Abstract
Purpose
Saw palmetto extracts are used for treating lower urinary tract symptoms in men despite level I evidence concluding that saw palmetto was ineffective in reducing lower urinary symptoms. We sought to determine whether higher doses of saw palmetto as studied in CAMUS affect serum PSA levels.
Materials and Methods
The CAMUS trial was a randomized, placebo-controlled double blind multi-centered North American trial conducted between June 5, 2008 and October 10, 2012 in which 369 men >45 years of age with AUA symptom score ≥ 8 and ≤ 24 were randomly assigned to placebo or dose escalation saw palmetto, which consisted of 320mg for first 24 weeks to 640mg for next 24 weeks to 960mg for last 24 weeks of this 72 week trial. Serum PSA levels (Beckman-Coulter) were obtained at baseline and at weeks 24, 48 and 72 and were compared between treatment groups using the pooled t and Fisher's exact tests.
Results
Serum PSA levels were similar at baseline for the placebo (1.93 ± 1.59 ng/ml) and saw palmetto groups (2.20 ± 1.95, p = 0.16). Changes in PSA levels over the course of the study were similar: placebo group mean change 0.16 ± 1.08 ng/ml and saw palmetto group mean change 0.23 ± 0.83 ng/ml (p value 0.50). Additionally, no differential effect on serum PSA levels was observed between treatment arms when groups were stratified by baseline PSA values.
Conclusions
Saw palmetto extract does not affect serum PSA levels more than placebo even at relatively high doses.
Keywords: saw palmetto extract, Serenoa repens, prostate specific antigen, benign prostatic hyperplasia
Introduction
Serum prostate specific antigen (PSA) is an important tool to evaluate patients with lower urinary tract symptoms (LUTS).1 Serum PSA has also been correlated with important benign prostatic hyperplasia (BPH) related outcomes such as prostate size and as a predictive marker for prostate cancer progression.2 As such, it is important for clinicians to consider the impact of potential BPH therapies on serum PSA levels.
Herbal medicines have been used in the treatment of men with enlarged prostates and bothersome LUTS for many years.3 One of the most common herbal treatments is extract of the fruit of the saw palmetto dwarf palm tree. While the active therapeutic ingredients of saw palmetto extract have not been identified, a variety of mechanisms including inhibition of types 1 and 2 5α-reductase and competitive binding to androgen receptors in prostatic cells have been proposed for its efficacy.4-7 Despite two recent randomized trials that show there was no significant effect of saw palmetto extract even at high doses of 320 mg (and up to 960 mg),8,9 saw palmetto remains widely utilized and available over the counter. Accordingly, it is important to understand the effects of saw palmetto on serum PSA. Therefore the purpose of this study was to evaluate the effect of increasing doses of saw palmetto extract on serum PSA levels among men enrolled in the Complementary and Alternative Medicine for Urologic Symptoms (CAMUS) trial. Primary results from the CAMUS trial have been published.9
Materials and Methods
The CAMUS trial was a randomized, double blind, two-arm trial that was conducted on a total of 369 men who were ≥ 45 years of age with a urinary flow rate above 4 mL/s, voided volume > 125 mL, American Urological Association (AUA) Symptom Score ≥ 8 and ≤ 24 at two screening visits. Study subjects were randomized to one of two treatment arms, either saw palmetto fruit extract or placebo for 72 weeks (Figure 1). The treatment arm receiving the saw palmetto fruit extract received one 320 mg gel cap daily for the first 24 weeks followed by two 320 mg gel caps for the second 24 weeks and three 320 mg gel caps daily for the final 24 weeks. The men receiving the placebo received the placebo matched in color and appearance and number of gel caps for all of the weeks of the trial. Adherence was estimated by pill counts at each visit (97.1%) and by tracking attendance at protocol-specified visits (97.0%). The subjects were recruited from 11 clinical centers in the United States and Canada. The study was approved by each site's and the data coordinating center's institutional review board. Men were ineligible for trial participation if they had received prior invasive treatment for BPH; had recent treatment with an α blocker (within 1 month), 5α-reductase inhibitor (within 3 months), or phytotherapy including saw palmetto extract (within 3 months); or recent treatment with other medications affecting LUTS. Additional exclusion criteria were as detailed previously.10
Figure 1.
