Abstract
Aims
To determine the optimal T1 mapping approach to assess myocardial fibrosis at 3T.
Methods and results
T1 mapping was performed at 3T using the modified look-locker-inversion sequence in 20 healthy volunteers and 20 patients with aortic stenosis (AS). Pre- and post-contrast myocardial T1, the partition coefficient (λ; ΔRmyocardium/ΔRblood, where ΔR = 1/post-contrast T1 − 1/pre-contrast T1), and extracellular volume fraction [ECV; λ (1 − haematocrit)] were assessed. After establishing the optimal time point and myocardial region for analysis, we compared the reproducibility of these T1 measures and their ability to differentiate asymptomatic patients with AS from healthy volunteers. There was no segmental variation across the ventricle in any of the T1 measures evaluated. λ and ECV did not vary with time, while post-contrast T1 was relatively constant between 15 and 30 min. Thus, mid-cavity myocardium at 20 min was used for subsequent analyses. ECV displayed excellent intra-, inter-observer, and scan–rescan reproducibility [intra-class correlation coefficients (ICC) 1.00, 0.97, and 0.96, respectively], as did λ (ICC 0.99, 0.94, 0.93, respectively). Moreover, ECV and λ were both higher in patients with AS compared with controls (ECV 28.3 ± 1.7 vs. 26.0 ± 1.6%, P < 0.001; λ 0.46 ± 0.03 vs. 0.44 ± 0.03, P = 0.02), with the former offering improved differentiation. In comparison, scan–rescan reproducibilities for pre- and post-contrast myocardial T1 were only modest (ICC 0.72 and 0.56) with no differences in values observed between cases and controls (both P> 0.05).
Conclusions
ECV appears to be the most promising measure of diffuse myocardial fibrosis at 3T based upon its superior reproducibility and ability to differentiate disease from health.
Keywords: diffuse myocardial fibrosis, t1 mapping, cardiac magnetic resonance imaging, aortic stenosis
Introduction
Myocardial fibrosis is a common pathological finding in a wide range of cardiovascular diseases and has been associated with an adverse prognosis.1–3 Using cardiovascular magnetic resonance (CMR), the late gadolinium enhancement (LGE) technique has become widely used to evaluate focal myocardial fibrosis. However in many conditions, including aortic stenosis, a more diffuse form of fibrosis predominates, which crucially is reversible and therefore a potential target for novel therapeutic strategies.4–7 LGE imaging has inherent limitations in assessing diffuse fibrosis because it relies upon detecting a difference in signal intensity between normal and fibrotic regions.8 Consequently, it has difficulty in discriminating areas of diffuse myocardial fibrosis, which tend to have an even distribution.
Recently, several T1 mapping approaches have been developed to quantify diffuse fibrosis. The first approach measures intrinsic myocardial T1 on the basis that T1 relaxation times are longer in regions of fibrosis (pre-contrast T1).9–11 Alternatively, myocardial T1 can be measured following gadolinium administration, which accumulates in fibrotic areas on account of the increased extracellular volume (post-contrast T1).12,13 However, post-contrast T1 is potentially confounded by individual variations in gadolinium kinetics and by the precise timing of imaging.8 As a result, investigators have proposed methods to correct for these factors using either blood-pool T1 values to derive the partition coefficient (λ),14 or plasma volume to calculate the contrast volume of distribution in the myocardium. The latter is commonly referred to as the myocardial extracellular volume fraction (ECV).15–20 Each of these approaches have been validated against the extent of myocardial fibrosis on histology.12,13,15,17,18,20 However, the optimal technique remains uncertain due to a lack of consistent acquisition sequences and disease states studied, whilst direct comparative studies are relatively lacking.20 In addition, there is insufficient reproducibility data (particularly scan–rescan) and few studies have been performed at 3T,21–24 which may offer potential improvements compared with 1.5T.25
Therefore, the purpose of this study was to perform a systematic and comprehensive assessment to determine the optimal T1 approach at 3T. In particular, we aimed to characterize the temporal and regional T1 profiles of the myocardium and to identify the optimal technique based upon its reproducibility and ability to differentiate asymptomatic patients with aortic stenosis from healthy volunteers. Patients with advanced symptoms and focal scarring were excluded so as to focus on patients in whom diffuse myocardial fibrosis is most likely to be of clinical interest.
Methods
Study participants
Twenty asymptomatic patients with mild-to-severe aortic stenosis were recruited from outpatient clinics at the Edinburgh Heart Centre (see Supplementary data online). Twenty healthy volunteers were recruited from the community and the University of Edinburgh. All individuals had normal renal function and a left ventricular ejection fraction within the normal range.
The exclusion criteria for patients with aortic stenosis were as follows: (i) other significant valvular heart disease (moderate to severe in nature), (ii) acquired or inherited cardiomyopathies, (iii) previous myocarditis and (iv) the presence of focal LGE. The exclusion criteria for healthy volunteers were as follows: (i) hypertension, (ii) diabetes mellitus, (iii) coronary artery disease (previous myocardial infarction, evidence of myocardial ischaemia, or >50% luminal stenosis in a major epicardial vessel) (iv) valvular heart disease, (v) cardiomyopathy or previous myocarditis, and (vi) the presence of focal LGE.
