Abstract
The assimilation hypothesis argues that second language learning recruits the brain network for processing the native language, whereas the accommodation hypothesis argues that learning a second language recruits brain structures not involved in native language processing. This study tested these hypotheses by examining brain activation of a group of native Chinese speakers, who were late bilinguals with varying levels of proficiency in English, when they performed a rhyming judgment to visually presented English word pairs (CE group) during fMRI. Assimilation was examined by comparing the CE group to native Chinese speakers performing the rhyming task in Chinese (CC group), and accommodation was examined by comparing the CE group to native English speakers performing the rhyming task in English (EE group). The CE group was very similar in activation to the CC group, supporting the assimilation hypothesis. Additional support for the assimilation hypothesis was the finding that higher proficiency in the CE group was related to increased activation in the Chinese network (as defined by the CC > EE), including the left middle frontal gyrus, the right inferior parietal lobule, and the right precuneus, and decreased activation in the English network (as defined by the EE > CC), including the left inferior frontal gyrus and the left inferior temporal gyrus. Although most of the results support assimilation, there was some evidence for accommodation as the CE group showed less activation in the Chinese network including the right middle occipital gyrus, which has been argued to be involved in holistic visuospatial processing of Chinese characters.
INTRODUCTION
Humans have a unique ability to learn more than one language—a skill that is thought to be mediated by functional and structural plasticity in the brain (Mechelli et al., 2004). The framework of accommodation and assimilation has been used to understand the brain's responses to learning a new language with a different writing system (Perfetti et al., 2007). Assimilation refers to using the procedures of the existing reading network in the acquisition of a new writing system, whereas accommodation refers to using new procedures for reading the new writing system (Perfetti & Liu, 2005; Piaget, 1983). In the current study, we examined the assimilation–accommodation pattern in a group of late Chinese–English bilinguals.
It has been of great interest to study the brain mechanisms that support English reading in Chinese speakers because of the significant differences between the two writing systems. Different from English, which follows the alphabetic principle with semiregular mapping between graphemes (letters) and phonemes (sounds), Chinese characters map to phonology at the syllable level, and no part of a character corresponds to a phoneme of the syllable. Chinese has phonetic radicals that do offer some cues to syllabic level pronunciation; yet, they do not have systematic mappings from character to syllable. Chinese is highly homophonic, as many written characters correspond to the same syllable. Therefore, efficient orthographic recognition plays an important role in successful Chinese reading. At the script level, English is a left–right linear layout of letters, whereas Chinese character forms are complex visual–spatial configurations in a two-dimensional square. Taken together, Chinese differs from English in the nature of the mapping between orthography and phonology, in which grapheme–phoneme correspondence procedures are involved in English and whole-character to whole-syllable mapping is involved in Chinese. Chinese also differs from English in the nature of the writing system, in which holistic visual analysis is more involved in Chinese, whereas fine-grained visual word form recognition is emphasized in both English and Chinese.
Perhaps, because of structural differences in the writing systems and how they map to phonology, different brain regions have been shown to be involved in Chinese reading compared with English reading in monolingual studies. At the phonological mapping level, studies have found that there is greater brain activation in the left inferior frontal gyrus (IFG) and left superior temporal gyrus (STG) in English reading than in Chinese reading, which has been associated with greater involvement of grapheme–phoneme correspondence reading procedures in English (Bolger, Perfetti, & Schneider, 2005; Tan, Laird, Karl, & Fox, 2005). On the other hand, there is greater brain activation in the left middle frontal gyrus (MFG) in Chinese reading than in English reading, which has been associated with the greater involvement of the character–syllable mapping procedures in Chinese (Bolger et al., 2005; Tan et al., 2005). At the script level, studies have shown greater activation in the left inferior temporal gyrus (ITG) in English than in Chinese perhaps because of the more detailed analysis of letters in English words, whereas there is greater activation in bilateral superior parietal lobule, right lingual gyrus, and right middle occipital gyrus in Chinese than in English perhaps because of the holistic visuospatial analysis in Chinese characters (Cao et al., 2010, 2012; Bolger et al., 2005; Tan et al., 2005).
There are a limited number of studies that have examined brain mechanisms of English reading in Chinese speakers. Interestingly, these studies have consistently revealed assimilation, that is, Chinese speakers appear to use their Chinese network to process English. One study found that English words, Chinese Pinyin (an alphabetic Chinese script), and Chinese characters activated the same brain network in Chinese–English bilinguals in both an orthographic search task and a semantic classification task (Ding et al., 2003), and similar results were replicated in a group of 10- to 12-year-old children (Xue, Dong, Jin, Zhang, & Wang, 2004). Two other studies found that both late and early Chinese–English bilinguals used one common network for both Chinese and English (Yang, Tan, & Li, 2011; Chee, Tan, & Thiel, 1999). Two other studies have found that late Chinese–English bilinguals engaged a region thought to be critical for reading Chinese (i.e., left MFG) in an English word reading task (Nelson, Liu, Fiez, & Perfetti, 2009) and an English word rhyming task (Tan et al., 2003), in comparison with a group of English monolinguals who showed substantial activation in the left IFG and left STG in the same English word task. However, there is also evidence for accommodation in Chinese–English bilinguals. One study found that there is reduced activity in the right visual cortex for English words compared with Chinese characters in Chinese–English bilinguals (Liu & Perfetti, 2003), probably because the visual form of English words does not require holistic visual analysis, which has been implicated as the right hemisphere function. Taken together, Chinese learners of English can assimilate the new language to their existing system with some accommodation at the written-form level. This may be because the brain network that copes with the relatively arbitrary mapping between orthography and phonology at the syllabic level in Chinese can be extended to the semiregular grapheme–phoneme correspondence in English.
Bilingual studies have often tried to determine whether proficiency in the second language is related to the differential recruitment of brain regions. Several studies have shown that high proficiency in the second language is associated with reduced activation in the left IFG, and this has been attributed to less effortful processing (Stein et al., 2006, 2009; Reiterer, Berger, Hemmelmann, & Rappelsberger, 2005; Tatsuno & Sakai, 2005). Proficiency also appears to be a factor influencing the patterns of assimilation and accommodation, but previous studies have provided mixed results. Some studies have found that increased proficiency in the second language is related to greater similarity to the first language during semantic judgment in a group of English–German bilinguals (Stein et al., 2009), during listening to stories in a group of Italian–English bilinguals (Perani et al., 1998), and during sentence production in a group of French–English bilinguals (Golestani et al., 2006). In contrast, there are also studies that have found that higher proficiency is related to greater accommodation. One study found that high proficiency in late English–Chinese bilinguals was associated with increased brain activation in two “Chinese” regions: the right superior parietal lobule and right lingual gyrus (Cao et al., 2012). Another study found that late English–Chinese bilinguals showed greater increased activation in another “Chinese” region (i.e., the left superior parietal lobule) with greater proficiency improvement from early learning to late learning (Deng, Booth, Chou, Ding, & Peng, 2008). To date, no published studies have examined how proficiency influences assimilation–accommodation patterns in Chinese–English bilinguals. Because the Chinese network appears to be effective in processing English as revealed by the previous studies showing assimilation, one may expect that high proficiency in English is characterized by greater assimilation in Chinese–English bilinguals.