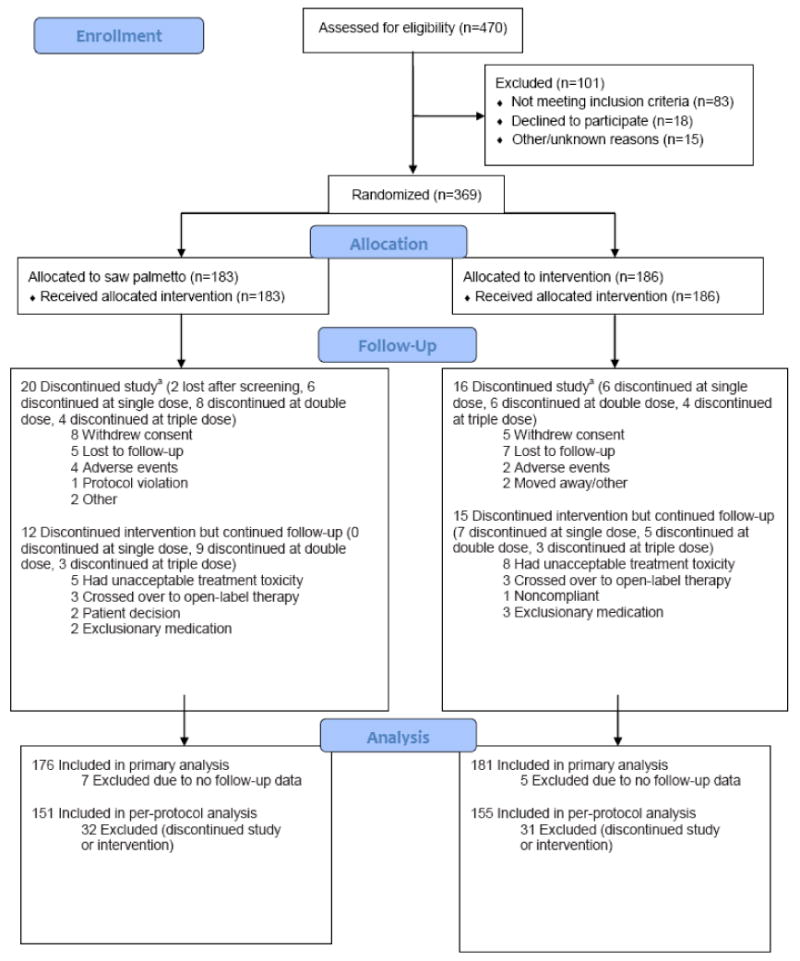
CONSORT diagram for the CAMUS trial.
aSeven participants in the saw palmetto extract group and 5 participants in the placebo group who discontinued the protocol provided no follow-up data.
Enrollment began in 2008 and was completed in 2010. The primary objective of the CAMUS Trial was to determine if saw palmetto extract reduces the AUA Symptom Score compared to placebo over 72 weeks. The following measurements were accessed to determine the impact of saw palmetto over time: BPH Impact Index, quality of life item score from IPSS, nocturia item score from the IPSS, peak urinary flow rate, post-void residual urine volume, erectile and ejaculatory function, International Continence Society (ICS) male Incontinence Scale, Jenkins Sleep Dysfunction Scale and National Institutes of Health Chronic Prostatitis Symptom Index (NIH CPSI). In addition, sera for PSA measurement were obtained at baseline and at weeks 24, 48 and 72. Serum PSA was measured in a central laboratory using the BeckmanC Access Immunoassay System (Beckman Coulter, Inc. Brea, CA).
