Abstract
Adipose tissue is a major regulator of bone metabolism and in the general population obesity is associated with greater bone mineral density (BMD). However, bone-fat interactions are multifactorial, and may involve pathways that influence both bone formation and resorption with competing effects on the skeleton. One such pathway involves adipocyte production of adipokines that regulate bone metabolism. In this study we determined the association between BMD, walking status, and circulating adipokines (adiponectin and leptin) in 149 men with chronic spinal cord injury (SCI). Although adipokine levels did not vary significantly based on walking status, there was a significant inverse association between adiponectin and BMD in wheelchair users independent of body composition. We found no association between adiponectin and BMD in the walkers and no association between leptin and BMD in either group. These findings suggest that for subjects with chronic SCI, walking may mitigate the effect of adiponectin mediated bone loss. For wheelchair users, adipose-derived adiponectin may contribute to SCI-induced osteoporosis because the osteoprotective benefits of obesity appear to require mechanical loading during ambulation.
Keywords: OSTEOPOROSIS, ADIPONECTIN, BIOMARKER, SPINAL CORD INJURY, REHABILITATION MEDICINE
Introduction
A complex system of cross-regulation exists linking fat and bone metabolism.(1,2) Adipocyte-derived hormones regulate bone cell activities(3–12) while the bone-derived protein osteocalcin stimulates expression of the adipokine adiponectin.(13,14) The role of adipokines in bone metabolism has been examined in vitro,(3,7–13) in animal models,(4,10,15) and in epidemiological studies,(16–31) with much of the focus on leptin and adiponectin. Leptin was originally described as the product of the obesity gene and the observed link between obesity and bone mineral density (BMD) led to the investigation of leptin’s role in bone.(32) Long considered to signal via both central nervous system(33) and peripheral pathways,(34) more recent evidence suggests that leptin mediates bone metabolism primarily via the peripheral pathway to stimulate bone formation.(35) The leptin receptor is expressed on osteoblasts(36) and has been shown to promote osteoblast over adipocyte differentiation in bone marrow stromal cells.(37) Leptin has also been shown to inhibit in vitro differentiation of human peripheral blood mononuclear cells (PBMC) into mature, functional osteoclasts.(7) Similarly, adiponectin is a polypeptide hormone produced by osteoblasts and adipocytes in both visceral and marrow fat depots.(3,38) Circulating levels of adiponectin are inversely associated with central adiposity and increase significantly with age.(39) Signaling via active receptors expressed on bone-forming cells,(3) adiponectin can directly stimulate osteoblastogenesis and indirectly inhibit osteoclastogenesis. It has been suggested that adiponectin may be a biomarker of both bone loss and fracture risk.(23,39–41) Higher adiponectin levels were associated with greater bone loss at the lumbar spine over the course of 1 year in 35 physically active older women.(22)
In general, greater body fat is associated with greater BMD(42,43) and this is often attributed to mechanical loading of the skeleton by fat mass and modulation of bone turn over by adipokines. Spinal cord injury (SCI) is a condition characterized by both obesity and severe osteoporosis, suggesting a disruption of the mechanisms linking fat mass and BMD. No information exists regarding the impact of paralysis on bone-fat interactions. Therefore, in this study we examined the association between circulating adiponectin or leptin levels and BMD based on the ability to walk in chronic SCI.
Materials and Methods
Subjects
We studied participants with chronic SCI who were enrolled in the Boston SCI-Health Study.(44,45) Subjects were recruited from veterans who receive care at our Veterans Affairs (VA) Medical Center facility, by advertisement in SCI consumer magazines, and by direct mail. Direct mail recruitment was sent to (1) persons who previously received medical care at our non-VA acute rehabilitation facility, (2) New England subscribers of New Mobility Magazine, and (3) members of the National Spinal Cord Injury Association. Participants were eligible if they were 22 years of age or older, 1 or more years after injury, were not ventilator dependent, did not have a tracheostomy, and had no other neuromuscular disease. A total of 196 participants with SCI were enrolled in the Boston SCI-Health Study between August 2009 and January 2011. For this study, we excluded females (n=29) and only included those who were 5 years or more postinjury, for a total of 149 participants.
Motor score
Motor level and completeness of injury were confirmed by physical exam at study entry by a trained rater according to the American Spinal Injury Association Impairment Scale (AIS). Participants were classified as AIS A or B (motor complete, no motor function below the neurological level of injury); AIS C (motor incomplete, motor function preserved below the neurological level, and more than half the key muscles below the neurological level are not strong enough to overcome gravity); or AIS D (motor incomplete, motor function preserved below the neurological level, and more than half the key muscles below the neurological level strong enough to overcome gravity). Injury severity was then classified in two categories: motor complete SCI (AIS A/B) or motor incomplete SCI (AIS C or D).
Dual X-ray absorptiometry for bone mineral density and body composition
We used a fifth-generation GE Healthcare Lunar (Madison, WI, USA) iDXA dual X-ray absorptiometry (DXA) scanner with enCore configuration version 12.3 to determine BMD and to assess body composition. Total fat mass (kg) and total lean mass (kg) were calculated by the system software from whole-body scans based on body weight measured at the time of scanning. Fractures are most common at the knee (distal femur or proximal tibia) after SCI. Therefore, BMD was determined at both SCI-specific (proximal tibia, distal femur) and standard (hip, radius) skeletal sites as described.(46) Unless there was a prior fracture or instrumentation, the nondominant lower extremity and radius were scanned. For the distal femur, the proximal edge of the region of interest (ROI) was set at 20% of the femur length (measured from the lateral femoral condyle), and the distal edge was set at the visible intersection between the patella and the femur, excluding the patella from the ROI. For the proximal tibia, the proximal edge was set at the most proximal point of contact between the tibia and fibular head sites, avoiding regions of overlap between the fibula and the tibia. Scans were obtained in triplicate at the proximal tibia and distal femur and averaged. Customized research software supplied by General Electric was used to determine BMD at the knee. As a standard procedure, a quality assurance phantom supplied by the manufacturer was measured at least every 2 days to confirm accuracy of the densitometer.