All clinical assessments and imaging studies were carried out at the Wellcome Trust Clinical Research Facility and the Clinical Research Imaging Centre, Edinburgh. Studies were performed with the approval of the local research ethics committee, and with the written informed consent from each participant.
Imaging protocols
Echocardiography
Transthoracic echocardiography was performed in all participants (iE33, Philips Medical Systems, The Netherlands) by two independent operators. The severity of aortic stenosis was classified using aortic valve jet velocity, mean pressure gradient, and the aortic valve area, according to the American Heart Association/American College of Cardiology guidelines.26 Diastolic function was assessed using pulse-wave Doppler [early (E) and late (A) mitral inflow velocities] and tissue Doppler imaging (average of medial and lateral annulus velocities of the mitral valve, e′).
CMR imaging
CMR was performed at 3T (MAGNETOM Verio, Siemens AG, Healthcare Sector, Erlangen, Germany) according to the study protocol (Supplementary data online). Short-axis cine images were obtained using a balanced steady-state free precession sequence (8-mm parallel slices with 2-mm spacing) for the assessment of left ventricular function and volumes.
T1 mapping was performed using the MOdified Look-Locker Inversion recovery (MOLLI; flip angle 35°; minimum TI 100 ms; TI increment of 80 ms; time delay of 150 ms with a heart beat acquisition scheme of 3-3-5) with built-in motion correction.27 A gradient echo field map and associated shim were performed to minimize off-frequency artefact. Short-axis T1 maps of the mid-cavity slice were acquired in diastole before and at 2, 5, 10, 15, 20, and 30 min following the administration of 0.1 mmol/kg of gadobutrol (Gadovist/Gadavist, Bayer Pharma AG, Germany). Additional basal and apical T1 maps were obtained in diastole at 0, 15, 20, and 30 min (see Supplementary data online). The basal slice was defined as the first complete ring of myocardium below the aortic outflow tract, and the mid-cavity slice as the most basal slice to include both papillary muscles. The apical slice was selected between the apex and the mid-cavity on the image least affected by trabeculations and partial volume averaging.
LGE imaging was performed between 8 and 15 min using two approaches: an inversion-recovery fast gradient-echo sequence and a phase-sensitive inversion recovery sequence, performed in two phase-encoding directions to differentiate true enhancement from artefact.28,29 The inversion time was optimized for each slice to achieve satisfactory nulling of the myocardium.
Imaging analysis
Assessment of the left ventricle and myocardial enhancement
The quantification of left ventricular function, volumes, and mass was performed using the Argus Ventricular Function software (Siemens AG Healthcare Sector). All volumes and mass were indexed to body surface area. Qualitative assessment of myocardial enhancement was assessed independently by two experienced operators (M.D. and S.M.).
Assessing the quality of T1 maps
The quality of T1 map data was examined using the individual inversion recovery images. All segments affected by off-resonance, excessive breathing motion artefacts not corrected by the inline motion correction, and mistriggering were excluded from the analysis.
Assessing myocardial T1 at multiple time points
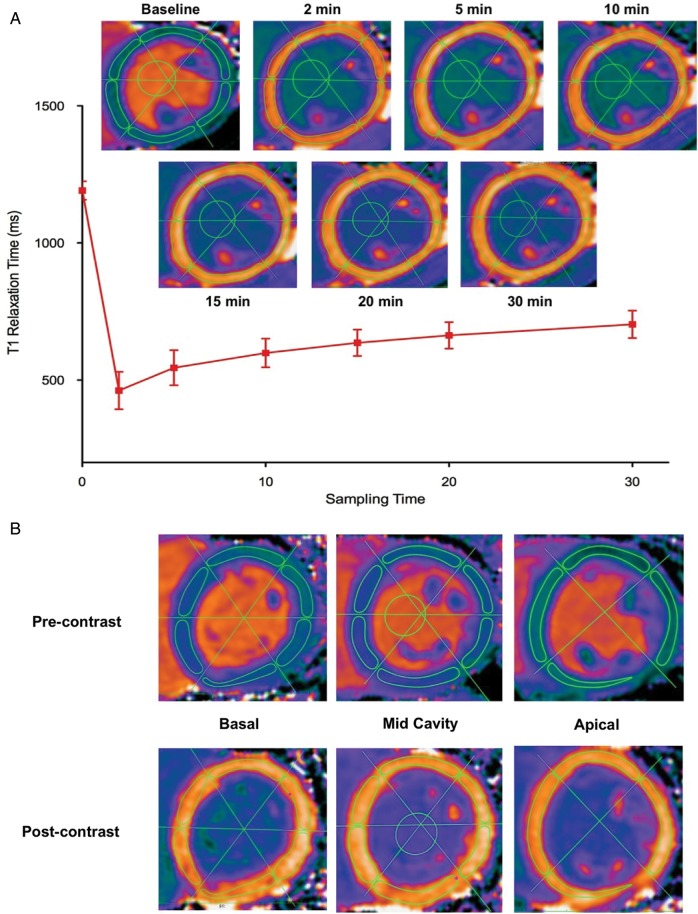
Myocardial T1 values were assessed at multiple time points to establish the optimal time point for post-contrast T1 mapping. This was defined at the flattest point on the T1 relaxation curve at which variation in T1 values with time was at a minimum. Mid-cavity motion-corrected T1 maps were analysed using a dedicated workstation (OsiriX version 4.1.1, Geneva, Switzerland). To minimize partial volume effects from surrounding tissues and blood pool, we standardized the windowing and placement of regions of interest (ROI) around the mid-cavity myocardium using a pre-defined protocol (Figure 1). The ROI were first drawn on the pre-contrast T1 maps and then copied onto each of the corresponding post-contrast T1 maps with stringent adjustments applied to avoid blood pool and artefact (Figure 1A). This approach ensured consistency in the placement of ROI across the different time points, allowing us to investigate the temporal variation in our T1 measures.