To examine the accommodation–assimilation hypothesis, it is very important to have a monolingual control group in both languages. First, language control refers to participants whose native language is the first language of the bilingual group. Second, language control refers to participants whose native language is the second language of the bilingual group. Assimilation may be implicated by greater similarity to the first-language control group than to the second-language control group, and vice versa for accommodation. In the bilingual literature, many studies have a first language control in which researchers asked the bilingual participants to read their first and second language and then compared their L1 and L2 (Jamal, Piche, Napoliello, Perfetti, & Eden, 2012; Leonard et al., 2010; Gandour et al., 2007; Liu, Dunlap, Fiez, & Perfetti, 2007; Xue et al., 2004; Perani et al., 2003; Pillai et al., 2003; Chee et al., 1999). Some studies have a second-language control group but do not have a first-language control group (Zhao et al., 2012). It is difficult to make definitive conclusions about accommodation and assimilation in these studies because of the lack of both control groups. There are two studies on late Chinese–English bilinguals that have both control groups (Nelson et al., 2009; Tan et al., 2003); however, the first-language control group was the bilingual participants themselves (i.e., within-subject), whereas the second-language control group was another group of participants (i.e., between-subject). Both of these studies found that the brain activation for the bilinguals when reading English is similar to their activation when reading Chinese but that their activation was different from a group of native English speakers reading English. These studies suggest a pattern of assimilation; however, the greater similarity to the Chinese network than to the English network may be because the former comparison is within-subject, whereas the latter comparison is between-subject. To effectively examine the assimilation versus accommodation hypothesis, three groups of participants are needed, namely, a group of bilinguals reading their second language, a group of first language controls, and a group of second language controls. In this design, comparisons to both control groups are between-subject, and therefore, greater similarity to one control group compared with another can be interpreted as assimilation or accommodation.
The current study aimed at examining the assimilation– accommodation hypothesis in Chinese–English bilinguals. We included three groups of participants, a group of native Chinese speakers performing an English word rhyming task (the CE group), a group of native Chinese speakers performing a Chinese word rhyming task (the CC group), and a group of native English speakers performing an English word rhyming task (the EE group). This design allowed us to define the Chinese network by comparing the CC to the EE group and the English network by comparing the EE to the CC group. We then could determine how the Chinese–English bilinguals recruited the Chinese or English network when performing a phonological task to English-written word forms. On the basis of the literature, we expected the CE group to be more similar to the CC group than to the EE group, which would be reflective of assimilation. To examine how proficiency modulates the pattern of assimilation/accommodation, we recruited bilingual participants with varying levels of reading proficiency in English. We expected that higher proficiency would be related to increasing recruitment of the Chinese network when reading English, again suggesting greater assimilation.
METHODS
Participants
Three groups of participants were involved in this study (see Table 1): the CE group was a group of 26 native Chinese speaking adults (mean age = 22.0 years, range = 19–27 years; 13 men) performing an English word rhyming judgment task, the CC group was a group of 20 native Chinese speaking adults (mean age = 21.0 years, range = 19–28 years; six men) performing a Chinese word rhyming judgment task, and the EE group was a group of 24 native English speaking adults (mean age = 21.5 years, range = 18–34 years; 13 men) performing an English word rhyming judgment task. Participants in the CE and CC groups were undergraduate or graduate students at Beijing Normal University, and participants in the EE group were undergraduate or graduate students at Northwestern University. The participants in the CE group and the CC group were late Chinese–English bilinguals with an average age of acquisition of English at 11.7 years (range: 9–13 years). The participants in the CE group had different levels of English proficiency, according to the ranges of raw scores on four subtests in a standardized English reading test, WJ III (Woodcock, McGrew, & Mather, 2001): vocabulary, Antonyms; vocabulary, Synonyms; word reading accuracy, Word ID; and pseudoword reading accuracy, Word Attack (see Table 1). The English proficiency in the CC group was not tested; however, it should be within the range of the CE group. Chinese reading proficiency was comparable in the CE group and the CC group according to their college entrance exam—the Chinese section. The limitation of the CC group serving as the L1 control was that participants in the CC group were also late Chinese–English bilinguals, and it has been suggested that learning a second language will modulate the brain network for the first language (Zou et al., 2012). Therefore, brain activation of the CC group may be slightly different than Chinese monolingual speakers. All participants met the following criteria: (1) native speakers of their language (English or Mandarin Chinese), (2) right-handed, (3) free of neurological disease or psychiatric disorders, (4) no attention deficit hyperactivity disorder, and (5) no learning disability. The institutional review board at Northwestern University and Beijing Normal University approved the informed consent procedures.
Table 1.
Demographic, Standardized Test, and Task Performance Measures for Participants in Each Group
| CE | CC | EE | |
|---|---|---|---|
| n | 26 | 20 | 24 |
| Age | 22 (2.1) | 21 (3.5) | 21.5 (2.2) |
| AoA | 11.7 [9–13] | – | – |
| WJ-III | |||
| Antonym | 13.2 [7–21] | – | – |
| Synonym | 9.7 [4–19] | – | – |
| Word ID | 40.8 [25–52] | – | – |
| Word Attack | 19.5 [9–27] | – | – |
| Accuracy (%) | 78.9 (10.0) | 92.6 (4.8) | 96.4 (4.0) |
| RT (msec) | 1220 (349) | 1224 (328) | 970 (314) |
EE = English speakers performing an English word rhyming task; CC = Chinese speakers performing a Chinese word rhyming task; CE = Chinese speakers performing an English word rhyming task; n = number of participants; AoA = age of acquisition for the second language. Antonym and Synonym are verbal measures from the Woodcock Johnson-III in English; Word ID, Word Attack, and Reading Fluency are reading measures from the WJ-III. Accuracy and RT indicate performance on the rhyming trials in the judgment task. Standard deviations are indicated by parentheses, and ranges are indicated in the brackets.
Tasks
During scanning, participants performed a rhyming judgment task to sequentially presented visual word pairs interspersed with perceptual control and baseline trials. Pairs of word stimuli either rhymed or did not rhyme. Participants were asked to respond as quickly and accurately as possible, using their right index finger for a yes (rhyme) response and their right middle finger for a no (nonrhyme) response. The presentation duration of all words was 800 msec, with a 200-msec interval between the two words. A red fixation cross appeared on the screen right after the offset of the second word, indicating the need to make a response. The response interval duration was variable (2200, 2600, or 2800 msec), such that each trial lasted for either 4000, 4400, or 4800 msec. Baseline trials (48) required the participant to press the “yes” button when a fixation cross at the center of the screen turned from black to red. Perceptual trials (24) were also included as part of a larger study but were not of interest in the present experiment. These trials required participants to determine whether two sequentially presented symbol patterns matched or mismatched by pressing the “yes” or “no” button. The timing for the perceptual control and baseline trials was the same as for the lexical trials. The order of presentation of lexical trials, perceptual trials, and baseline trials and the variation of response interval were optimized for event-related designs by OptSeq (surfer.nmr.mgh.harvard.edu/optseq/).
In English, all words were monosyllabic without homophones. There were 24 trials in each of four conditions and included two rhyming conditions, such that the words in each pair had similar orthographic and phonological endings (O+P+, e.g., late–hate) or different orthographic but similar phonological endings (O−P+, e.g., jazz–has), and two nonrhyming conditions, such that words had different orthographic and phonological endings (O−P−, e.g., press–list) or similar orthographic but different phonological endings (O+P−, e.g., pint–mint). However, we were only interested in the two rhyming conditions in the current study, because responses in the nonrhyming conditions may or may not involve phonology but would strongly engage nonlinguistic decision processes. Orthographic similarity was manipulated in the rhyming conditions so that participants could not base their decision solely on orthographic overlap. All words were matched across conditions for written word frequency and the sum of their written bigram frequency (English Lexicon Project, elexicon.wustl.edu).