For subjects age 50 years or older, T-score was used to classify hip bone density (total hip and femoral neck) according to the World Health Organization (WHO) definitions of normal (T-score≥−1), osteopenia (T-score<−1 and>−2.5), and osteoporosis (T-score≤−2.5). For subjects under the age of 50 years, Z-score was used to classify hip BMD as normal (Z-score>−2) or as lower than expected for age and sex (Z-score≤−2).
Biochemical analyses
Subjects were asked to undergo testing in a fasting state and efforts were made to collect samples in the morning before a meal. For subject safety, individuals were advised to have a light meal or snack if fasting could worsen a medical condition (orthostatic hypotension). In all cases information was collected on time since last meal or snack. Plasma samples were drawn into an EDTA tube and immediately delivered to the core blood research laboratory at our facility. The samples were centrifuged for 15 minutes at 2600 rpm (1459g) at 4°C and stored at −80°C until batch analysis. All biochemical analyses were performed at the Clinical & Epidemiologic Research Laboratory, Department of Laboratory Medicine at Children’s Hospital in Boston (Boston, MA, USA), a state-of-the-art reference laboratory that specializes in microanalysis. Assays were performed in duplicate and any duplicate with >10% coefficient of variation (CV) was repeated. Total adiponectin was quantified by ELISA assay (Alpco Diagnostics, Salem, NH, USA) with a detection limit of 0.075 ng/mL. Leptin was quantified by ultrasensitive ELISA assay (R&D Systems, Minneapolis, MN, USA) with a detection limit of 7.8 pg/mL. Total osteocalcin was measured as an indicator of bone formation by electrochemiluminescence immunoassay on a 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN, USA) with a detection limit of 0.50 ng/mL. C-telopeptide was measured as an indicator of bone resorption by electrochemiluminescence immunoassay on a 2010 Elecsys autoanalyzer (Roche Diagnostics) with a detection limit of 0.01 ng/mL. The amount of 25-hydroxyvitamin D (25 OH vitamin D) was quantified by enzyme immunoassay (Immunodiagnostic Systems Inc., Fountain Hills, AZ, USA) with a detection limit of 2.0 ng/mL. One wheelchair user had an osteocalcin level of 87.8 ng/mL. One walker and one wheelchair user had leptin levels of 122,358.5 and 91,812.2 pg/mL, respectively. These values were considered to be outliers and were removed from subsequent osteocalcin or leptin analyses.
Variable definition
Information regarding SCI, medical history, medication use, and fracture history was obtained by questionnaire at the time of DXA scan. Participants were weighed and supine length measured for the calculation of body mass index (BMI). In subjects with severe joint contractures, length was self-reported (n=14). Usual mobility mode (more than 50% of the time) was considered in the following two categories: wheelchair use (motorized wheelchair or hand-propelled wheelchair) or walking (with aid such as crutch, cane or walk without assistance). For fracture history, information was collected on timing (before SCI, at time of SCI, or after SCI), and location. Fractures were categorized as osteoporotic (ie, those occurring from standing height or less or in the absence of trauma) or traumatic fracture. Digit and rib fractures were excluded. When available, medical records were used to confirm self-reported fracture history (20/40 fractures). Osteoporotic fractures that occurred after SCI were considered in the analysis. Bisphosphonate use was assessed as ever/never. Hip BMD was used to categorize osteoporosis status as normal bone density, osteopenia, or osteoporosis/BMD lower than expected for age. For body composition, total lean mass (kg) and total fat mass (kg) were included in the analyses.
Statistical analysis
All analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, USA). Statistical t tests or χ2 tests were used to compare subject characteristics as appropriate. General linear models (PROC GLM) were applied to assess associations between adiponectin and osteocalcin. To account for the multiple measurements of bone density measured within the same person at the four sites below the level of injury (total hip, femoral neck, proximal tibia, and distal femur), a mixed model procedure with repeated measures and unstructured correlation was used to assess predictors of BMD. Factors with a p value of <0.10 in the univariate mixed models, as well as factors that were deemed clinically significant (body composition), were included in the multivariable models assessing the association of BMD and adiponectin or leptin (PROC MIXED). Factors with a p value of <0.05 were considered statistically significant and any factor with a p value of >0.05 was removed from the model. Linear trend p values for the BMD of each quartile of adiponectin among the walkers and wheelchair users were derived using an ordinal variable coded based on the median of each quartile.
Results
Subject characteristics
Subject characteristics are presented in Table 1. All participants were male and the majority were white. Ages ranged from 27.1 to 87.6 years, and injury duration ranged from 5.1 to 60.8 years. A total of 54 subjects walked independently or with an assistive device and 95 subjects used a wheelchair. Those who walked were significantly older than those who used a wheelchair (62.6 versus 51.3 years old, p<0.0001). Years since injury did not vary between the two groups (p=0.63). Participants who walked had a significantly higher lean mass (p<0.0001) and BMI (p=0.01) than those who used a wheelchair. There was no difference in bisphosphonate use or levels of vitamin D, cross-linked C-telopeptide (CTX), osteocalcin, adiponectin, or leptin between the two groups (p=0.08–0.87). A majority of subjects (77%) had not consumed anything for at least 8 hours prior to testing. Adiponectin and leptin levels did not vary significantly based on time since last meal or snack (p=0.27 for adiponectin and p=0.88 for leptin).
Table 1.