Statistical Analysis
The primary analysis was based on the modified intention-to-treat population that included all eligible participants who took at least 1 dose of study drug and had at least one follow-up assessment. Treatment groups were compared with respect to demographics and baseline measures using Pearson chi-squared test for categorical variables and the pooled t test for continuous variables. Treatment groups, categorized by baseline PSA levels, were also compared with rises in PSA over the study period using Fisher's exact test. Lastly, CAMUS participants were categorized by percentage change in PSA levels from baseline to week 72, baseline to week 24, week 24 to week 48, and week 48 to week 72 by treatment arm and the treatment groups were compared using Fisher's exact test. Statistical significance in this study was set as p < 0.05, with all reported p values being two-sided. Analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
A total of 470 men attended a first screening visit after 1032 men had been pre-screened. Of these, 369 men were randomized (Figure 1) with PSA data being available for 368 men throughout the study period. Study participants had a mean age of 61 years (SD 8.4) and a mean serum PSA of 2.07 ng/ml (SD 1.78) (Table 1). No significant differences in demographic and socioeconomic factors were observed between the saw palmetto and placebo groups.
Table 1.
Baseline characteristics of participants in the modified intention-to-treat analysis.a
| Participants | ||||
|---|---|---|---|---|
| Characteristics | Total (N=357) |
Saw Palmetto Extract (n=176) |
Placebo (n=181) |
p value |
| Age, y | 60.97 (8.40) | 61.25 (8.72) | 60.7 (8.08) | 0.54 |
| Race/ethnicity, No. (%) | 0.42 | |||
| White | 284 (79.6) | 145 (82.4) | 139 (76.8) | |
| Black | 41 (11.5) | 17 (9.7) | 24 (13.3) | |
| Hispanic, Latino or otherb | 32 (9.0) | 14 (8.0) | 18 (9.9) | |
| Education, No. (%) | 0.64c | |||
| <High school | 13 (3.6) | 6 (3.4) | 7 (3.9) | |
| High school graduate | 38 (10.6) | 20 (11.4) | 18 (9.9) | |
| Some college | 60 (16.8) | 26 (14.8) | 34 (18.8) | |
| College graduate | 99 (27.7) | 48 (27.3) | 51 (28.2) | |
| Postcollege | 142 (39.8) | 75 (42.6) | 67 (37.0) | |
| No repsonse | 5 (1.4) | 1 (0.6) | 4 (2.2) | |
| PSA level, ng/ml | 2.07 (1.78) | 2.20 (1.95) | 1.93 (1.59) | 0.16 |
Abbreviations: No, number; PSA, prostate-specific antigen; y, year
Data are presented as mean (SD) unless otherwise specified.
Other included American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, or unknown or not reported.
P value based on Wilcoxon rank sum test.
Serum PSA levels were similar between the placebo (1.93 ± 1.59 ng/ml) and saw palmetto groups (2.20 ± 1.95 ng/ml, p = 0.16) at baseline. Further, the PSA mean change from baseline was similar between groups throughout the study (Table 2). The PSA changed by a mean of 0.23 ± 0.83 ng/ml in the saw palmetto group whereas the mean change in the placebo group was 0.16 ± 1.08 ng/ml at 72 weeks (p = 0.50). Saw palmetto was not more effective than placebo in decreasing mean PSA scores from baseline through 24 and 48 weeks as well (Table 2).
Table 2.
Mean PSA change from baseline through weeks 24, 48 and 72 for CAMUS participants by treatment arm.
| Time period | Saw Palmetto (SD) | Placebo (SD) | p-value* |
|---|---|---|---|
| Baseline to 24 weeks | 0.06 (0.59) | 0.14 (0.99) | 0.40 |
| Baseline to 48 weeks | 0.06 (1.10) | 0.02 (0.83) | 0.72 |
| Baseline to 72 weeks | 0.23 (0.83) | 0.16 (1.08) | 0.50 |
Abbreviations: SD = standard deviation
2-sided pooled t-test
After stratifying baseline PSA values into 3 groups (0≤PSA<2.5, 2.5≤PSA<4, 4≤PSA≤10), the saw palmetto and placebo groups had similar results for percentage of patients with any PSA rise, median PSA rise and percentage of patients with any PSA rise exceeding relevant National Comprehensive Cancer Network (NCCN)11 guidelines over the entire study period (Table 3).