Figure 1.
Methodology for measuring myocardial T1 at multiple time points and in multiple segments of the left ventricle (A) Measurement of myocardial T1 at multiple time points. ROI were drawn within the borders on the pre-contrast myocardial T1 maps and then copied onto the corresponding post-contrast images at all time points. Minor adjustments were made to avoid artefact and blood pool. An ROI was also drawn in the left ventricular blood pool in order to calculate the partition coefficient (λ) and extracellular volume fraction (ECV) at each time point. This approach demonstrated excellent intra- and inter-observer reproducibility. (B) Assessment of regional variation in T1 measures. Using the anterior and inferior ventricular insertion points as well as the mid-point of the ventricular cavity as reference points, three intersecting lines were drawn to divide the left ventricle into 16 segments. ROI were drawn onto the basal (six segments), mid-cavity (six segments), and apical (four segments) pre-contrast T1 maps with the standardized approach described above. Subsequently, the ROI were copied onto the 20-min post-contrast T1 maps. Pre- and post-contrast T1, λ, and ECV values were assessed in each segment
Derivation of the partition coefficient and ECV
Myocardial λ and ECV were also calculated at all time points. These measures were derived from pre- and post-contrast myocardial T1 values corrected for blood-pool T1 (measured at the mid-cavity, Figure 1) and haematocrit (sampled at the time of CMR), according to:
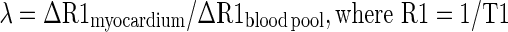 |
(1) |
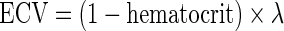 |
(2) |
Assessing myocardial T1 in multiple segments
Using a standardized approach, the basal, mid-cavity, and apical T1 maps were divided into segments according to the standard 17-segment model recommended by the American College of Cardiology/American Heart Association.30 We excluded the true apex because it was not possible to avoid partial volume effects. ROI were drawn in each of the 16 segments on the pre-contrast T1 map and subsequently, copied onto the post-contrast T1 map at the optimal time point established from the above analysis (Figure 1B). Pre- and post-contrast myocardial T1, λ, and ECV values were assessed in each segment of the left ventricle.
Comparison of T1 measures
Reproducibility analyses: intra-, inter-observer, and scan–rescan
We established the reproducibility of our image analysis technique and of the different T1 measures from a random sample of five healthy volunteers and five patients with aortic stenosis. In assessing intra-observer reproducibility, a single observer repeated analysis after an interval of >2 weeks to minimize recall bias. For inter-observer reproducibility, two independent observers performed separate blinded analyses.
Scan–rescan reproducibility was assessed in 10 healthy volunteers. Repeat scans were performed at least 7 days after the first scan and haematocrit samples were collected on both scan days. Images were analysed as above by a single observer.
Statistical analysis
A formal sample size estimation was not performed because this was an exploratory study to optimize a novel technique and to determine the T1-derived measure with the most potential to assess diffuse myocardial fibrosis.
The distribution of all continuous variables was assessed for normality using the Shapiro–Wilk test. Continuous variables were presented as mean ± standard deviation or median with inter-quartile range as appropriate. Comparison of continuous variables was performed using Student's t-test or the Mann–Whitney U test for non-parametric data. Categorical variables were expressed as percentages and compared using the χ2 test. In all T1-derived measures, we compared the values across all the segments using one-way analysis of variance (ANOVA) with post hoc Bonferroni adjustment. We examined the potential influence of heart rate and age on T1 measures using univariate linear analysis and adjusted for the effects of age and haematocrit using multivariate linear regression.
Reproducibility analysis (intra-, inter-observer, and scan–rescan) was performed using intra-class correlation coefficients (ICC). ICC values between 0.50 and 0.75 indicated moderate reliability and values >0.75 good reliability. For clinical measures, excellent ICCs of >0.90 are required to ensure sufficient reliability.31 Fixed and proportional biases with 95% limits of agreement were assessed using Bland–Altman analyses.
All statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA) and SPSS version 19 (SPSS, Inc., Chicago, IL). A two-sided P < 0.05 was considered statistically significant.
Results
Patients with aortic stenosis were older than healthy volunteers (median age 75 vs. 55 years, P < 0.01) and there were an equal number of males and females (Table 1). On average, they had moderate aortic stenosis (a mean aortic valve area of 1.2 ± 0.6 cm2; peak aortic valve velocity 3.3 ± 0.9 m/s) with an increased left ventricular mass index and indices of diastolic dysfunction compared with healthy volunteers (Table 1).
Table 1.