Participant Characteristics
| Variable | Walkers (n ± 54) | Wheelchair users (n ± 95) |
|---|---|---|
| Demographics | ||
| Age (years) (mean ± SD) | 62.6 ± 12.0 | 51.3 ± 12.5* |
| Years since injury (mean ± SD) | 22.1 ± 13.2 | 21.1 ± 11.8 |
| White, n (%) | 47 (87.0 %) | 87 (91.6 %) |
| Injury severity, n (%) | ||
| Motor complete SCI | 2 (3.7 %) | 74 (77.9 %) |
| Motor incomplete SCI | 52 (96.3 %) | 21 (22.1 %) |
| Body composition (kg) (mean ± SD) | ||
| Weight | 88.53 ± 16.50 | 83.76 ± 20.35 |
| Total lean mass | 55.80 ± 80.02 | 49.95 ± 82.35* |
| Total fat mass | 29.69 ± 10.71 | 31.20 ± 14.04 |
| BMI (kg/m2) (mean ± SD) | 28.5 ± 5.0 | 26.1 ± 5.7** |
| 25 OH vitamin D (ng/mL) (mean ± SD) | 23.8 ± 12.7 | 23.5 ± 8.0 |
| Normal (≥20 ng/mL) | 31 (57.4 %) | 62 (65.3 %) |
| Deficient (<20 ng/mL) | 23 (42.6 %) | 33 (34.7 %) |
| BMD (g/cm2) (mean ± SD) | ||
| SCI-specific skeletal sites | ||
| Distal femur | 0.91 ± 0.18 | 0.64 ± 0.19b,* |
| Proximal tibia | 1.02 ± 0.21 | 0.63 ± 0.22c,* |
| Traditional sites | ||
| Total hip | 0.99 ± 0.19a | 0.73 ± 0.20d,* |
| Femur neck | 0.93 ± 0.15a | 0.75 ± 0.20d,* |
| Radius | 0.98 ± 0.12 | 0.98 ± 0.10c |
| Hip bone density classification, n (%)e | ||
| Normal | 26 (48.1 %) | 12 (12.6 %)* |
| Osteopenia | 19 (35.2 %) | 9 (9.5 %) |
| Osteoporosis/BMD lower than expected for age | 8 (14.8 %) | 67 (70.5 %) |
| Hip BMD not available | 1 (1.9 %) | 7 (7.4 %) |
| Bisphosphonate use, n (%) | 5 (9.3 %) | 18 (19.0 %) |
| Post SCI osteoporotic fracture history, n (%) | 9 (16.7 %) | 31 (32.6 %)** |
| Markers of bone turnover (ng/mL) (mean ± SD) | ||
| Osteocalcin | 19.5 ± 8.1 | 19.0 ± 7.6b |
| C-telopeptide | 0.31 ± 0.13 | 0.36 ± 0.22 |
| Adiponectin (ng/mL) (mean ± SD) | 5730.9 ± 3355.8 | 5849.2 ± 3283.2 |
| Leptin (pg/mL) (mean ± SD) | 13517.1 ± 13480.0a | 13016.6 ± 11796.5b |
SCI=spinal cord injury; BMI=body mass index; BMD=bone mineral density.
n=53.
n=94.
n=93.
n=88.
Based upon Z-score or T-score at the total hip or femoral neck.
Significantly different from walkers, p<0.0001.
Significantly different from walkers, p<0.0001.
BMD could not be determined in 1 subject at the distal femur, 2 subjects at the proximal tibia, 8 subjects at the hip, and 2 subjects at the radius due to knee/hip replacement, fixation rods, heterotrophic ossification, spasms, or contractures preventing proper scan positioning. Lower extremity BMD (distal femur, proximal tibia, total hip and femur neck) was significantly lower in the wheelchair users compared to the walkers (p<0.0001), but there was no significant difference in radius BMD between the 2 groups (p=0.97). Walkers were more likely to have their hip BMD classified as normal compared to wheelchair users. Wheelchair users were more likely to be classified as osteoporotic/low BMD (p<0.0001) and to report post-SCI osteoporotic fractures (p=0.01).
Clinical factors associated with lower extremity BMD
Factors associated with BMD varied according to the ability to walk. There was no significant association between BMD and age, injury duration, vitamin D level or status, or total fat mass (p=0.12–0.99; Table 2). For the walkers, BMD was positively associated with body weight (p=0.01), BMI (p=0.03), and lean mass (p=0.0008). There was no association between BMD and CTX (p=0.36) or osteocalcin (p=0.16). Adiponectin and leptin were not significantly associated with BMD in the walkers and remained nonsignificant after adjusting for lean mass (p=0.36, Table 3A, p=0.14, Table 3B, respectively). These results remained unchanged when also adjusting for BMI or when excluding active bisphosphonate users from the analyses. There was no significant linear trend in mean BMD among the quartiles of adiponectin and mean BMD in the walkers after adjusting for lean mass (p=0.87, Table 4).
Table 2.
Univariate Factors Associated With BMD (g/cm2) Based on Walking Status
| Walkers (n=54) | Wheelchair users (n=95) | |||
|---|---|---|---|---|
| Variable | β ± SE | p | β ± SE | p |
| Age (years) | −0.0023 ± 0.0015 | 0.12 | 0.0011 ± 0.0012 | 0.37 |
| Injury duration (years) | 0.00080 ± 0.0014 | 0.56 | −0.0019 ± 0.0015 | 0.19 |
| Weight (kg) | 0.0028 ± 0.0010 | 0.01 | 0.0015 ± 0.00084 | 0.07 |
| Lean mass (kg) | 0.0074 ± 0.0021 | 0.0008 | 0.0046 ± 0.0020 | 0.03 |
| Fat mass (kg) | 0.0022 ± 0.0017 | 0.20 | 0.0013 ± 0.0012 | 0.29 |
| BMI (kg/m2) | 0.0076 ± 0.0034 | 0.03 | 0.0049 ± 0.003 | 0.11 |
| 25 OH vitamin D (ng/mL) | −0.00028 ± 0.04 | 0.85 | −0.0013 ± 0.002 | 0.53 |
| Markers of bone turnover (ng/mL) | ||||
| C-telopeptide | 0.13 ± 0.14 | 0.36 | −0.19 ± 0.076 | 0.01 |
| Osteocalcin | 0.0031 ± 0.0022 | 0.16 | −0.0054 ± 0.0022 | 0.02 |
| Mean BMD ± SE | p | mean BMD ± SE | p | |
| 25 OH vitamin D | 0.99 | 0.52 | ||
| Normal (≥20 ng/mL) | 0.875 ± 0.027 | 0.684 ± 0.021 | ||
| Deficient (<20 ng/mL) | 0.875 ± 0.023 | 0.708 ± 0.029 | ||
Bone density was obtained from a repeated measures regression model based on proximal tibia, distal femur, total hip, and femoral neck BMD.