Table 3.
The number and percent of CAMUS participants categorized by baseline PSA levels and by treatment arm with rises in PSA over the study period.
| Saw Palmetto | Placebo | ||
|---|---|---|---|
| Baseline PSA (ng/ml) | % (n/N) with any PSA rise for entire study period | % (n/N) with any PSA rise for entire study period | p value |
| 0≤PSA<2.5 | 83.3 (110/132) | 91.2 (125/137) | 0.07* |
| 2.5≤PSA<4 | 92.6 (25/27) | 96.0 (24/25) | 1.00* |
| 4≤PSA≤10 | 95.8 (23/24) | 82.6 (19/23) | 0.19* |
| Baseline PSA (ng/ml) | Median of PSA rise for entire study period | Median of PSA rise for entire study period | |
| 0≤PSA<2.5 | 0.135 | 0.08 | |
| 2.5≤PSA<4 | -0.08 | -0.09 | |
| 4≤PSA≤10 | 0.66 | 0.09 | |
| Baseline PSA (ng/ml) | %(n/N) with annualized PSA rise for any PSA testing interval that exceeded the relevant NCCN cutpoints† | %(n/N) with annualized PSA rise for any PSA testing interval that exceeded the relevant NCCN cutpoints† | p value |
| 0≤PSA<2.5 | 56.8 (75/132) | 55.5 (76/137) | 0.90* |
| 2.5≤PSA<4 | 81.5 (22/27) | 88.0 (22/25) | 0.71* |
| 4≤PSA≤10 | 95.8 (23/24) | 73.9 (17/23) | 0.05* |
| Baseline PSA (ng/ml) | % (n/N) with annualized PSA rise exceeding the relevant NCCN cutpoints† during the 48-72 week time period | % (n/N) with annualized PSA rise exceeding the relevant NCCN cutpoints† during the 48-72 week time period | p value |
| 0≤PSA<2.5 | 32.2 (37/132) | 26.0 (32/137) | 0.32* |
| 2.5≤PSA<4 | 53.9 (14/25) | 52.0 (13/25) | 1.00* |
| 4≤PSA≤10 | 81.0 (17/24) | 55.0 (11/23) | 0.10* |
P values are for comparison of saw palmetto and placebo groups and are based on Fisher's exact test (twosided).
For men with a PSA <4 ng/ml, a PSA velocity ≥0.35 ng/ml/year is suspicious for cancer. For men with a PSA of 4-10 ng/ml, a PSA velocity ≥0.75 ng/ml/year is suspicious for cancer.
The overall change in PSA between baseline and the last visit as a decrease or percent increase by treatment assignment was also examined (Table 4). There were no significant differences in percent change from baseline to week 72, baseline to week 24, and week 48 to week 72. From week 24 to week 48, there was a significantly higher percentage of decreases in PSA levels among subjects in the saw palmetto group compared to the placebo group (p=0.004, Fisher's exact test) and a significantly higher percentage of small increases (25% or less) in the placebo group compared to saw palmetto (p=0.002, Fisher's exact test).
Table 4.
The number and percent of CAMUS participants categorized by percentage change in PSA levels from baseline to week 72, baseline to week 24, week 24 to week 48, and week 48 to week 72 by treatment arm.