In Chinese, all words consisted of two characters without homophones at the word level. As with the English stimuli, there were 24 trials in each of four conditions, two rhyming and two nonrhyming. Rhyming was defined as the same rhyme for the second character of the word. Similar orthography was defined as sharing a phonetic radical for the second character of the word. The two rhyming conditions included one with similar orthography and phonology (O+P+, e.g., 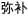 /mi2bu3/,
/mi2bu3/, 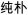 /chun2pu3/) and one with different orthography and similar phonology (O−P+, e.g.,
/chun2pu3/) and one with different orthography and similar phonology (O−P+, e.g., 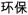 /huan2bao3/,
/huan2bao3/, 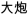 /da4pao4/). The two nonrhyming conditions included one with different orthography and phonology (O−P−, e.g.,
/da4pao4/). The two nonrhyming conditions included one with different orthography and phonology (O−P−, e.g.,  /sun3huai4/,
/sun3huai4/, 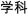 /xue2ke1/) and one with similar orthography and different phonology (O+P−, e.g.,
/xue2ke1/) and one with similar orthography and different phonology (O+P−, e.g., 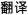 /fan1yi4/,
/fan1yi4/,  /xuan3ze2/). In half of the trials of the four lexical conditions (rhyming and nonrhyming), the second character of the first and second words had the same tone (e.g.,
/xuan3ze2/). In half of the trials of the four lexical conditions (rhyming and nonrhyming), the second character of the first and second words had the same tone (e.g., 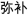 /mi2bu3/,
/mi2bu3/, 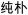 /chun2pu3/), and in the other half, they had different tones (e.g.,
/chun2pu3/), and in the other half, they had different tones (e.g., 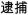 /dai4bu3/,
/dai4bu3/, 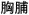 /xiong1pu2/). The two-character words and the second character of those words were matched on several variables across conditions including adult written frequency (Beijing Language and Culture University, 1990), and number of strokes. As with the English task, we included only the rhyming trials in the data analysis.
/xiong1pu2/). The two-character words and the second character of those words were matched on several variables across conditions including adult written frequency (Beijing Language and Culture University, 1990), and number of strokes. As with the English task, we included only the rhyming trials in the data analysis.
MRI Data Acquisition
Participants lay in the scanner with their head position secured with foam padding. An optical response box was placed in the participant's dominant right hand, and a compression alarm ball was placed in the left hand. The head coil was positioned over the participant's head so that they could effectively use the mirror to view the projection screen at the rear of the scanner. Images for the CE and CC groups were acquired using a 3.0-T Siemens scanner (Siemens Healthcare, Erlangen, Germany) at Beijing Normal University, and images for the EE group were acquired using an identical scanner at Northwestern University. Identical scanning protocols were used at the two locations. Gradient echo localizer images were acquired to determine the placement of the functional slices. For the functional images, a susceptibility weighted single-shot EPI method with BOLD was used with the following scan parameters: echo time = 20 msec, flip angle = 80°, matrix size = 120 × 128, field of view = 220 × 206.3 mm, slice thickness = 3 mm (0.48-mm gap), number of slices = 32, and repetition time [TR] = 2000 msec. These parameters resulted in a 1.7 × 1.7 × 3 mm voxel size. One hundred forty-five whole-brain volumes were acquired each run using an interleaved bottom-to-top sequence, with one complete volume collected every 2 sec (TR = 2000 msec). A high-resolution, T1-weighted 3-D image was also acquired with the following parameters: TR = 2300 msec, echo time = 3.36 msec, flip angle = 9°, matrix size = 256 × 256, field of view = 256 mm, slice thickness = 1 mm, number of slices = 160, and resulting voxel size = 1 × 1 × 1 mm. The acquisition of the anatomical scan took approximately 9 min.
Image Analysis
Data analysis was performed using SPM8 (www.fil.ion.ucl. ac.uk/spm). The following steps were used for data preprocessing: (1) slice timing correction for interleaved acquisition using sinc interpolation; (2) fourth degree b-splice interpolation for realignment to the first volume; (3) trilinear coregistration with the anatomical image; (4) segmentation of the anatomical image; (5) normalization of all brains to the standard T1 Montreal Neurological Institute adult template with voxel size = 2 × 2 × 2 mm (12 linear affine parameters for brain size and position, eight nonlinear iterations and nonlinear basis functions); and (6) 4 × 4 × 8 mm FWHM Gaussian kernel smoothing up to one volume, where movements that exceeded 3 mm in any of the x, y, or z dimensions were replaced with the mean of the images immediately before and after the outlying volume. Participants with >1 volume with >3 mm of movement were excluded from the study.
Statistical analyses at the first level were calculated using an event-related design with four lexical trial types, the perceptual control trials, and the baseline trials as six conditions of interest. A high-pass filter with a cutoff period of 128 sec was applied. Trials were modeled using a canonical hemodynamic response function. Data from each participant were entered into a general linear model using an event-related analysis procedure. Group results were obtained using random effects analyses by combining subject-specific summary statistics across the group as implemented in SPM8. The effect of rhyming trials (including O+P+ and O−P+) versus the baseline trials was tested using a one-sample t test separately for each group (CE, CC, and EE). Group comparisons were conducted on the contrast of rhyming minus baseline using two-sample t tests: CC versus EE, EE versus CE, and CC versus CE. To determine whether reading proficiency in the second language in the CE group was associated with brain activation, correlations were calculated with raw scores on word reading accuracy (i.e., Word ID), pseudoword reading accuracy (i.e., Word Attack), and accuracy on the rhyming trials for the experimental task. The main effect of CC minus EE and EE minus CC served as masks in these correlation analyses because we were interested if second-language reading skill was correlated with brain activation for the Chinese or English network, respectively. All reported results were at uncorrected p < .001, voxels > 15, which equals FWE-corrected p < .05 in our data according to AlphaSim (Cox, 1996).
RESULTS
Behavioral Performance
ANOVAs of Group (EE, CC, and CE) were calculated on the rhyming task separately for Accuracy and RT (see Table 1). For Accuracy, there was a significant main effect of Group (F(2, 67) = 44.498, p < .001). Multiple comparisons showed that the CC and EE groups had a higher accuracy than the CE group (t(44) = 5.748, p < .001 for CC > CE; t(48) = 8.167, p < .001 for EE > CE). The EE group also had a higher accuracy than the CC group (t(42) = 2.911, p < .01). For RT, there was a significant main effect of Group (F(2, 67) = 4.050, p < .05). Multiple comparisons showed that the EE group had a faster RT than the CE group (t(48) = 2.652, p < .05) and the CC group (t(42) = 2.619, p < .05). The CE group and the CC group were not significantly different.
Brain Activation Patterns
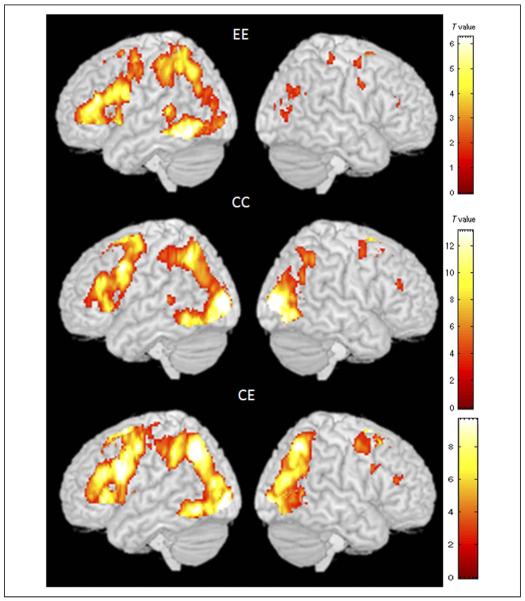
Results for the contrast of rhyming judgment greater than baseline for the EE, CC, and CE groups are presented in Table 2, and Figure 1 shows the brain activation maps for each group. There was greater activation for the rhyming compared with baseline in a similar network across the three groups. The left hemisphere network included inferior/middle occipital gyri, inferior temporal/fusiform gyri, inferior/middle frontal gyri, and inferior parietal lobule (IPL). The right hemisphere network included middle occipital gyrus, IPL, and MFG. Figure 1 shows clearly that the brain activation pattern in the CE group is more similar to the CC group than to the EE group, especially in the right occipito-parietal regions.
Table 2.