BMD=bone mineral density; BMI=body mass index.
Table 3.
Association Between Adipokines and Bone Density Based on Walking Status
| β ± SE | p | |
|---|---|---|
| (A) Adiponectin, BMD (g/cm2) per mg/dL | ||
| Walkers (n=54) | ||
| Unadjusted | −0.00764 ± 0.0532 | 0.89 |
| Fully adjusteda | −0.0460 ± 0.0496 | 0.36 |
| Wheelchair users (n=95) | ||
| Unadjusted | −0.179 ± 0.0494 | 0.0005 |
| Fully adjusteda | −0.159 ± 0.0532 | 0.004 |
| (B) Leptin, BMD (g/cm2) per mg/dL | ||
| Walkers (n=54) | ||
| Unadjusted | 0.0242 ± 0.0133 | 0.08 |
| Fully adjusteda | 0.0186 ± 0.0122 | 0.14 |
| Wheelchair users (n=95) | ||
| Unadjusted | −0.00276 ± 0.0123 | 0.82 |
| Fully adjusteda | −0.0168 ± 0.0132 | 0.20 |
Bone density was obtained from a repeated measures regression model based on proximal tibia, distal femur, total hip, and femoral neck BMD.
BMD=bone mineral density.
Adjusted for lean mass (kg).
Table 4.
Mean Bone Density by Adiponectin Quartile Based on Walking Status
| Walkers | Wheelchair users | |||
|---|---|---|---|---|
| Mean BMD (g/cm2) ± SE | p for trend | Mean BMD (g/cm2) ± SE | p for trend | |
| Adiponectin quartiles (mg/dL) | 0.87* | 0.002* | ||
| Quartile 1 (≤0.3350) | 0.897 ± 0.032 | 0.776 ± 0.033 | ||
| Quartile 2 (0.3350 to ≤0.4916) | 0.901 ± 0.031 | 0.703 ± 0.035 | ||
| Quartile 3 (0.4916 to ≤0.7336) | 0.852 ± 0.031 | 0.703 ± 0.032 | ||
| Quartile 4 (>0.7336) | 0.910 ± 0.035 | 0.616 ± 0.032 | ||
Mean bone density was obtained from a repeated measures regression model based on proximal tibia, distal femur, total hip, and femoral neck BMD. Adiponectin quartiles based on its distribution in the entire cohort.
BMD=bone mineral density.
Adjusted for lean mass (kg).
For wheelchair users, there was no association between BMD and body weight or BMI (p=0.07–0.11, Table 2). BMD was positively associated with lean mass (p=0.03) and negatively associated with CTX (p=0.01) and osteocalcin (p=0.02). BMD was negatively associated with adiponectin (p=0.0005) and remained significant after adjusting for lean mass (p=0.004, Table 3A). Leptin was not significantly associated with BMD (p=0.82) and remained nonsignificant after adjusting for lean mass (p=0.20, Table 3B). These results remained unchanged when adjusting for BMI or when excluding active bisphosphonate users from the analyses. There was an inverse linear trend in BMD level with adiponectin quartiles that remained significant after adjusting for lean mass (p=0.002, Table 4). A similar analysis demonstrated no consistent relationship between leptin quartiles and BMD in either walkers or wheelchair users (results not shown).
Adiponectin and history of post-SCI osteoporotic fracture
Fifteen fractures occurred during high-impact events including motor vehicle accidents, falls down flights of stairs, and skiing accidents. These fractures were considered traumatic and were not included in the analysis. There was no association between adiponectin and post-SCI osteoporotic fracture history in either group (walkers p=0.1, wheelchair users p=0.08). When considering only those who reported a history of fracture, there was no association between adiponectin and time since fracture (walkers p=0.82, wheelchair users p=0.77).
Association between osteocalcin and adiponectin
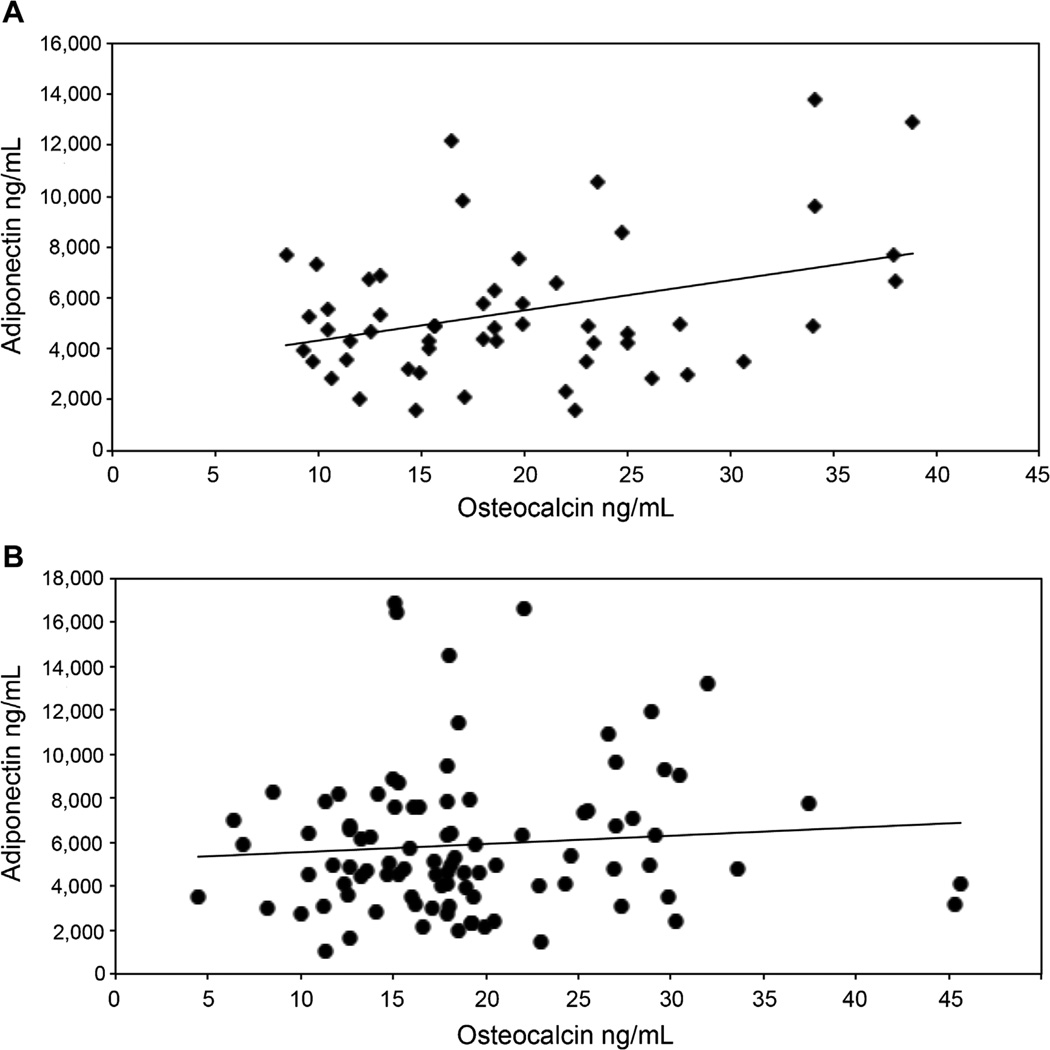
For walkers, osteocalcin was positively associated with adiponectin (p=0.01) and this remained significant after adjusting for age and fat mass (p=0.02, R2=0.32; Fig. 1A, B). For wheelchair users, the association between osteocalcin and adiponectin was not significant (p=0.19, age and fat mass adjusted p=0.20). These results remained unchanged when excluding active bisphosphonate users from the analyses.
Fig. 1.
Association between adiponectin and osteocalcin by walking status. Greater osteocalcin levels are significantly associated with greater adiponectin levels (A) in subjects with SCI who walk (p=0.02, R2=0.32), (B) but not those who use a wheelchair (p=0.38). These relationships did not change when excluding subjects taking a bisphosphonate.
Discussion