| Saw Palmetto | Placebo | ||||
|---|---|---|---|---|---|
| Visit Week | Change | Frequency | % | Frequency | % |
| Baseline - Week 72 | Any decrease | 53 | 37 | 62 | 43 |
| 0-25% increase | 48 | 33 | 50 | 34 | |
| 25-50% increase | 24 | 17 | 21 | 14 | |
| 50-75% increase | 15 | 10 | 9 | 6 | |
| 75-100% increase | 1 | 1 | 2 | 1 | |
| >100% increase | 4 | 3 | 2 | 1 | |
|
| |||||
| Baseline - Week 24 | Any decrease | 83 | 49 | 80 | 47 |
| 0-25% increase | 58 | 35 | 61 | 36 | |
| 25-50% increase | 17 | 10 | 17 | 10 | |
| 50-75% increase | 7 | 4 | 5 | 3 | |
| 75-100% increase | 1 | 1 | 2 | 1 | |
| >100% increase | 2 | 1 | 6 | 4 | |
|
| |||||
| Week 24 - Week 48 | Any decrease | 98 | 61* | 73 | 46* |
| 0-25% increase | 38 | 24* | 64 | 40* | |
| 25-50% increase | 15 | 9 | 11 | 7 | |
| 50-75% increase | 5 | 3 | 7 | 4 | |
| 75-100% increase | 2 | 1 | 2 | 1 | |
| >100% increase | 2 | 1 | 2 | 1 | |
|
| |||||
| Week 48 - Week 72 | Decrease | 53 | 37 | 64 | 45 |
| 0-25% increase | 55 | 39 | 51 | 36 | |
| 25-50% increase | 23 | 16 | 17 | 12 | |
| 50-75% increase | 4 | 3 | 7 | 5 | |
| 75-100% increase | 2 | 1 | 1 | 1 | |
| >100% increase | 5 | 4 | 2 | 1 | |
p < 0.05 Fisher's exact test
Discussion
Our trial evaluated the effects of saw palmetto on LUTS and other secondary BPH-related outcomes including the effect of this extract on serum PSA. We found that saw palmetto extract did not have a greater effect than placebo on serum PSA over the course of the study. Even after increasing the saw palmetto extract to three times the standard dose over the course of the study, no significant effects on PSA were observed Although a small but statistically significant effect of decreasing PSA was noted when comparing saw palmetto at a dose of 640 mg to placebo from week 24 to week 48, this likely is not clinically meaningful as the effect was small and no effect was seen at other saw palmetto doses.
It has been previously shown that treatment with standard doses of saw palmetto (320 mg/day) for 3 months is sufficient to result in a significant reduction in prostate dihydrotestosterone (DHT) levels and significant increases in prostate testosterone levels compared with an untreated group, supporting the 5α-reductase inhibitory effect of this medication.4 Similar results have also been shown in men treated with saw palmetto (320 mg/day) for 6 months (prostate tissue DHT levels were reduced by 32% from 6.49 to 4.40 ng/g [p<0.005] in the saw palmetto group compared with no significant change in a placebo group),12 whereas finasteride and dutasteride reduce prostatic DHT by approximately 90%.13 In light of saw palmetto's potentially anti-androgenic,7 a number of studies have examined the effects of this herbal supplement on serum PSA in addition to other BPH-related outcomes.8,14-18 The majority of these studies evaluated the effects of a standard 320 mg dose of saw palmetto over a 6-12 month period (with one study evaluating the effects of saw palmetto over 24 months) and found that saw palmetto did not affect serum PSA values. While we observed similar results, our study has several strengths that are important to highlight in interpreting these findings. In addition to this being a randomized, double-blinded, two-arm trial, the dose of saw palmetto was escalated to three times the standard over the course of the study with each dose being evaluated for its effect on PSA over an adequate duration of therapy (24 weeks).
The results of this study are clinically relevant to men's health. While there has been convincing data establishing the lack of therapeutic benefit of saw palmetto in alleviating LUTS,8,9,19 many men continue to take this medication.20 By definitively establishing the lack of an effect on PSA by saw palmetto, even at supra-standard doses, our study confirms that clinicians need not adjust serum PSA levels in men taking this herbal supplement, who opt to undergo such testing for prostate cancer early detection.
