:35998–36010. doi: 10.1074/jbc.M111.254177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-xL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D-OD, Vais H, Cheung K-H, Foskett JK. Nuclear patch-clamp electrophysiology of Ca2+ channels. Cold Spring Harb Protoc. 2013a doi: 10.1101/pdb.prot073064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D-OD, Vais H, Cheung K-H, Foskett JK. Patch-clamp electrophysiology of intracellular Ca2+ channels. Cold Spring Harb Protoc. 2013b doi: 10.1101/pdb.top066217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko SM, Yarotskyy VV, Kovalenko TN, Kostyuk PG, Thomas RC. Spontaneously active and InsP3-activated ion channels in cell nuclei from rat cerebellar Purkinje and granule neurones. J Physiol. 2005;565:897–910. doi: 10.1113/jphysiol.2004.081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV, Bare DJ, Mignery GA, Blatter LA. IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J Physiol. 2007;584:601–611. doi: 10.1113/jphysiol.2007.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]