Baseline clinical, echocardiographic and CMR characteristics
| Healthy volunteers | Aortic stenosis | P value | |
|---|---|---|---|
| Clinical | |||
| Males, n (%) | 10 (50) | 10 (50) | 1.00 |
| Median age, years (IQR) | 55 (22, 65) | 71 (53, 75) | <0.01 |
| Hypertension, n (%) | 0 | 12 (60) | – |
| Diabetes mellitus, n (%) | 0 | 3 (15) | – |
| Coronary artery disease, n (%) | 0 | 4 (20) | – |
| Systolic blood pressure, mmHg | 140 ± 12 | 153 ± 25 | 0.05 |
| Heart rate, beats/min | 67 ± 11 | 62 ± 10 | 0.14 |
| Haematocrit | 0.41 ± 0.03 | 0.39 ± 0.04 | 0.05 |
| Medications | |||
| Aspirin, n (%) | 0 | 6 (30) | – |
| ACEI/ARB, n (%) | 0 | 6 (30) | – |
| Beta blockers, n (%) | 0 | 3 (15) | – |
| Statin therapy, n (%) | 0 | 8 (40) | – |
| Echocardiography | |||
| Aortic valve area, cm2 | 2.4 ± 0.6 | 1.2 ± 0.6 | < 0.01 |
| Mean pressure gradient, mmHg | 4 ± 1 | 25 ± 16 | < 0.01 |
| Peak velocity, m/s | 1.4 ± 0.2 | 3.3 ± 0.9 | < 0.01 |
| Mean e′, cm/s | 10.7 ± 4.1 | 6.5 ± 2.5 | < 0.01 |
| Median E/e′ (IQR) | 7.1 (6.0, 8.4) | 12.3 (8.6, 17.0) | 0.03 |
| Cardiac magnetic resonance | |||
| Indexed EDV, mL/m2 | 76 ± 14 | 74 ± 18 | 0.65 |
| Indexed ESV, mL/m2 | 28 ± 7 | 23 ± 9 | 0.07 |
| Stroke volume, mL/m2 | 48 ± 8 | 51 ± 12 | 0.43 |
| Indexed LV mass, g/m2 | 67 ± 14 | 81 ± 18 | < 0.01 |
| LV mass/EDV, g/mL | 0.88 ± 0.10 | 1.11 ± 0.20 | <0.01 |
EDV, end diastolic volume; ESV, end systolic volume; IQR, interquartile range; ACEI, angiotensin-converting enzyme inhibitors; ARB angiotensin receptor blockers.
A total of 1215 myocardial segments were analysed (606 in healthy volunteers and 609 in patients with aortic stenosis). 5.1% of segments were rejected because of artefact.
Influence of heart rate and age on T1 measures
Across the range of heart rates in our study, there was no correlation between heart rate and pre-contrast myocardial T1 (r = −0.23, P = 0.16), suggesting that incomplete restoration of magnetization due to fast heart rates and long T1 values was not an important factor. In healthy volunteers (age range 19–75) there was no correlation between age and any of the T1 measures investigated (pre-contrast myocardial T1, r = −0.09, P = 0.70; post-contrast myocardial T1, r = −0.25, P = 0.29; λ, r = 0.16, P = 0.52; and ECV, r = 0.25, P = 0.29).
Effects of time on post-contrast T1 values
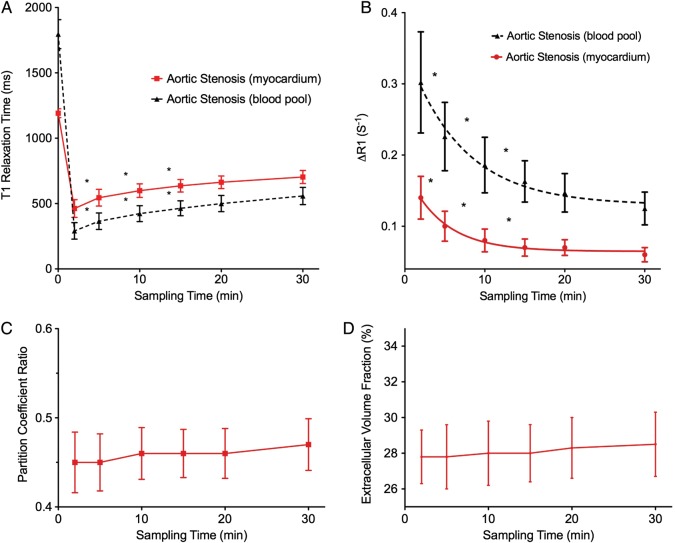
Post-contrast T1 values in the blood pool and myocardium were lower than pre-contrast values, and demonstrated an exponential return to baseline with time (Figure 2; Supplementary data online). In particular, the initial 15-min post-contrast MOLLIs were characterized by rapid changes in T1 values but thereafter the relaxation curve appeared to plateau such that subsequent changes were minimal. Indeed, T1 values at 20 min did not differ significantly from those at 15 and 30 min (Figure 2). On this basis, 20 min was the time point used for subsequent comparisons.
Figure 2.