Brain Activations for the Contrast of Rhyming Minus Baseline for Each Group: EE, CC, and CE
| Anatomical Region | H | BA | Voxels | Z score | x | y | z |
|---|---|---|---|---|---|---|---|
| EE | |||||||
| Inferior occipital gyrus, fusiform gyrus, ITG | L | 37, 19 | 3,256 | inf. | −46 | −60 | −12 |
| IFG | L | 46, 45, 44 | 2,614 | 6.87 | −48 | 30 | 18 |
| Superior parietal lobule, IPL | L | 7, 40 | 166 | 6.17 | −26 | −64 | 44 |
| STG | L | 22 | 16 | 5.79 | −56 | −40 | 8 |
| Medial frontal gyrus, cingulate gyrus, superior frontal gyrus | L | 6, 8, 32 | 399 | 5.65 | −4 | 12 | 52 |
| Posterior cingulate, lingual gyrus | L/R | 30, 18, 19 | 2,457 | 5.35 | −4 | −70 | 8 |
| Superior frontal gyrus, MFG, precentral gyrus | L | 6, 4, 3, 2, 1 | 176 | 5.26 | −22 | −6 | 52 |
| Parahippocampal gyrus | L | 27 | 61 | 3.38 | −14 | −34 | 0 |
| Precentral gyrus | L | 6 | 231 | 4.53 | −56 | 0 | 40 |
| Cingulate gyrus | L | 24 | 39 | 4.34 | −4 | 0 | 28 |
| Precentral gyrus | R | 6 | 25 | 4.01 | 54 | −4 | 36 |
| MFG | R | 6 | 27 | 3.91 | 26 | −4 | 56 |
| Postcentral gyrus | R | 3 | 15 | 3.81 | 40 | −34 | 60 |
| Middle occipital gyrus | R | 19 | 28 | 3.73 | 38 | −82 | 6 |
| Precentral gyrus | R | 4 | 16 | 3.71 | 30 | −32 | 58 |
| Parahippocampal gyrus | L | 30 | 15 | 3.56 | −20 | −52 | 0 |
| Cuneus | L | 18 | 15 | 3.51 | −16 | −80 | 24 |
| CC | |||||||
| Middle occipital gyrus, cuneus, precuneus | R | 18, 19 | 7,809 | Inf. | 24 | −92 | 4 |
| MFG | L | 9 | 2,984 | 6.85 | −48 | 10 | 36 |
| Medial frontal gyrus, superior frontal gyrus | L | 6 | 812 | 6.25 | −2 | 16 | 48 |
| Caudate | R | – | 64 | 4.33 | 28 | −36 | 8 |
| Parahippocampal gyrus | L | 27 | 58 | 4.31 | −16 | −32 | −4 |
| STG | L | 22 | 31 | 4.26 | −50 | −36 | 6 |
| IPL, precuneus | L | 40, 7 | 836 | 6.65 | −30 | −58 | 46 |
| MFG | R | 6 | 127 | 3.87 | 32 | −4 | 52 |
| Culmen | – | – | 42 | 3.84 | 0 | −48 | −10 |
| MFG | R | 46 | 40 | 3.80 | 40 | 32 | 22 |
| CE | |||||||
| Superior parietal lobule | L | 7 | 1,034 | Inf. | −22 | −62 | 46 |
| Lingual gyrus, inferior occipital gyrus, ITG | L | 19, 37 | 407 | 7.83 | −16 | −90 | −6 |
| Medial frontal gyrus, superior frontal gyrus | L/R | 6 | 557 | 7.67 | −4 | 14 | 50 |
| IFG, MFG | L | 46, 9, 45 | 1,102 | 7.61 | −44 | 12 | 26 |
| IFG, insula | L | 47, 13 | 133 | 7.51 | −28 | 28 | 0 |
| IPL | L | 40 | 171 | 6.27 | −42 | −42 | 44 |
| Parahippocampal gyrus | L | 27 | 4.70 | −16 | −32 | −4 | |
| MFG | R | 6 | 194 | 4.56 | 26 | −6 | 52 |
| Superior parietal lobule, middle temporal gyrus, precuneus | R | 7, 39, 37 | 487 | 7.17 | 26 | −64 | 50 |
| Lingual gyrus, middle occipital gyrus | R | 18, 19 | 77 | 6.82 | 18 | −90 | −2 |
H = hemisphere; L = left; R = right; BA = Brodmann's area.
Figure 1.
Brain activation maps for the contrast of rhyming > baseline in the EE, CC, and CE groups. The EE group is native English speakers performing an English visual rhyming task, the CC group is native Chinese speakers performing a Chinese visual rhyming task, and the CE group is native Chinese speakers performing the English visual word rhyming task. The activation maps illustrate that the CE group is most similar to the CC group, particularly for occipito-parietal cortex in the right hemisphere, showing that the second language (i.e., English) is most similar to the Chinese language network.
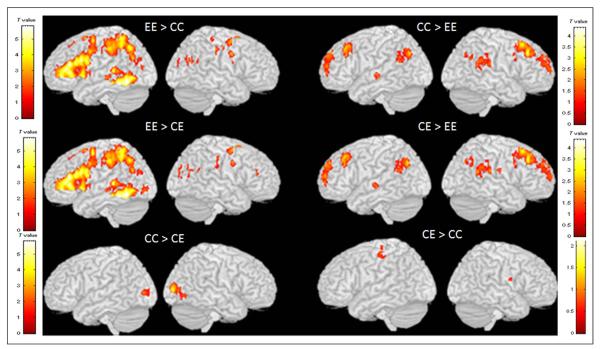
Group comparisons on the contrast of rhyming judgment greater than baseline are presented in Table 3, and Figure 2 shows the brain maps for these comparisons. In general, the brain maps are very similar for the contrasts of EE > CC and EE > CE as well as for the contrasts of CC > EE and CE > EE, showing that CE and CC are similar to each other. The EE group showed greater activation than both the CC and CE groups in the left IFG, left ITG, left posterior STG, and left inferior/superior parietal lobule. In contrast, both the CC and CE groups showed greater activation than the EE group in the bilateral middle frontal gyri, bilateral IPL, and left anterior STG. The comparison between the CC and CE groups resulted in little difference as shown in Figure 2. The CC group showed greater activation than the CE group in the bilateral middle occipital gyri. In summary, the activation maps within each group and the differences between groups showed that the Chinese speakers making English rhyme judgments activated a network that was very similar to that activated by Chinese speakers making Chinese rhyme judgments. This is very different from the network activated by English speakers making rhyming judgments in English.
Table 3.