We examined BMD and circulating adipokine levels based on walking status in 149 men with chronic SCI. There was a significant inverse relationship between adiponectin and BMD in wheelchair users independent of body composition. We found no association between adiponectin and BMD in walkers and no association between leptin and bone density in either group. Wheelchair users also had lower BMD at the knees (distal femur and proximal tibia) and hips (total hip and femur neck), were more likely to have osteoporosis at the hip, and were more likely to report a history of osteoporotic fracture compared to those who walk. BMD at the radius was not significantly different between the two groups. Adiponectin and leptin levels also did not vary significantly based on walking status. We found no association between age, vitamin D level, or vitamin D status (normal versus deficient) and BMD in either walkers or wheelchair users. We found that lower extremity BMD increased with lean mass in both groups. Body weight and BMI were positively associated with BMD in walkers but not in wheelchair users.
Our findings are in agreement with previous reports of a negative association between BMD and adiponectin in men that is independent of body composition.(40) In the current study, we detected this association in paralyzed men with chronic SCI. We found no association between adiponectin and bone in subjects who walk. Although we found no association between adiponectin and bone in subjects who walk, it is possible that differences in study population and design could account for this finding. Other studies have found associations in the elderly, and included men generally older than in our cohort.(39–41) Associations have been reported between adiponectin and lumbar spine BMD,(23) a site that we did not study because spinal instrumentation or arthritic changes are common after SCI, making reliable spine imaging difficult. Furthermore, the lumbar spine is not a clinically relevant skeletal site in this population because compression fractures rarely occur after SCI. In addition, other studies in the general population included a much larger numbers of subjects. Therefore, it is possible that a weaker association exists between bone and adiponectin in men with SCI who walk that we could not detect.
SCI has been considered a model of accelerated aging because it prematurely leads to reduced mobility.(45) In this respect, it is possible that wheelchair users have bone-fat interactions that are more similar to elderly men than younger men with SCI who walk. Adipose tissue may serve a dual role in the context of bone-fat interactions: (1) contributor to total body weight and therefore a source of mechanical loading; and (2) an endocrine organ that produces adipokines. Motor complete SCI results in pure mechanical unloading. In this case adipose tissue functions as an endocrine organ without the accompanying mechanical loading. It is possible that elderly men have changes in functional status associated with less mobility than younger, healthy males. In this case, bone-fat interactions in elderly men may more resemble wheelchair users. Most studies do not address walking status when examining adipokines and bone. The Swedish based MrOs study assessed physical activity in the week prior to testing and found adiponectin levels were greater in subjects who were more physically active, suggesting a relationship between mechanical loading and adipokine production.(39) This is in contrast to our finding that adipokine levels did not vary based on the ability to walk.
A negative association has been demonstrated between adiponectin and BMD in healthy older men, postmenopausal women,(17,18,20,25,28,31) adolescents,(20) and people with metabolic syndrome.(47) In some studies this relationship is mediated by body mass, fat mass, lean mass, or BMI and does not remain significant after adjusting for these factors.(18,20,23,28,48) Adiponectin has also been implicated in the development of osteoporosis.(23,39–41) Adiponectin was associated with fracture risk in elderly men participating in the MrOS study (Sweden) and in the Health ABC study (USA).(39,41) There was no relationship between adiponectin and fracture in elderly women in the Health ABC study, suggesting a gender effect. The relationship between adipokine levels and bone loss or fracture risk is unknown in SCI. In this study we found no association between adiponectin levels and history of osteoporotic fracture occurring after SCI. Future work is needed to determine if adiponectin levels are predictive of incident osteoporotic fractures in this population.