Variation of different T1 measures with time. (A) Myocardium and blood-pool T1. Post-contrast T1 values are dramatically reduced with the administration of contrast, followed by an exponential increase in values towards baseline. Significant changes in T1 were observed in the first 15 min following contrast administration, while a plateau phase was observed between 15 and 30 min where values remained relatively unchanged. (B) Change in T1 relaxation rates (ΔR1) in the myocardium and blood pool. During the first 15 min, significant changes in ΔR1 values were observed in the myocardium and blood pool. This was followed by values that remained relatively stable between 15 and 30 min. (C and D) Partition coefficient and ECV. Both measures were constant at all the time points examined, suggesting contrast equilibrium between the blood pool and myocardium occurs as early as 2 min. *Denotes significant difference in values (P < 0.05) between the two adjacent time points
Interestingly, λ or ECV values were constant at all time points evaluated, reflecting a constant relationship between the myocardial and blood-pool T1 relaxation times (Figure 2).
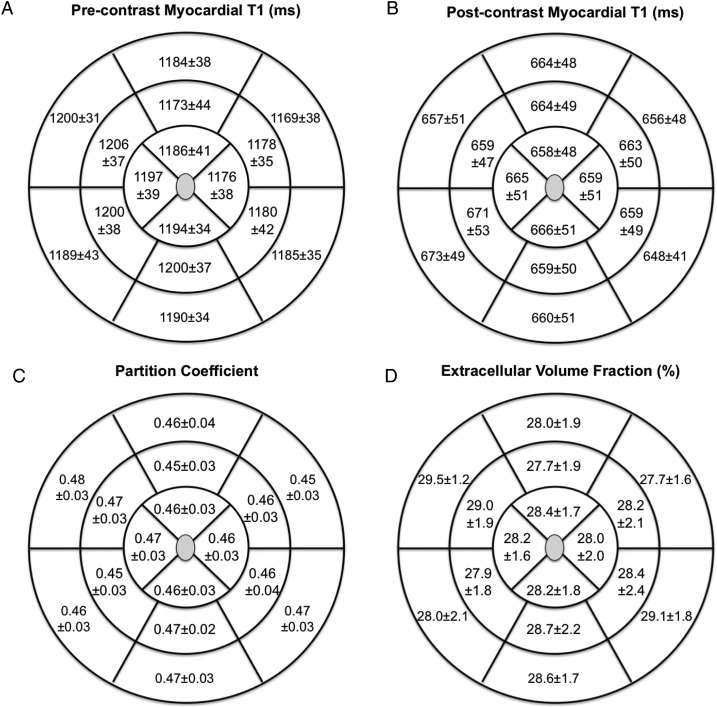
Regional variation in T1 measures
In healthy volunteers, there was no variation in any of the T1 measures across the 16 segments of the left ventricle (P > 0.1 in all measures; all pairwise comparisons with Bonferroni corrections), with similar results demonstrated in patients with aortic stenosis (Figure 3). Specifically, there were no differences across any of the myocardial slices or between segments within the same slice (P > 0.1 for all measures; all pairwise comparisons with Bonferroni corrections). Indeed, T1 measures in the mid-cavity were representative of those assessed across the entire left ventricular myocardium (λ 0.46 ± 0.03 vs. 0.46 ± 0.03, P = 1.00; ECV 28.4 ± 1.7 vs. 28.3 ± 1.9%, P = 0.61). Thus, the mid-cavity myocardium was used for subsequent comparisons.
Figure 3.
Variation in the different T1 measures across the left ventricular myocardium in patients with aortic stenosis. There were no differences in the pre- (A) or post-contrast (B) myocardial T1, partition coefficient (C), and the ECV values (D) across the 16 segments. ICC values were >0.90 for each myocardial segment
Comparison of T1 measures
Reproducibility analysis: intra-, inter-observer, and scan–rescan
Intra- and inter-observer reproducibilities were excellent for pre- and post-contrast T1 values, with no fixed or proportional biases and narrow limits of agreement (Table 2). However, scan–rescan reproducibilities for pre- and post-contrast T1 values were modest (ICC 0.72 and 0.56, respectively).
Table 2.
Reproducibility analysis
| T1 measure | Intra-observer |
Inter-observer |
Scan–rescan |
|||
|---|---|---|---|---|---|---|
| Mean difference | ICC | Mean difference | ICC | Mean difference | ICC | |
| Blood-pool pre-contrast T1, ms | 0.6 (−6.0 to 7.2) | 1.00 (1.00–1.00) | 2.6 (−6.1 to 11.3) | 1.00 (0.99–1.00) | 43.6 (−91.7 to 178.9) | 0.65 (0.12 to 0.90) |
| Myocardial pre-contrast T1, ms | −1.8 (−14.7 to 11.1) | 0.99 (0.96–1.00) | −5.7 (−14.9 to 3.5) | 0.99 (0.75–1.00) | 16.1 (−25.9 to 58.1) | 0.72 (0.16 to 0.93) |
| Blood-pool post-contrast T1, ms | 0.4 (−1.7 to 2.5) | 1.00 (1.00–1.00) | −0.3 (−2.2 to 1.6) | 1.00 (1.00–1.00) | 14.0 (−99.0 to 127.0) | 0.58 (−0.02 to 0.88) |
| Myocardial post-contrast T1, ms | 1.4 (−6.7 to 9.5) | 1.00 (0.99–1.00) | 4.5 (−0.7 to 15.5) | 1.00 (0.98–1.00) | 17.8 (−76.9 to 112.5) | 0.56 (−0.01 to 0.87) |
| Partition coefficient | −0.003 (−0.012 to 0.007) | 0.99 (0.96–1.00) | −0.011 (−0.03 to 0.01) | 0.94 (0.31–0.99) | −0.003 (−0.02 to 0.02) | 0.93 (0.76 to 0.98) |
| ECV, % | −0.05 (−0.34 to 0.24) | 1.00 (1.00–1.00) | −0.54 (−1.47 to 0.39) | 0.97 (0.60–0.99) | 0.09 (−1.16 to 1.34) | 0.96 (0.85 to 0.99) |
ECV, extracellular volume fraction.