Brain Activations for the Comparisons between the EE, CC, and CE Groups on the Contrast of P+ Minus Baseline
| Anatomical Region | H | BA | Voxels | Z score | x | y | z |
|---|---|---|---|---|---|---|---|
| EE > CC | |||||||
| ITG, fusiform gyrus, inferior occipital gyrus | L | 37, 19 | 646 | 7.26 | −46 | −60 | −12 |
| IFG | L | 46, 44, 45 | 1,925 | 6.13 | −50 | 30 | 18 |
| STG, middle temporal gyrus | L | 22, 21 | 109 | 5.47 | −56 | −40 | 8 |
| Medial frontal gyrus | L | 8 | 657 | 5.99 | −4 | 12 | 52 |
| Superior parietal lobule, precuneus | L | 7 | 1,379 | 5.45 | −26 | −64 | 44 |
| Superior frontal gyrus | L | 6 | 44 | 4.42 | −22 | −6 | 52 |
| Posterior cingulate, lingual gyrus | L/R | 31, 18 | 1,301 | 5.45 | 12 | −62 | −2 |
| Middle temporal gyrus | L | 21 | 24 | 4.31 | −46 | −28 | −6 |
| Precentral gyrus | L | 6 | 133 | 4.24 | −42 | −12 | 46 |
| Precentral gyrus | R | 6 | 54 | 4.82 | 54 | −4 | 36 |
| CC > EE | |||||||
| MFG | R | 9 | 643 | 5.69 | 26 | 28 | 38 |
| Precuneus | L | 7 | 80 | 4.72 | −38 | −70 | 34 |
| IPL | R | 40 | 224 | 4.36 | 54 | −44 | 26 |
| MFG | L | 9 | 61 | 4.35 | −40 | 22 | 40 |
| MFG | L | 8 | 134 | 4.33 | −28 | 26 | 46 |
| Superior frontal gyrus | L | 10 | 141 | 4.25 | −18 | 54 | 28 |
| MFG | R | 6 | 72 | 4.18 | 42 | 12 | 56 |
| Precuneus, IPL | R | 7, 39, 40 | 55 | 3.89 | 40 | −70 | 36 |
| Postcentral gyrus | R | 4 | 24 | 3.69 | 56 | −16 | 24 |
| STG, supramarginal gyrus | L | 22, 40 | 56 | 3.67 | −42 | −54 | 22 |
| IFG | R | 44 | 15 | 3.66 | 50 | 18 | 12 |
| EE > CE | |||||||
| ITG, fusiform gyrus, inferior occipital gyrus | L | 19, 37 | 666 | 7.14 | −46 | −60 | −12 |
| IFG | 46, 45 | 1,783 | 6.16 | −48 | 30 | 18 | |
| STG, middle temporal gyrus | L | 22, 21 | 142 | 5.58 | −56 | −40 | 8 |
| Superior parietal lobule, IPL | L | 7, 40 | 1,225 | 5.14 | −26 | −64 | 44 |
| STG | L | 22 | 78 | 5.06 | −54 | 8 | −4 |
| IFG | L | 47 | 74 | 4.76 | −26 | 30 | −4 |
| Lingual gyrus, posterior cingulate | R | 18, 30 | 1,354 | 4.75 | 12 | −62 | −2 |
| Superior frontal gyrus | L | 6 | 68 | 4.68 | −22 | −6 | 52 |
| Medial frontal gyrus, cingulate gyrus, superior frontal gyrus | L | 6, 32 | 153 | 4.49 | −4 | 12 | 52 |
| Inferior occipital gyrus | L | 18 | 15 | 3.89 | −22 | −90 | −4 |
| Middle occipital gyrus | L | 18 | 24 | 3.80 | −30 | −88 | −4 |
| Precentral gyrus | R | 6 | 17 | 3.66 | 54 | −4 | 36 |
| Precentral gyrus | L | 6 | 54 | 4.06 | −42 | −12 | 44 |
| CE > EE | |||||||
| MFG | R | 9 | 604 | 5.52 | 26 | 30 | 40 |
| Precuneus, IPL | L | 7, 39 | 78 | 4.83 | −38 | −70 | 34 |
| STG, IPL | R | 22, 40 | 232 | 4.62 | 54 | −50 | 20 |
| Cingulate gyrus | L/R | 24, 32 | 341 | 4.56 | −10 | −36 | 38 |
| MFG | L | 8, 9 | 177 | 4.50 | −26 | 26 | 46 |
| Superior frontal gyrus | L | 10 | 143 | 4.42 | −18 | 54 | 28 |
| MFG | L | 9 | 79 | 4.35 | −40 | 24 | 40 |
| MFG | R | 6 | 102 | 3.93 | 42 | 12 | 56 |
| STG | L | 22 | 51 | 3.89 | −44 | −56 | 22 |
| Postcentral gyrus | R | 3 | 40 | 3.89 | 62 | −16 | 18 |
| Insula | R | 13 | 26 | 3.66 | 58 | −32 | 20 |
| IPL | R | 40 | 29 | 3.63 | 44 | −62 | 44 |
| Insula | L | 13 | 19 | 3.54 | −42 | −20 | −4 |
| CC > CE | |||||||
| Middle occipital gyrus, inferior occipital gyrus | R | 18, 19 | 247 | 5.52 | 24 | −92 | 4 |
| Middle occipital gyrus, cuneus | L | 18, 19 | 72 | 4.25 | −26 | −86 | 4 |
| CE > CC | |||||||
| Postcentral gyrus | L | 3 | 58 | 3.92 | −46 | −24 | 58 |
| Precentral gyrus | R | 4 | 15 | 3.86 | 56 | −6 | 16 |
| Precentral gyrus | L | 6 | 17 | 3.80 | −36 | −24 | 70 |
Figure 2.
Comparisons between the EE, CC, and CE groups. These comparisons illustrate that the CE group shows little difference from the CC group, showing that the second language (i.e., English) is most similar to the Chinese language network.
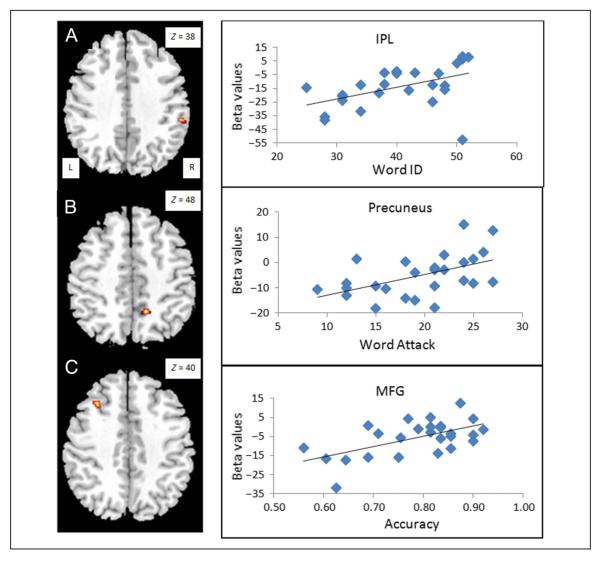
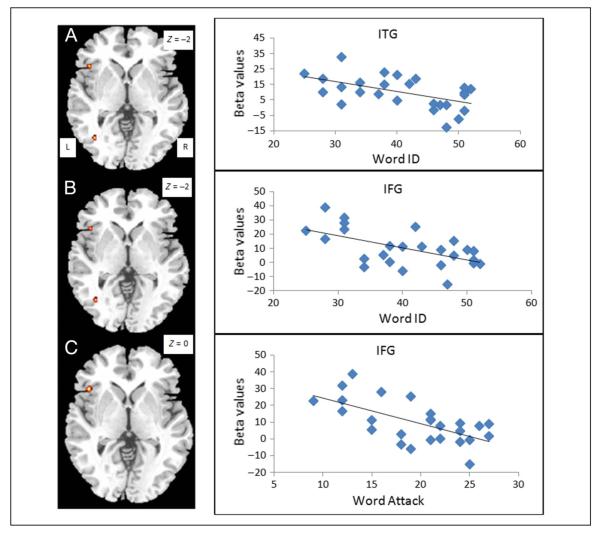
Table 4 presents findings from the brain–behavior correlation analyses within the CE group. We found a significant positive correlation with the score on Word ID in the right IPL (see Figure 3A), with the score on Word Attack in the right precuneus (see Figure 3B), and with the accuracy on the rhyming trials in two clusters in the left MFG and one cluster in the left medial frontal gyrus (see Figure 3C) when these analyses were constrained by an inclusive mask of CC > EE. We found no positive brain–behavior correlations with an inclusive mask of EE > CC. We found a significant negative correlation with the score on Word ID in the left ITG and left IFG (see Figure 4A and B) and a significant negative correlation with Word Attack in the left IFG (see Figure 4C) when these analyses were constrained by an inclusive mask of EE > CC. The negative correlations overlapped for Word ID and Word Attack in the left IFG. We found no negative brain–behavior correlations with an inclusive mask of CC > EE. In summary, higher skill in English for the Chinese speakers was associated with greater activation in the Chinese network and less activation in the English network.
Table 4.
Brain Activations that Showed Significant Correlations with Reading Scores and Accuracy in the CE Group
| Anatomical Region | H | BA | Voxels | Z score | x | y | z |
|---|---|---|---|---|---|---|---|
| Positive | |||||||
| Word ID | |||||||
| IPL | R | 40 | 15 | 3.91 | 56 | −34 | 38 |
| Word Attack | |||||||
| Precuneus | R | 7 | 15 | 4.12 | 12 | −52 | 48 |
| Accuracy | |||||||
| Medial frontal gyrus | L | 6 | 19 | 4.05 | 22 | 38 | 34 |
| MFG | L | 9 | 21 | 3.91 | −30 | 20 | 40 |
| MFG | L | 9 | 17 | 3.71 | −32 | 32 | 32 |
| Negative | |||||||
| Word ID | |||||||
| ITG | L | 19, 37 | 15 | 4.01 | −38 | −66 | −2 |
| IFG | L | 47 | 15 | 3.55 | −44 | 22 | 0 |
| Word Attack | |||||||
| IFG | L | 47 | 15 | 3.91 | −44 | 22 | 0 |
Figure 3.