A potential limitation of this study is that study participants used only one formulation of saw palmetto, whereas many potential forms of the supplement exist. Although it is possible that other formulations of saw palmetto may have an effect on serum PSA levels, this has not been supported by other studies that have evaluated this outcome.14-18 It should also be noted that this trial's sample size was not chosen with consideration of the sample size needed to achieve adequate power for the secondary analysis considered here. Instead the sample size was based on CAMUS' primary objective of comparing the effect of saw palmetto with placebo on lower urinary tract symptoms. Those sample size considerations are given in the publication reporting on that study.9 Some of our results that were trending towards significant could be significant if a larger study saw a similar effect. For example, the comparison of the two treatment groups among those with initial PSA < 2.5 ng/ml with respect to the proportion with any PSA rise (table 3) would have been statistically significant if the same effect was seen (83% vs 91%) in a sample 20% larger (about 160/group). Lastly, while it has been suggested that polymorphisms in the 5α-reductase and androgen receptor genes may influence serum PSA levels,21-24 we did not account for a potential differential effect of saw palmetto on such polymorphisms and PSA levels in our study population. However, to the best of our knowledge, there is no published evidence that suggests that saw palmetto would act differently on polymorphisms of 5α-reductase or the androgen receptor.
Conclusions
In this randomized, double-blinded, two-arm trial, saw palmetto was evaluated at 3 different doses (320 mg, 640 mg and 960 mg) for 24 weeks at a time and was found to have had no effect on serum PSA levels compared with placebo.
Acknowledgments
Funding/Support: This study was funded by cooperative agreements from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: U01 DK63795, U01 DK63797, U01 DK63825, U01 DK63835, U01 DK63866, U01 DK63833, U01 DK63862, U01 DK63840, U01 DK63883, U01 DK63831, U01 DK63778 and U01 DK63788. Support was also provided by the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements, NIH. Saw palmetto fruit extract and matching placebo was donated by Rottapharm/Madaus, Cologne, Germany. This study was conducted under an Investigational New Drug Application from the Food and Drug Administration. Rottapharm/Madaus had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation or approval of the manuscript. Rottapaharm/Madaus provided nonbinding comments to the authors on a draft of the manuscript. NIH scientists representing the funding agencies did participate in the design and conduct of the study as well as review and approval of the manuscript.
Abbreviations
- AUA
American Urological Association
- BPH
Benign prostatic hyperplasia
- CAMUS
Complementary and Alternative Medicine for Urologic Symptoms
- DHT
Dihydrotestosterone
- ICS
International Continence Society
- LUTS
Lower urinary tract symptoms
- NCCN
National Comprehensive Cancer Network
- NIH CPSI
National Institutes of Health Chronic Prostatitis Symptom Index
- PSA
Prostate specific antigen
- SD
Standard deviation
CAMUS Study Group
Steering Committee Chair
-
Massachusetts General Hospital
Michael J. Barry, MD
Data Coordinating Center
-
University of Alabama at Birmingham
O. Dale Williams, PhD (Director)
Sreeletha Meleth, PhD (Associate Director)
Alan Cantor, PhD
Clinical Sites
-
New York University
Andrew McCullough, MD (PI through 12/3/10)
Christopher Kelly, MD (PI as of 12/3/10)
Brianne Goodwin, BSN, RN (Study Coordinator)
Laurie Mantor (Study Coordinator)
Artrit Butuci (Research Data Associate)
-
Northern California Kaiser Permanente
Andrew L. Avins, MD, MPH
Harley Goldberg, DO (Co-I)
Luisa Hamilton (Study Coordinator)
Cynthia Huynh (Research Associate)
-
Northwestern University Feinberg School of Medicine
Kevin T. McVary, MD (PI)
Robert Brannigan, MD (Co-I)
Brian Helfand, MD, PhD (Consultant)
Maria Velez (Study Coordinator)
Nancy Schoenecker, RN, CCRC (Clinical Research Coordinator)
-
Queens University
J. Curtis Nickel, MD (PI)
Alvaro Morales (Co-I)