Conversely, all measures of reproducibility were excellent for λ and ECV. ICC values were > 0.90 with no fixed biases and narrow confidence limits (Table 2). The scan–rescan variability was ± 3%. Furthermore, the ICC values for both λ and ECV were >0.90 in each of the 16 myocardial segments.
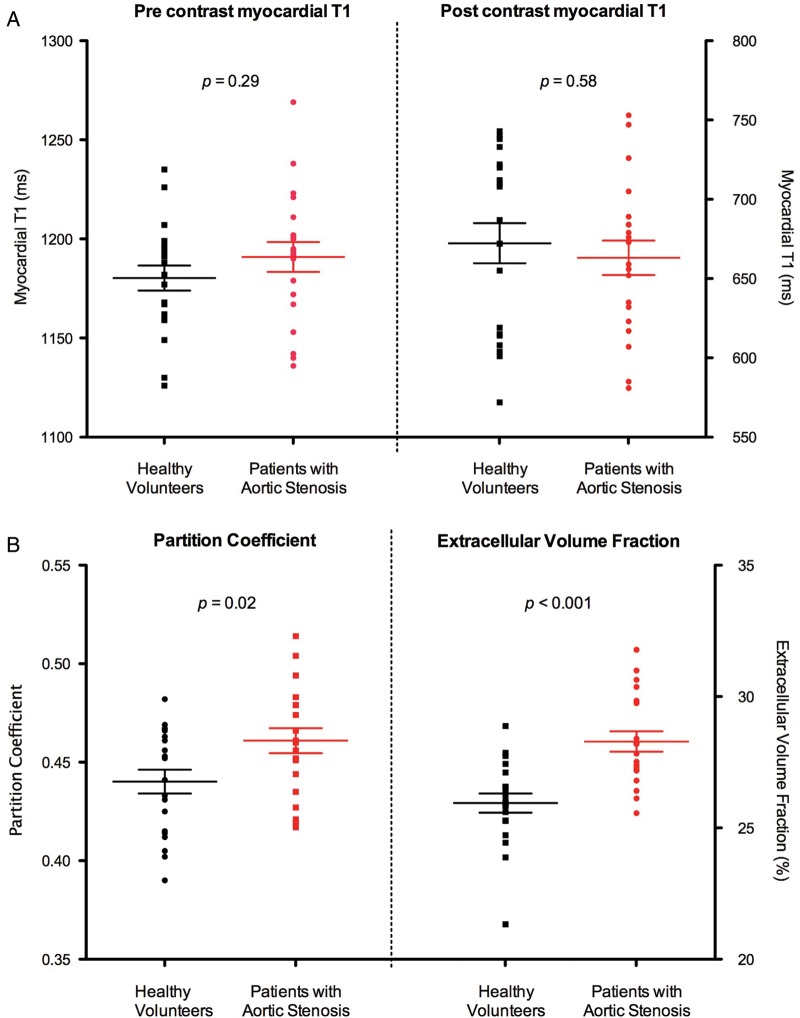
Ability to differentiate patients with aortic stenosis from healthy volunteers
Pre-contrast myocardial T1 values were similar in healthy volunteers and patients with aortic stenosis (1180 ± 28 vs. 1191 ± 34 ms, P = 0.29) as were post-contrast T1 values (672 ± 56 vs. 663 ± 43 ms, P = 0.59; Figure 4A). However, λ and ECV values were higher in patients with aortic stenosis compared with healthy volunteers (λ 0.46 ± 0.03 vs. 0.44 ± 0.03, P = 0.02; ECV 28.3 ± 1.7 vs. 26.0 ± 1.6%, P < 0.001; Figure 4B; Supplementary data online), with the latter appearing to offer better differentiation. The absolute increase in the ECV values in patients with aortic stenosis was 2.1% compared with healthy volunteers (95% CI: 0.6–3.6%, P = 0.009) after adjustment for age and haematocrit levels. Moreover, a correlation was observed between ECV and diastolic function (ECV and E/e′ r = 0.63, P < 0.01; ECV and e′ r = −0.50, P < 0.01), providing indirect support for increased myocardial fibrosis in patients with aortic stenosis. The other T1-related measures did not demonstrate such an association (P > 0.05 for all).
Figure 4.
Ability of the T1 measures to differentiate patients with aortic stenosis from healthy volunteers. (A) There was no significant difference in pre- and post-contrast myocardial T1 values between healthy volunteers and patients with aortic stenosis. (B) Partition coefficient and ECV values were significantly higher in patients with aortic stenosis compared with healthy volunteers. Results in mean ± SEM
Discussion
Using a standardized methodology and patient cohort, we have systematically compared commonly used T1 measures at multiple time points and in multiple regions across the left ventricle. We have shown that pre-contrast T1 was limited by an inability to differentiate patients with aortic stenosis from healthy volunteers, while post-contrast myocardial T1 lacked sufficient scan–rescan reproducibility. In comparison, λ and in particular ECV demonstrated excellent reproducibility and were increased in patients with aortic stenosis compared with healthy volunteers.