Positive brain–behavioral correlations for reading skill measures within the CE group. (A) Correlation with standardized word reading (i.e., Word ID) at the right IPL. (B) Correlation with standardized pseudoword reading (i.e., Word Attack) at the right precuneus. (C) Correlation with accuracy on the rhyming task at the left MFG. All of these positive correlations overlap with CC greater than EE (see Figure 2), showing that better performance in the second language (i.e., English) is associated with greater recruitment of the Chinese language network.
Figure 4.
Negative brain–behavioral correlations for reading skill measures within the CE group. Correlation with standardized word reading (i.e., Word ID) at the left ITG (A) and the left IFG (B). Correlation with standardized pseudoword reading (i.e., Word Attack) at (C) the left IFG. All of these negative correlations overlap with EE greater than CC (see Figure 2), showing that worse performance in the second language is associated with recruitment of the English language network.
DISCUSSION
We investigated the assimilation–accommodation hypothesis in second language learning by comparing brain activation of a group of bilingual Chinese speakers performing a rhyming task in English (CE) with native Chinese speakers performing a rhyming task in Chinese (CC) and with native English speakers performing the rhyming task in English (EE). We found that the CE group was more similar to the CC group than to the EE group in the direct group comparisons, suggesting assimilation. The brain–behavior correlations also revealed that higher proficiency in the CE group was related to greater involvement of the Chinese network and reduced involvement of the English network, suggesting increased assimilation and decreased accommodation. However, we found accommodation at the word-form level, reflected by reduced activation in the right middle occipital gyrus in the CE group in comparison with the CC group. In summary, our study suggests that late Chinese–English bilinguals use their native language network to process the second language, and higher proficiency is characterized by greater involvement of the native language network. Our finding of assimilation and accommodation is consistent with previous studies on Chinese–English bilinguals using different tasks, namely a visual word rhyming judgment task (Tan et al., 2003), a passive viewing task (Nelson et al., 2009; Liu et al., 2007), a lexical decision task (Yang et al., 2011), a grammatical judgment task (Chen, Shu, Liu, Zhao, & Li, 2007), or a word-stem completion task (Chee et al., 1999). However, unlike the previous studies, our results are based on direct comparisons at the whole-brain level of the bilingual Chinese–English group with two control groups. A comparison with the CC and EE groups is necessary to directly test the assimilation–accommodation hypothesis. Another novel aspect of our study is that we showed that greater assimilation was related to higher proficiency in the second language.
The main finding of our current study is the assimilation in the late Chinese–English bilinguals. A very similar network was involved in the bilinguals' first and second language reading, which is distinct from the native English speakers' network. The EE group showed greater activation in the English network including the left ITG, STG, and IFG than the CC group and the CE group, whereas the CC and CE groups showed greater activation than the EE group in the left MFG, which is thought to be a crucial region in Chinese reading involved in lexical retrieval and integration (Cao et al., 2009; Tan et al., 2001). This is consistent with previous studies that have found that Chinese–English bilinguals activated the left MFG for English word reading, whereas native English speakers activated the left IFG and STG for the same task (Nelson et al., 2009; Tan et al., 2003). Actually, a number of neuroimaging studies have found that the first and second language networks are similar (for a review, see Abutalebi, 2008; Green & Abutalebi, 2008), suggesting that the L2 seems to be acquired through the same neural structures responsible for the L1 acquisition.
Moreover, in our study, we found that higher proficiency in the second language is associated with increased assimilation and decreased accommodation. This is consistent with previous studies that have found that higher proficiency is associated with greater assimilation (Stein et al., 2009; Perani et al., 1998). We found that higher accuracy on the English word rhyming task was associated with greater activation in the left MFG. Previous studies on Chinese L1 readers have found that adults show greater activation in this region than children (Cao et al., 2009, 2010), suggesting that the left MFG is associated with higher proficiency. It appears that expertise in reading L1 and L2 is related to the involvement of the same region, suggesting that reading L1 and L2 shares the same underlying procedures.
We also found that higher proficiency, as measured by word reading accuracy, in the second language was associated with greater activation for the Chinese–English speakers reading English in the right IPL, a region in the Chinese network (as defined by CC > EE). The right IPL has been suggested to be involved in spatial perception and visual–motor integration in both the clinical literature (for a review, see Critchley, 1953) and the neuroimaging literature (Caspers et al., 2011; Fernandez-Ruiz, Goltz, DeSouza, Vilis, & Crawford, 2007; Andersen & Buneo, 2002; Colby & Olson, 1999), whereas the left IPL has been found to be involved in semantic and phonological processing in language (Price, Wise, Watson, Patterson, & Howard, 1994; Geschwind, 1970). One recent study found that the right IPL was more activated in participants from Mainland China for simple Chinese characters than traditional Chinese characters that were matched for familiarity and visual complexity, and the only difference between them was that there was both reading and writing experiences with the simple characters, but there was only reading experience for the traditional characters (Zhai, Ren, Xiao, Deng, & Xu, 2011). Another recent study found that the right IPL was more activated in literate population than illiterate population in a Chinese character discrimination task (Wu et al., 2012), again suggesting that only people with the experience of writing show activation in the right IPL during recognition. Our study suggests that this region plays a more important role for Chinese character recognition than English word recognition (i.e., CC > EE), probably because there is a greater emphasis on writing in the classroom instruction during reading acquisition for Chinese to cope with the complexity of the visual form. The current study suggests that, because of the influence of the first language, Chinese–English bilinguals apply this strategy when they learn English as well. We also found that higher proficiency, as measured by pseudoword reading accuracy, was associated with greater activation in the right precuneus/superior parietal lobule, which is also a region in the Chinese network (as defined by CC > EE). The right precuneus/superior parietal lobule has been thought to be associated with visuospatial analysis (LaBar, Gitelman, Parrish, & Mesulam, 1999; Alivisatos & Petrides, 1997; Cohen et al., 1996). This region is especially important for Chinese because of the complex visuospatial configuration of Chinese characters, as this region has been found to be more involved in processing Chinese characters as compared with English words (Liu et al., 2007), more involved in Chinese adults than Chinese children during character reading (Cao et al., 2010), and more involved in Chinese L2 learners with a higher performance (Cao et al., 2012). Taken together, higher English proficiency in Chinese–English bilinguals appears to be associated with a greater involvement of the right parietal regions that are more involved in Chinese than in English for the processes of visual–spatial and visual–motor coordination.
In contrast, we found that Chinese–English bilinguals with higher proficiency in English showed reduced activation in two regions of the English network (as defined by EE > CC), namely, the left IFG and left ITG. The left IFG is associated with phonological processing in English reading including phonological manipulation and working memory, and the left ITG is associated with orthographic recognition (Price, 2012; Pugh et al., 1996). These two regions have been found to be essential in English reading, because they are more involved in English reading than in Chinese reading (Bolger et al., 2005; Tan et al., 2005), more involved in adult reading than in child reading (Shaywitz et al., 2007; Booth et al., 2004), and more involved in higher skill child readers than lower skill child readers (Booth et al., 2003). Our finding of reduced activation in these important English regions in high-proficiency bilinguals implicates that the bilinguals use a different procedure from native English speakers. Taken together, higher proficiency in English is characterized by increased assimilation and decreased accommodation in the Chinese– English bilinguals.
The only evidence of accommodation from the current study is found in the visual form areas. The CE group showed significantly reduced activation in the bilateral middle occipital gyrus than the CC group. It suggests that English words are less demanding in visual analysis than Chinese characters. This is consistent with one previous study that found that Chinese–English bilinguals showed reduced activity in the right visual cortex for English words compared with Chinese characters, suggesting accommodation at the visual-form level (Liu & Perfetti, 2003). The right visuo-orthographic regions have been found to be more involved in Chinese than in English because of the visual complexity of Chinese characters (Bolger et al., 2005; Tan et al., 2005). In several training studies of English speakers who are Chinese L2 learners, accommodation at the visual-form level has also been demonstrated by the involvement of visuo-orthographic regions in the “Chinese” network including the right fusiform gyrus and right middle occipital gyrus when reading Chinese characters (Cao et al., 2012; Zhao et al., 2012; Deng, Chou, Ding, Peng, & Booth, 2011; Nelson et al., 2009; Liu et al., 2007). Higher proficiency in Chinese L2 learners is also associated with greater accommodation in regions involved in the Chinese visual form processing (Cao et al., 2012).