D. Robert Siemens, MD (Co-I)
Joe Downey, MSc, CCRP (Study Coordinator)
Janet Clark-Pereira, CCRP (Study Coordinator)
-
University of Colorado Denver
E. David Crawford, MD (PI)
Shandra S. Wilson, MD (Co-I)
Paul D. Maroni, MD (Co-I)
Patricia DeVore, BS (Clinical Research Coordinator)
Cliff Jones (Clinical Research Coordinator)
-
University of Iowa
Karl J. Kreder, MD, MBA (PI)
Victoria Sharp, MD, MBA (Co-I)
Diane Meyerholz, RN, BSN (Study Coordinator)
Mary Eno, RN (Study Coordinator)
-
University of Maryland
Michael J. Naslund, MD (PI)
Ganine Markowitz-Chrystal, MS, CCRC (Study Coordinator)
-
University of Texas, Southwestern Medical Center
Claus G. Roehrborn, MD (PI)
Brad Hornberger, PA-C (Co-I)
Allison Beaver, RN (Study Coordinator)
Suzie Carter (Data Manager)
-
Washington University School of Medicine
Gerald L. Andriole, MD (PI)
Vivien Gardner, RN, BSN (Study Coordinator)
Karen Whitmore (Supervisor Patient Services)
-
Weill Cornell Medical College
Steven A. Kaplan, MD (PI)
Alexis E. Te, MD (Co-I)
Noreen Buckley, NP, CCRC (Study Coordinator)
Maritza Rodriquez (Medical Assistant)
-
Yale University School of Medicine
Harris E. Foster, Jr., MD (PI)
John W. Colberg, MD (Co-I)
Karen Stavris, RN MSN, CCRC (Study Coordinator)
Biostatistics Consultant
-
University of Arkansas for Medical Sciences
Jeannette Y. Lee, PhD
National Institutes of Health
-
National Institute of Diabetes, Digestive & Kidney Diseases
John W. Kusek, PhD
Leroy M. Nyberg, PhD (through 9/2/09)
-
National Center for Complementary and Alternative Medicine
Catherine M. Meyers, MD
-
Office of Dietary Supplements
Joseph M. Betz, PhD
Data Safety Monitoring Board
-
University of Minnesota VA Medical Center
Timothy J. Wilt, MD, MPH (Chair)
-
University of Illinois at Chicago
Harry H.S. Fong, Ph.D.
-
University of Chicago
Glenn S. Gerber, MD
-
University of Virginia
Mikel Gray, RN, PhD, CUNP, FAAN
-
HeteroGeneity LLC
Freddie Ann Hoffman, MD
-
University of North Carolina
Gary Koch, PhD
-
University of California at Los Angeles
Mark Litwin, MD, MPH
-
US Environmental Protection Agency
Warren E. Lux, MD
-
Harvard Medical School
Michael P. O’Leary, MD, MPH
-
Intercultural Cancer Council
Col (Ret.) James E. Williams, Jr.
-
Hines VA Hospital Cooperative Studies Program Coordinating Center
Domenic Reda, PhD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185(5):1793–1803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 2.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 3.Dedhia RC, McVary KT. Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2008;179(6):2119–2125. doi: 10.1016/j.juro.2008.01.094. [DOI] [PubMed] [Google Scholar]
- 4.Di Silverio F, Monti S, Sciarra A, et al. Effects of long-term treatment with Serenoa repens (Permixon) on the concentrations and regional distribution of androgens and epidermal growth factor in benign prostatic hyperplasia. Prostate. 1998;37(2):77–83. doi: 10.1002/(sici)1097-0045(19981001)37:2<77::aid-pros3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Gerber GS. Saw palmetto for the treatment of men with lower urinary tract symptoms. J Urol. 2000;163(5):1408–1412. [PubMed] [Google Scholar]
- 6.Buck AC. Is there a scientific basis for the therapeutic effects of serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J Urol. 2004;172(5 Pt 1):1792–1799. doi: 10.1097/01.ju.0000140503.11467.8e. [DOI] [PubMed] [Google Scholar]
- 7.Pais P. Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5alpha-reductase II. Adv Ther. 2010;27(8):555–563. doi: 10.1007/s12325-010-0041-6. [DOI] [PubMed] [Google Scholar]
- 8.Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354(6):557–566. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 9.Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. Jama. 2011;306(12):1344–1351. doi: 10.1001/jama.2011.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J, Andriole G, Avins A, et al. Redesigning a large-scale clinical trial in response to negative external trial results: the CAMUS study of phytotherapy for benign prostatic hyperplasia. Clin Trials. 2009;6(6):628–636. doi: 10.1177/1740774509352199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oncology NCCNCPGi. Prostate Cancer Early Detection Version 1.2011. http://www.nccn.org.