It has been suggested that BMI does not accurately reflect changes in body composition after SCI. In one report participants with SCI were 13% fatter per unit of BMI compared to age and sex matched controls.(49) Our data are consistent with this finding in that wheelchair users had greater total fat and a lower BMI than the walkers. Even though it underestimates adiposity, BMI is still recommended for body composition assessment in SCI clinical trials with epidemiological, neurological, and functional outcomes.(50) In this study we did not use BMI to identify individuals with SCI who were overweight or obese. Instead, we considered BMI to reflect mechanical loading of bone during ambulation due to total body mass. We did find that relationships between bone and body composition differ based on walking status. Body mass and BMI are positively associated with BMD in men with chronic SCI who walk, and this finding is in agreement with reports in the general population. In contrast, we observed a dissociation between body weight and BMD in paralyzed men that is not unexpected given that obesity and fractures are both common in this population. Osteocalcin is a marker of bone formation and is thought to link bone and fat metabolism by regulating adiponectin expression.(13,14) In the current study we also found a significant association between osteocalcin and adiponectin in the walkers only. These findings suggest that the mechanical loading associated with walking is critical for cross-regulation of bone and fat metabolism.
Despite the widely held belief that obesity is osteoprotective, many studies have suggested detrimental effects of obesity on bone. In the general population, obesity in adolescence causes decreased bone strength relative to body weight, and fractures may be more common in both obese children(51) and obese adults.(52) Although not directly assessed, decreased physical activity and therefore decreased mechanical loading may account for the findings reported in these studies. It is possible that, in the absence of mechanical loading to stimulate bone formation, adipokine-mediated bone resorption predominates promoting bone loss. This is supported in the current study by the significant inverse association between markers of bone turnover and BMD in the wheelchair users only.
In this study we found no independent relationship between leptin and BMD after adjusting for body composition. Leptin has been studied in the context of SCI but none have focused on associations between leptin and bone.(53–59) Leptin is reportedly greater in men with SCI compared to age- and BMI-matched controls and is positively correlated with fat mass. In contrast to leptin, there is a significant inverse relationship between adiponectin and BMD in wheelchair users independent of body composition.
In this study ambulatory status is closely related to the severity of neurological injury. Bone is densely innervated and it is possible that loss of neural input contributes to bone loss in the setting of chronic SCI. Similarly, there are other putative signaling pathways that may work separately or synergistically with adiponectin to influence bone metabolism following paralysis. Future work focused on differences in myokine or inflammatory cytokine expression based on ambulatory status is warranted. Furthermore, additional work is required to assess adiponectin as a biomarker of fracture risk and to determine if adiponectin contributes to bone loss in motor complete SCI or other conditions associated with decreased mechanical loading of the lower extremity, including cerebral palsy, prolonged bed rest, or microgravity.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (R21HD057030 and R21HD057030-02S1), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1R01AR059270-01), the Department of Education, NIDRR (H133N110010), the Office of Research and Development, Rehabilitation Research and Development (Merit Review Grant B6618R), and the Massachusetts Veterans Epidemiology Research and Information Center, Cooperative Studies Program, Department of Veterans Affairs. We thank Sam Davis, clinical research coordinator and technician, Boston VA Healthcare System, for assisting with bone density scans; and Rachael Burns and Kara Loo, research assistants, Boston VA Healthcare System, for collection of anthropometric data.
Footnotes
All authors state that they have no conflicts of interest.
Authors’ roles: Study design: LM, RB, DG, EG, and RZ. Study conduct: AD, JD, and AL. Data collection: AD, JD, and AL. Data analysis: LM, EG, AD, and DG. Data interpretation: LM, RB, EG, AL, DG, and RZ. Drafting manuscript: LM, RB, EG, AL, AD, and RZ. Revising manuscript content: LM, AD, RB, EG, DG, AL, JD, and RZ. Approving final version of manuscript: LM, AD, JD, AL, DG, EG, RB, and RZ. RB, EG, AD, DG, RZ, and LM take responsibility for the integrity of the data analysis.
References
- 1.Duque G. Bone and fat connection in aging bone. Curr Opin Rheumatol. 2008;20:429–434. doi: 10.1097/BOR.0b013e3283025e9c. [DOI] [PubMed] [Google Scholar]
- 2.Rosen CJ, Klibanski A. Bone, fat, body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–849. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 5.Guo LJ, Xie H, Liao EY. Effect of adiponectin on human osteoblast differentiation. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:731–736. Chinese. [PubMed] [Google Scholar]
- 6.Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Kajimura D, Myers MG, Jr, Chua SC, Jr, Wang Q, Kim JK, Kaestner KH, Karsenty G. An osteoblast-dependent mechanism contributes to the leptin regulation of insulin secretion. Ann N Y Acad Sci. 2009;1173(Suppl 1):E20–E30. doi: 10.1111/j.1749-6632.2009.05061.x. [DOI] [PubMed] [Google Scholar]
- 7.Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 8.Lee HW, Kim SY, Kim AY, Lee EJ, Choi JY, Kim JB. Adiponectin stimulates osteoblast differentiation through induction of COX2 in mesenchymal progenitor cells. Stem Cells. 2009;27:2254–2262. doi: 10.1002/stem.144. [DOI] [PubMed] [Google Scholar]