Connectionist models have suggested that universal statistical learning rules can apply to all writing systems with some accommodation at the visual word-form level (Zevin & Seidenberg, 2006). Consistent with this argument, our study found that the first and second language networks are similar, but we also showed that second language learning is characterized by changes in the first language network to accommodate to the special features of the new visual word form. However, evidence suggests that accommodation not only happens at the visual word-form level but also in brain regions involved in mapping from visual word forms to linguistic representations. For example, studies have found that English L1 speakers showed greater activation in the left MFG for their second language Chinese than their first language (Cao et al., 2012; Nelson et al., 2009; Liu et al., 2007). The left MFG has been thought to be a crucial region in Chinese reading, which is associated with lexical retrieval and integration. It appears that assimilation is not sufficient in English learners of Chinese, and new procedures related with the function of left MFG need to be recruited. A recent study on equal Spanish–English bilinguals also found that English was associated with greater activation in the left MFG as compared with Spanish (Jamal et al., 2012), suggesting that the less-regular mapping between orthography and phonology in English compared with Spanish required additional neural resources. A critical remaining question is what controls the balance of accommodation and assimilation, that is, the extent to which assimilation is sufficient or accommodation is necessary in learning a second language.
Computational modeling provides some insight to the underlying causes of the balance of assimilation and accommodation when learning a second language. Computational modeling has shown that, although the same statistical learning rules can be applied in different writing systems, different numbers of hidden units are required in different languages. Yang, McCandliss, Shu, and Zevin (2009) have presented a general connectionist model of Chinese print-to-sound translation that implements the same functional architecture and learning rules as models that have been previously applied to English (Yang et al., 2009; Zevin & Seidenberg, 2006; Harm & Seidenberg, 1999). Compared with models trained in English, models required more neural resources (i.e., hidden units) to effectively learn the greater number of arbitrary relations between orthography and phonology in Chinese. We speculate that, when a system learns to read a language with an arbitrary mapping between orthography and phonology, more hidden units are involved, and this system can effectively handle the learning of a new orthography that involves a semiregular mapping between orthography and phonology, such as in the case of Chinese–English bilinguals. This may explain the extensive assimilation in our Chinese–English bilinguals when reading English. However, if a system has learned to read a language with semiregular mapping between orthography and phonology, it may require more resources or additional brain regions to deal with the arbitrary mapping between orthography and phonology in a less-transparent language, such as in the case of English–Chinese bilinguals. Taken together, the second language learning is asymmetrical across writing systems, and the pattern of assimilation and accommodation may depend on the nature of the mapping between orthography and phonology in both the first language and the second language.
The relations between the first language and the second language may not be the only factor influencing assimilation/accommodation. One study suggests that age of acquisition plays an important role in the balance of assimilation/accommodation (Das, Padakannaya, Pugh, & Singh, 2011), because they found that only early simultaneous exposure to reading distinct orthographies (i.e., Hindi and English) results in orthography-specific activation (i.e., left ITG for English and left IPL for Hindi). However, this result is not consistent with the Chinese training studies in English speakers reviewed above, because the participants in these studies started to learn Chinese in college and they still showed orthographic-specific activation (i.e., accommodation). Another factor that might influence the pattern of assimilation and accommodation is the usage of the second language. In our study, although some participants' proficiency in English is very high, they do not use English on a daily basis. Accommodation may be expected in readers who frequently use English such as Chinese immigrants in English-speaking countries.
In conclusion, we found that Chinese–English speakers relied on the Chinese network to read English, and higher proficiency in English was associated with increased involvement of the Chinese network and reduced involvement of the English network, suggesting that English is assimilated into the Chinese network in Chinese–English bilinguals. However, some accommodation appeared to occur at the visual word-form level as the Chinese–English speakers showed less involvement of the right occipital cortex proposed to be involved in the holistic visual–spatial processing of Chinese characters.
Acknowledgments
This work was supported by the BNU Open Project from the State Key Laboratory of Cognitive Neuroscience and Learning to Fan Cao and by the China National Natural Science Foundation (31000500) to Li Liu.
REFERENCES
- Abutalebi J. Neural aspects of second language representation and language control. Acta Psychologica Sinica. 2008;128:466–478. doi: 10.1016/j.actpsy.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Alivisatos B, Petrides M. Functional activation of the human brain during mental rotation. Neuropsychologia. 1997;35:111–118. doi: 10.1016/s0028-3932(96)00083-8. [DOI] [PubMed] [Google Scholar]
- Andersen R, Buneo CA. Intentional maps in posterior parietal cortex. Annual Review of Neuroscience. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: Universal structures plus writing system variation. Human Brain Mapping. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. The relation between brain activation and lexical performance. Human Brain Mapping. 2003;19:155–169. doi: 10.1002/hbm.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. Journal of Cognitive Neuroscience. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Lee R, Shu H, Yang Y, Xu G, Li K, et al. Cultural constraints on brain development: Evidence from a developmental study of visual word processing in Mandarin Chinese. Cerebral Cortex. 2010;20:1223–1233. doi: 10.1093/cercor/bhp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Peng D, Liu L, Jin Z, Fan N, Deng Y, et al. Developmental differences of neurocognitive networks for phonological and semantic processing in Chinese word reading. Human Brain Mapping. 2009;30:797–809. doi: 10.1002/hbm.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Vu M, Lung Chan DH, Lawrence JM, Harris LN, Guan Q, et al. Writing affects the brain network of reading in Chinese: A functional magnetic resonance imaging study. Human Brain Mapping. 2012 doi: 10.1002/hbm.22017. doi:10.1002/hbm.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Rick T, von Kapri A, Kuhlen T, Huang R, et al. Probabilistic fibre tract analysis of cytoarchitectonically defined human inferior parietal lobule areas reveals similarities to macaques. Neuroimage. 2011;58:362–380. doi: 10.1016/j.neuroimage.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Tan EW, Thiel T. Mandarin and English single word processing studied with functional magnetic resonance imaging. Journal of Neuroscience. 1999;19:3050–3056. doi: 10.1523/JNEUROSCI.19-08-03050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Shu H, Liu Y, Zhao J, Li P. ERP signatures of subject–verb agreement in L2 learning. Bilingualism: Language and Cognition. 2007;10:161–174. [Google Scholar]
- Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thompson WL, Anderson AK, et al. Changes in cortical activity during mental rotation: A mapping study using functional MRI. Brain. 1996;119:89–100. doi: 10.1093/brain/119.1.89. [DOI] [PubMed] [Google Scholar]
- Colby CL, Olson CR. Spatial cognition. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental neuroscience. Academic Press; San Diego, CA: 1999. pp. 1363–1407. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Critchley M. The parietal lobes. Hafner; New York: 1953. [Google Scholar]