Over recent years, multiple T1-derived parameters have been derived to assess diffuse myocardial fibrosis. We have attempted to optimize and compare these different techniques at 3T using a standardized technique with meticulous attention paid to avoid blood pool and artefact. This approach demonstrated excellent reproducibility, with respect to the entire ventricle and within individual myocardial segments, indicating that both global and regional T1 can be measured. Furthermore, pre- and post-contrast T1, λ, and ECV values did not vary across segments in the left ventricle. This is as anticipated given the diffuse distribution of interstitial fibrosis.
We have also characterized the temporal variation of T1 measures. Post-contrast T1 demonstrated the characteristic exponential increase back to baseline following gadolinium administration. The rate of this recovery appears to be determined by the blood concentration of gadolinium (equilibrium between blood pool and myocardial T1 occurred as early as 2 min), which depends on its volume of distribution and renal clearance. Whilst the first 15 min following injection were characterized by large variations in myocardial and blood-pool T1 values, a plateau phase ensued between 15 and 30 min during which T1 values were relatively constant. We therefore investigated whether meaningful comparisons could be made between serial scans using post-contrast T1 values at 20 min. Unfortunately, the scan–rescan reproducibility of post-contrast T1 was modest. This is likely the consequence of inter-day variation in gadolinium pharmacokinetics relating to glomerular filtration rate, the patient's volume status and diet. Such variation will have had a major impact on our post-contrast T1 values potentially obscuring any differences attributable to diffuse fibrosis. Indeed, this is the likely explanation for the lack of difference in values between patients with aortic stenosis and healthy volunteers. Whilst complex kinetic models have been developed in an attempt to correct for some of these factors these are based upon multiple assumptions and data acquired at 1.5 not 3T.32
An alternate T1 technique is therefore necessary to overcome variations in gadolinium kinetics. One approach is pre-contrast myocardial T1. In this study, pre-contrast myocardial T1 demonstrated improved scan–rescan reproducibility; but, like post-contrast T1, was unable to differentiate patients with aortic stenosis from healthy volunteers. This probably reflects the reduced sensitivity of pre-contrast techniques that rely on the inherent T1 properties of healthy myocardium and fibrosis. In comparison, a recent study demonstrated that pre-contrast myocardial T1 values (using the shortened modified MOLLI sequence) were higher in patients with severe aortic stenosis compared with healthy volunteers.10 Most likely this reflects the more advanced disease state in their study population. Indeed, there was no increase in the values amongst their patients with moderate stenosis, who had a similar degree of hypertrophy to our cohort (81 ± 18 vs. 82 ± 17 g/m2).
A second approach is to correct post-contrast myocardial T1 values for variation in the pharmacokinetics of gadolinium. λ appears to be an effective method by using a ratio of myocardial and blood-pool T1 values. Indeed λ demonstrated excellent reproducibility, indicating that inter-day variation in gadolinium kinetics can be accounted for by this approach. Furthermore, λ values are increased in patients with aortic stenosis compared with healthy volunteers. This probably reflects the improved sensitivity of contrast-enhanced techniques, based on the accumulation of gadolinium in regions of fibrosis and the resultant shortening of T1.8
ECV translates λ into a percentage of the myocardium affected by diffuse fibrosis and is in many ways easier to conceptualize. Furthermore, it corrects for the effects of plasma volume, which can vary considerably from day to day (perhaps accounting for some of the scan–rescan variation in pre- and post-contrast T1 values). ECV demonstrated excellent reproducibility in this study and appears to further improve the differentiation between patients with aortic stenosis and healthy volunteers.
Both λ and ECV appear to possess all the necessary attributes for the measurement of diffuse fibrosis at 3T. Interest surrounds such techniques in the assessment of novel anti-fibrotic agents in aortic stenosis 33 and our scan–rescan reproducibility will be of use when estimating the required sample sizes for such studies. These will require the excellent reproducibility provided by λ and ECV to ensure that any difference detected between scans is attributed to the intervention. Moreover, the slowly progressive nature of fibrosis means that any treatment differences are likely to be small, so that the improved sensitivity of λ and ECV in particular over pre-contrast T1 will also be important.
Study limitations
By design we did not recruit patients with end-stage aortic stenosis, as we believe that T1 mapping will be more relevant to those with less severe disease in whom myocardial fibrosis is more likely to be reversible with novel anti-fibrotic therapies. Unfortunately, this has limited our ability to validate the various T1 measures against histology. However, good correlations for each technique have previously been established with histology, and supported in this study by increased ECV and λ in regions of LGE (Supplementary data online). Furthermore, we have demonstrated a close association between ECV and markers of diastolic dysfunction. It is therefore reasonable to assume that our T1-derived measures are markers of diffuse myocardial fibrosis particularly, as we have carefully excluded individuals with pathologies that might have confounded our results.