- 12.Marks LS, Hess DL, Dorey FJ, Luz Macairan M, Cruz Santos PB, Tyler VE. Tissue effects of saw palmetto and finasteride: use of biopsy cores for in situ quantification of prostatic androgens. Urology. 2001;57(5):999–1005. doi: 10.1016/s0090-4295(00)01052-9. [DOI] [PubMed] [Google Scholar]
- 13.Rittmaster R, Hahn RG, Ray P, Shannon JB, Wurzel R. Effect of dutasteride on intraprostatic androgen levels in men with benign prostatic hyperplasia or prostate cancer. Urology. 2008;72(4):808–812. doi: 10.1016/j.urology.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Carraro JC, Raynaud JP, Koch G, et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. Prostate. 1996;29(4):231–240. doi: 10.1002/(SICI)1097-0045(199610)29:4<231::AID-PROS4>3.0.CO;2-E. discussion 241-232. [DOI] [PubMed] [Google Scholar]
- 15.Gerber GS, Zagaja GP, Bales GT, Chodak GW, Contreras BA. Saw palmetto (Serenoa repens) in men with lower urinary tract symptoms: effects on urodynamic parameters and voiding symptoms. Urology. 1998;51(6):1003–1007. doi: 10.1016/s0090-4295(98)00143-5. [DOI] [PubMed] [Google Scholar]
- 16.Marks LS, Partin AW, Epstein JI, et al. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. J Urol. 2000;163(5):1451–1456. [PubMed] [Google Scholar]
- 17.Gerber GS, Fitzpatrick JM. The role of a lipido-sterolic extract of Serenoa repens in the management of lower urinary tract symptoms associated with benign prostatic hyperplasia. BJU Int. 2004;94(3):338–344. doi: 10.1111/j.1464-410X.2004.04962.x. [DOI] [PubMed] [Google Scholar]
- 18.Djavan B, Fong YK, Chaudry A, et al. Progression delay in men with mild symptoms of bladder outlet obstruction: a comparative study of phytotherapy and watchful waiting. World J Urol. 2005;23(4):253–256. doi: 10.1007/s00345-005-0005-7. [DOI] [PubMed] [Google Scholar]
- 19.Tacklind J, MacDonald R, Rutks I, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;(2):CD001423. doi: 10.1002/14651858.CD001423.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1–23. [PubMed] [Google Scholar]
- 21.Schatzl G, Madersbacher S, Gsur A, et al. Association of polymorphisms within androgen receptor, 5alpha-reductase, and PSA genes with prostate volume, clinical parameters, and endocrine status in elderly men. Prostate. 2002;52(2):130–138. doi: 10.1002/pros.10101. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Meyers DA, Sterling DA, et al. Association studies of serum prostate-specific antigen levels and the genetic polymorphisms at the androgen receptor and prostate-specific antigen genes. Cancer Epidemiol Biomarkers Prev. 2002;11(7):664–669. [PubMed] [Google Scholar]
- 23.Jesser C, Mucci L, Farmer D, et al. Effects of G/A polymorphism, rs266882, in the androgen response element 1 of the PSA gene on prostate cancer risk, survival and circulating PSA levels. Br J Cancer. 2008;99(10):1743–1747. doi: 10.1038/sj.bjc.6604690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savblom C, Giwercman A, Malm J, et al. Association between polymorphisms in the prostate-specific antigen (PSA) promoter and release of PSA. Int J Androl. 2009;32(5):479–485. doi: 10.1111/j.1365-2605.2008.00882.x. [DOI] [PubMed] [Google Scholar]