- Das T, Padakannaya P, Pugh KR, Singh NC. Neuroimaging reveals dual routes to reading in simultaneous proficient readers of two orthographies. Neuroimage. 2011;54:1476–1487. doi: 10.1016/j.neuroimage.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Booth JR, Chou T-L, Ding G-S, Peng D-L. Item-specific and generalization effects on brain activation when learning Chinese characters. Neuropsychologia. 2008;46:1864–1876. doi: 10.1016/j.neuropsychologia.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Chou TL, Ding GS, Peng DL, Booth JR. The involvement of occipital and inferior frontal cortex in the phonological learning of Chinese characters. Journal of Cognitive Neuroscience. 2011;23:1998–2012. doi: 10.1162/jocn.2010.21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Perry C, Peng D, Ma L, Li D, Xu S, et al. Neural mechanisms underlying semantic and orthographic processing in Chinese–English bilinguals. NeuroReport. 2003;14:1557–1562. doi: 10.1097/00001756-200308260-00003. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Goltz HC, DeSouza JF, Vilis T, Crawford JD. Human parietal “reach region” primarily encodes intrinsic visual direction, not extrinsic movement direction, in a visual motor dissociation task. Cerebral Cortex. 2007;17:2283–2292. doi: 10.1093/cercor/bhl137. [DOI] [PubMed] [Google Scholar]
- Gandour J, Tong Y, Talavage T, Wong D, Dzemidzic M, Xu Y, et al. Neural basis of first and second language processing of sentence-level linguistic prosody. Human Brain Mapping. 2007;28:94–108. doi: 10.1002/hbm.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. The clinical syndromes of the cortical connections. Modern Trends in Neurology. 1970;5:29–40. [PubMed] [Google Scholar]
- Golestani N, Alario FX, Meriaux S, Le Bihan D, Dehaene S, Pallier C. Syntax production in bilinguals. Neuropsychologia. 2006;44:1029–1040. doi: 10.1016/j.neuropsychologia.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Green DW, Abutalebi J. Understanding the link between bilingual aphasia and language control. Journal of Neurolinguistics. 2008;21:558–576. [Google Scholar]
- Harm MW, Seidenberg MS. Phonology, reading, and dyslexia: Insights from connectionist models. Psychological Review. 1999;106:491–528. doi: 10.1037/0033-295x.106.3.491. [DOI] [PubMed] [Google Scholar]
- Jamal NI, Piche AW, Napoliello EM, Perfetti CA, Eden GF. Neural basis of single-word reading in Spanish–English bilinguals. Human Brain Mapping. 2012;33:235–245. doi: 10.1002/hbm.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam MM. Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparison within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Leonard MK, Brown TT, Travis KE, Gharapetian L, Hagler DJ, Jr., Dale AM, et al. Spatiotemporal dynamics of bilingual word processing. Neuroimage. 2010;49:3286–3294. doi: 10.1016/j.neuroimage.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dunlap S, Fiez J, Perfetti C. Evidence for neural accommodation to a writing system following learning. Human Brain Mapping. 2007;28:1223–1234. doi: 10.1002/hbm.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Perfetti CA. The time course of brain activity in reading English and Chinese: An ERP study of Chinese bilinguals. Human Brain Mapping. 2003;18:167–175. doi: 10.1002/hbm.10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RS, et al. Neurolinguistics: Structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Modern Chinese word frequency corpus . Beijing Language and Culture University; 1990. [Google Scholar]
- Nelson JR, Liu Y, Fiez J, Perfetti CA. Assimilation and accommodation patterns in ventral occipitotemporal cortex in learning a second writing system. Human Brain Mapping. 2009;30:810–820. doi: 10.1002/hbm.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Abutalebi J, Paulesu E, Brambati S, Scifo P, Cappa SF, et al. The role of age of acquisition and language usage in early, high-proficient bilinguals: An fMRI study during verbal fluency. Human Brain Mapping. 2003;19:170–182. doi: 10.1002/hbm.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Paulesu E, Galles NS, Dupoux E, Dehaene S, Bettinardi V, et al. The bilingual brain: Proficiency and age of acquisition of the second language. Brain. 1998;121:1841–1852. doi: 10.1093/brain/121.10.1841. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Liu Y. Orthography to phonology and meaning: Comparisons across and within writing systems. Reading and Writing. 2005;18:193–210. [Google Scholar]
- Perfetti CA, Liu Y, Fiez J, Nelson J, Bolger DJ, Tan L-H. Reading in two writing systems: Accommodation and assimilation in the brain's reading network. Bilingualism: Language and Cognition. 2007;10:131–146. [Google Scholar]
- Piaget J. Piaget's theory. Handbook of child psychology. John Wiley & Sons; New York: 1983. [Google Scholar]
- Pillai JJ, Araque JM, Allison JD, Sethuraman S, Loring DW, Thiruvaiyaru D, et al. Functional MRI study of semantic and phonological language processing in bilingual subjects: Preliminary findings. Neuroimage. 2003;19:565–576. doi: 10.1016/s1053-8119(03)00151-4. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Watson JDG, Patterson K, Howard D, Frackowiak RS. Brain activity during reading: The effects of exposure duration and task. Brain. 1994;117:1255–1269. doi: 10.1093/brain/117.6.1255. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Reiterer S, Berger ML, Hemmelmann C, Rappelsberger P. Decreased EEG coherence between prefrontal electrodes: A correlate of high language proficiency? Experimental Brain Research. 2005;163:109–113. doi: 10.1007/s00221-005-2215-z. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, Fulbright RK, et al. Age-related changes in reading systems of dyslexic children. Annals of Neurology. 2007;61:363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- Stein M, Dierks T, Brandeis D, Wirth M, Strik W, Koenig T. Plasticity in the adult language system: A longitudinal electrophysiological study on second language learning. Neuroimage. 2006;33:774–783. doi: 10.1016/j.neuroimage.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Stein M, Federspiel A, Koenig T, Wirth M, Lehmann C, Wiest R, et al. Reduced frontal activation with increasing 2nd language proficiency. Neuropsychologia. 2009;47:2712–2720. doi: 10.1016/j.neuropsychologia.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Karl L, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta-analysis. Human Brain Mapping. 2005;25:83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH. The neural system underlying Chinese logograph reading. Neuroimage. 2001;13:836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, et al. Neural systems of second language reading are shaped by native language. Human Brain Mapping. 2003;18:158–166. doi: 10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuno Y, Sakai KL. Language-related activations in the left prefrontal regions are differentially modulated by age, proficiency, and task demands. Journal of Neuroscience. 2005;25:1637–1644. doi: 10.1523/JNEUROSCI.3978-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock–Johnson III Tests of Achievement. The Riverside Publishing Company; Itasca, IL: 2001. [Google Scholar]
- Wu J, Li X, Yang J, Cai C, Sun H, Guo Q. Prominent activation of the bilateral inferior parietal lobule of literate compared with illiterate subjects during Chinese logographic processing. Experimental Brain Research. 2012;219:327–337. doi: 10.1007/s00221-012-3094-8. [DOI] [PubMed] [Google Scholar]
- Xue G, Dong Q, Jin Z, Zhang L, Wang Y. An fMRI study with semantic access in low proficiency second language learners. NeuroReport. 2004;15:791–796. doi: 10.1097/00001756-200404090-00010. [DOI] [PubMed] [Google Scholar]
- Yang J, McCandliss BD, Shu H, Zevin JD. Simulating language-specific and language-general effects in a statistical learning model of Chinese reading. Journal of Memory and Language. 2009;61:238–257. doi: 10.1016/j.jml.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Tan L, Li P. Lexical representation of nouns and verbs in the late bilingual brain. Journal of Neurolinguistics. 2011;24:674–682. [Google Scholar]
- Zevin JD, Seidenberg MS. Simulating consistency effects and individual differences in nonword naming: A comparison of current models. Journal of Memory and Language. 2006;54:145–160. [Google Scholar]
- Zhai H-C, Ren J, Xiao S-Y, Deng B-P, Xu X-X. Brain activations of the processing of the “reading-only without writing” character. Acta Psychologica Sinica. 2011;43:132–142. [Google Scholar]
- Zhao J, Li QL, Wang JJ, Yang Y, Deng Y, Bi HY. Neural basis of phonological processing in second language reading: An fMRI study of Chinese regularity effect. Neuroimage. 2012;60:419–425. doi: 10.1016/j.neuroimage.2011.12.074. [DOI] [PubMed] [Google Scholar]
- Zou L, Abutalebi J, Zinszer B, Yan X, Shu H, Peng D, et al. Second language experience modulates functional brain network for the native language production in bimodal bilinguals. Neuroimage. 2012;62:1367–1375. doi: 10.1016/j.neuroimage.2012.05.062. [DOI] [PubMed] [Google Scholar]