- 9.Lee WY, Rhee EJ, Oh KW, Kim SY, Jung CH, Yun EJ, Baek KH, Kang MI, Kim SW. Identification of adiponectin and its receptors in human osteoblast-like cells and association of T45G polymorphism in exon 2 of adiponectin gene with lumbar spine bone mineral density in Korean women. Clin Endocrinol (Oxf) 2006;65:631–637. doi: 10.1111/j.1365-2265.2006.02641.x. [DOI] [PubMed] [Google Scholar]
- 10.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331:520–526. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 11.Peng M, Chen S, Fang W, Yu X. Effects of leptin on the expression of alpha1 (I) collagen gene in human osteoblast-like MG63 cells. Biochem Cell Biol. 2010;88:683–686. doi: 10.1139/O10-007. [DOI] [PubMed] [Google Scholar]
- 12.Zeadin MG, Butcher MK, Shaughnessy SG, Werstuck GH. Leptin promotes osteoblast differentiation and mineralization of primary cultures of vascular smooth muscle cells by inhibiting glycogen synthase kinase (GSK)-3beta. Biochem Biophys Res Commun. 2012;425:924–930. doi: 10.1016/j.bbrc.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Schafer AL, Sellmeyer DE, Schwartz AV, Rosen CJ, Vittinghoff E, Palermo L, Bilezikian JP, Shoback DM, Black DM. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study) J Clin Endocrinol Metab. 2011;96:E1982–E1989. doi: 10.1210/jc.2011-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhou P, Kimondo JW. Adiponectin and osteocalcin: relation to insulin sensitivity. Biochem Cell Biol. 2012;90:613–620. doi: 10.1139/o2012-022. [DOI] [PubMed] [Google Scholar]
- 15.Ealey KN, Kaludjerovic J, Archer MC, Ward WE. Adiponectin is a negative regulator of bone mineral and bone strength in growing mice. Exp Biol Med (Maywood) 2008;233:1546–1553. doi: 10.3181/0806-RM-192. [DOI] [PubMed] [Google Scholar]
- 16.Adiponectin is a metabolic link between obesity and bone mineral density. Exp Biol Med (Maywood) 2008;233:vi. [PubMed] [Google Scholar]
- 17.Ağbaht K, Gürlek A, Karakaya J, Bayraktar M. Circulating adiponectin represents a biomarker of the association between adiposity and bone mineral density. Endocrine. 2009 Jun;35(3):371–379. doi: 10.1007/s12020-009-9158-2. [DOI] [PubMed] [Google Scholar]
- 18.Basurto L, Galvan R, Cordova N, Saucedo R, Vargas C, Campos S, Halley E, Avelar F, Zarate A. Adiponectin is associated with low bone mineral density in elderly men. Eur J Endocrinol. 2009;160:289–293. doi: 10.1530/EJE-08-0569. [DOI] [PubMed] [Google Scholar]
- 19.Dennison EM, Syddall HE, Fall CH, Javaid MK, Arden NK, Phillips DI, Cooper C. Plasma leptin concentration and change in bone density among elderly men and women: the Hertfordshire Cohort Study. Calcif Tissue Int. 2004;74:401–406. doi: 10.1007/s00223-002-0017-x. [DOI] [PubMed] [Google Scholar]
- 20.Huang KC, Cheng WC, Yen RF, Tsai KS, Tai TY, Yang WS. Lack of independent relationship between plasma adiponectin, leptin levels and bone density in nondiabetic female adolescents. Clin Endocrinol (Oxf) 2004;61:204–208. doi: 10.1111/j.1365-2265.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 21.Jen KL, Buison A, Darga L, Nelson D. The relationship between blood leptin level and bone density is specific to ethnicity and menopausal status. J Lab Clin Med. 2005;146:18–24. doi: 10.1016/j.lab.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Jurimae J, Kums T, Jurimae T. Adipocytokine and ghrelin levels in relation to bone mineral density in physically active older women: longitudinal associations. Eur J Endocrinol. 2009;160:381–385. doi: 10.1530/EJE-08-0673. [DOI] [PubMed] [Google Scholar]
- 23.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Yano S, Sugimoto T. Relationships between serum adiponectin levels versus bone mineral density, bone metabolic markers, and vertebral fractures in type 2 diabetes mellitus. Eur J Endocrinol. 2009;160:265–273. doi: 10.1530/EJE-08-0642. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman BA, Warren MP, Dominguez JE, Wang J, Heymsfield SB, Pierson RN. Bone density and amenorrhea in ballet dancers are related to a decreased resting metabolic rate and lower leptin levels. J Clin Endocrinol Metab. 2002;87:2777–2783. doi: 10.1210/jcem.87.6.8565. [DOI] [PubMed] [Google Scholar]
- 25.Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646–651. doi: 10.1016/s8756-3282(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz MT, de la Piedra C, Barrios V, Garrido G, Argente J. Changes in bone density and bone markers in rhythmic gymnasts and ballet dancers: implications for puberty and leptin levels. Eur J Endocrinol. 2004;151:491–496. doi: 10.1530/eje.0.1510491. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulou F, Krassas GE, Kalothetou C, Koliakos G, Constantinidis TC. Serum leptin values in relation to bone density and growth hormone-insulin like growth factors axis in healthy men. Arch Androl. 2004;50:97–103. [PubMed] [Google Scholar]
- 28.Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92:1517–1523. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- 29.Soot T, Jurimae T, Jurimae J. Areal bone density in young females with different physical activity patterns: relationships with plasma leptin and body composition. J Sports Med Phys Fitness. 2007;47:65–69. [PubMed] [Google Scholar]
- 30.Ushiroyama T, Ikeda A, Hosotani T, Higashiyama T, Ueki M. Inverse correlation between serum leptin concentration and vertebral bone density in postmenopausal women. Gynecol Endocrinol. 2003;17:31–36. [PubMed] [Google Scholar]
- 31.Zoico E, Zamboni M, Di Francesco V, Mazzali G, Fantin F, De Pergola G, Zivelonghi A, Adami S, Bosello O. Relation between adiponectin and bone mineral density in elderly post-menopausal women: role of body composition, leptin, insulin resistance, and dehydroepiandrosterone sulfate. J Endocrinol Invest. 2008;31:297–302. doi: 10.1007/BF03346361. [DOI] [PubMed] [Google Scholar]