The patients with aortic stenosis were significantly older than healthy volunteers in our study, but the differences in ECV and λ values between patients with aortic stenosis and healthy volunteers were independent of age. This is also consistent with previous studies.15,34
Scan–rescan reproducibility was only assessed in healthy volunteers, but not in patients with aortic stenosis. However, both patients with aortic stenosis and healthy volunteers demonstrated similar T1 relaxation profiles in the myocardium and blood pool. Furthermore, we did not observe any proportional bias in healthy volunteers. Therefore, we believe that the scan–rescan reproducibility in patients with aortic stenosis would be similar to that in healthy volunteers.
Conclusions
In the CMR assessment of diffuse myocardial fibrosis, pre- and post-contrast myocardial T1 have limitations. In contrast, λ and in particular ECV demonstrate excellent reproducibility and an ability to differentiate patients with aortic stenosis from healthy volunteers. Among all the T1-derived measures evaluated, ECV appears to have the most potential for the assessment of diffuse myocardial fibrosis at 3T.
Supplementary data
Supplementary data are available at European Heart Journal – Cardiovascular Imaging online.
Conflict of interest: P.J.W. is a full time employee with Siemens Healthcare.
Funding
M.R.D. and D.E.N. are supported by a Clinical Lectureship (CH/09/002) and Chair (CH/09/002) respectively from the British Heart Foundation (BHF). C.W.L.C is supported by the NRF-MOH Healthcare Research Scholarship (PhD) from the National Research Foundation-Ministry of Health, Singapore. Funding to pay the Open Access publication charges for this article was provided by the British Heart Foundation.
Supplementary Material
References
- 1.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–9. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 2.Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. J Am Coll Cardiol Imag. 2012;5:370–7. doi: 10.1016/j.jcmg.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 4.Hein S, Arnon E, Kostin S, Schonburg M, Elsaser A, Polyakova V, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–91. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 5.Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–7. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 6.Lopez B, Gonzalez A, Diez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–54. doi: 10.1161/CIRCULATIONAHA.109.912774. [DOI] [PubMed] [Google Scholar]
- 7.Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79:744–55. doi: 10.1161/01.cir.79.4.744. [DOI] [PubMed] [Google Scholar]
- 8.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JP, et al. Myocardial T1 mapping: Application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007;58:34–40. doi: 10.1002/mrm.21272. [DOI] [PubMed] [Google Scholar]
- 10.Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–37. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. J Cardiovasc Magn Reson. 2013;6:488–97. doi: 10.1016/j.jcmg.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–80. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 13.Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, et al. T1 mapping in cardiomyopathy at cardiac MR: Comparison with endomyocardial biopsy. Radiology. 2012;265:724–32. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218:703–10. doi: 10.1148/radiology.218.3.r01fe18703. [DOI] [PubMed] [Google Scholar]
- 15.Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, et al. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268–78. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 1: Evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63–74. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation. 2010;122:138–44. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 18.White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, et al. T1 Mapping for Myocardial Extracellular Volume Measurement by CMR: Bolus only versus primed infusion technique. J Am Coll Cardiol Imag. 2013;6:955–62. doi: 10.1016/j.jcmg.2013.01.011. doi:10.1016/j.jcmg.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Flett AS, Sado DM, Quarta G, Mirabel M, Pellerin D, Herrey AS, et al. Diffuse myocardial fibrosis in severe aortic stenosis: An equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovas Imaging. 2012;33:819–26. doi: 10.1093/ehjci/jes102. [DOI] [PubMed] [Google Scholar]
- 20.Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373–83. doi: 10.1161/CIRCIMAGING.112.000192. [DOI] [PubMed] [Google Scholar]
- 21.Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, et al. T1 mapping of the myocardium: Intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson. 2012;14:26–35. doi: 10.1186/1532-429X-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawel N, Nacif M, Zavodni A, Jones J. T1 mapping of the myocardium: Intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012;14:27–37. doi: 10.1186/1532-429X-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69–80. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P, et al. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: Sample size considerations for clinical trials. J Cardiovasc Magn Reson. 2012;14:90–8. doi: 10.1186/1532-429X-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oshinski JN, Delfino JG, Sharma P, Gharib AM, Pettigrew RI. Cardiovascular magnetic resonance at 3.0T: Current state of the art. J Cardiovasc Magn Reson. 2010;12:55–68. doi: 10.1186/1532-429X-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Xue HH, Shah SS, Greiser AA, Guetter CC, Littmann AA, Jolly M-PM, et al. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med. 2012;67:1644–55. doi: 10.1002/mrm.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber A, Hayes C, Spannagl B, Rieber J, Klauss V, Schoenberg SO, et al. Phase-sensitive inversion recovery single-shot balanced steady-state free precession for detection of myocardial infarction during a single breathhold. Acad Radiol. 2007;14:1500–8. doi: 10.1016/j.acra.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 30.Cerqueira MD, Weisman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 31.Portney LG, Watkins MP. New Jersey, United States: Pearson; 2009. Foundations of clinical research. [Google Scholar]
- 32.Gai N, Turkbey EB, Nazarian S, van der Geest RJ, Liu C-Y, Lima JAC, et al. T1 mapping of the gadolinium-enhanced myocardium: adjustment for factors affecting interpatient comparison. Magn Reson Med. 2010;65:1407–15. doi: 10.1002/mrm.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–63. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 34.Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–41. doi: 10.1136/heartjnl-2012-302346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.