- 32.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 33.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 34.Khosla S. Leptin—central or peripheral to the regulation of bone metabolism? Endocrinology. 2002;143:4161–4164. doi: 10.1210/en.2002-220843. [DOI] [PubMed] [Google Scholar]
- 35.Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, Iwaniec UT. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013;28:22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enjuanes A, Supervia A, Nogues X, Diez-Perez A. Leptin receptor (OB-R) gene expression in human primary osteoblasts: confirmation. J Bone Miner Res. 2002;17:1135. doi: 10.1359/jbmr.2002.17.6.1135. [DOI] [PubMed] [Google Scholar]
- 37.Thomas T. Leptin: a potential mediator for protective effects of fat mass on bone tissue. Joint Bone Spine. 2003;70:18–1821. doi: 10.1016/s1297-319x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 38.Yokota T, Meka CS, Medina KL, Igarashi H, Comp PC, Takahashi M, Nishida M, Oritani K, Miyagawa J, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins. J Clin Invest. 2002;109:1303–1310. doi: 10.1172/JCI14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson H, Oden A, Lerner UH, Jutberger H, Lorentzon M, Barrett-Connor E, Karlsson MK, Ljunggren O, Smith U, McCloskey E, Kanis JA, Ohlsson C, Mellstrom D. High serum adiponectin predicts incident fractures in elderly men: Osteoporotic Fractures in Men (MrOS) Sweden. J Bone Miner Res. 2012;27:1390–1396. doi: 10.1002/jbmr.1591. [DOI] [PubMed] [Google Scholar]
- 40.Araneta MR, von Mühlen D, Barrett-Connor E. Sex differences in the association between adiponectin and BMD, bone loss, and fractures: the Rancho Bernardo study. J Bone Miner Res. 2009;24:2016–2022. doi: 10.1359/JBMR.090519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW, Kanaya AM, Harris TB, Bauer DC, Cauley JA. Adipokines and the risk of fracture in older adults. J Bone Miner Res. 2011;26:1568–1576. doi: 10.1002/jbmr.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8:567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 43.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31:547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 44.Battaglino RA, Sudhakar S, Lazzari AA, Garshick E, Zafonte R, Morse LR. Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone. 2012;51:600–605. doi: 10.1016/j.bone.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, Garshick E, Battaglino RA. Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res. 2012;27:352–359. doi: 10.1002/jbmr.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morse LR, Lazzari AA, Battaglino R, Stolzmann KL, Matthess KR, Gagnon DR, Davis SA, Garshick E. Dual energy X-ray absorptiometry of the distal femur may be more reliable than the proximal tibia in spinal cord injury. Arch Phys Med Rehabil. 2009;90:827–831. doi: 10.1016/j.apmr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iacobellis G, Iorio M, Napoli N, Cotesta D, Zinnamosca L, Marinelli C, Petramala L, Minisola S, D’Erasmo E, Letizia C. Relation of adiponectin, visfatin and bone mineral density in patients with metabolic syndrome. J Endocrinol Invest. 2011;34:e12–e15. doi: 10.1007/BF03346703. [DOI] [PubMed] [Google Scholar]
- 48.Jurimae J, Jurimae T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26:618–623. doi: 10.1007/s00774-008-0861-5. [DOI] [PubMed] [Google Scholar]
- 49.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr, Waters RL, Bauman WA. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol. 2003;95:2398–2407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- 50.Gater DR, Kody CL. Body composition assessment in spinal cord injury clinical trials. Top Spinal Cord Inj Rehabil. 2005;11:36–49. [Google Scholar]
- 51.Petit MA, Beck TJ, Hughes JM, Lin HM, Bentley C, Lloyd T. Proximal femur mechanical adaptation to weight gain in late adolescence: a six-year longitudinal study. J Bone Miner Res. 2008;23:180–188. doi: 10.1359/JBMR.071018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raska I, Jr, Broulik P. The impact of diabetes mellitus on skeletal health: an established phenomenon with in established causes? Prague Med Rep. 2005;106:137–148. [PubMed] [Google Scholar]
- 53.Bauman WA, Spungen AM, Zhong YG, Mobbs CV. Plasma leptin is directly related to body adiposity in subjects with spinal cord injury. Horm Metab Res. 1996;28:732–736. doi: 10.1055/s-2007-979889. [DOI] [PubMed] [Google Scholar]
- 54.Gezici AR, Ergun R, Karakas A, Gunduz B. Serum leptin levels following acute experimental spinal cord injury. J Spinal Cord Med. 2009;32:416–421. doi: 10.1080/10790268.2009.11753205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang TS, Wang YH, Chen SY. The relation of serum leptin to body mass index and to serum cortisol in men with spinal cord injury. Arch Phys Med Rehabil. 2000;81:1582–1586. doi: 10.1053/apmr.2000.9173. [DOI] [PubMed] [Google Scholar]
- 56.Jeon JY, Steadward RD, Wheeler GD, Bell G, McCargar L, Harber V. Intact sympathetic nervous system is required for leptin effects on resting metabolic rate in people with spinal cord injury. J Clin Endocrinol Metab. 2003;88:402–407. doi: 10.1210/jc.2002-020939. [DOI] [PubMed] [Google Scholar]
- 57.Maimoun L, Puech AM, Manetta J, Badiou S, Paris F, Ohanna F, Rossi M, Sultan C. Circulating leptin concentrations can be used as a surrogate marker of fat mass in acute spinal cord injury patients. Metabolism. 2004;53:989–94. doi: 10.1016/j.metabol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Maruyama Y, Mizuguchi M, Yaginuma T, Kusaka M, Yoshida H, Yokoyama K, Kasahara Y, Hosoya T. Serum leptin, abdominal obesity and the metabolic syndrome in individuals with chronic spinal cord injury. Spinal Cord. 2008;46:494–499. doi: 10.1038/sj.sc.3102171. [DOI] [PubMed] [Google Scholar]
- 59.Wang YH, Huang TS, Liang HW, Su TC, Chen SY, Wang TD. Fasting serum levels of adiponectin, ghrelin, and leptin in men with spinal cord injury. Arch Phys Med Rehabil. 2005;86:1964–1968. doi: 10.1016/j.apmr.2005.04.017. [DOI] [PubMed] [Google Scholar]