Abstract
Background & Aims
It is important to increase our understanding of gustatory detection of dietary fat and its contribution to fat preference. We studied the roles of the fat taste receptors CD36 and GPR120 and their interactions via Ca2+ signaling in fungiform taste bud cells (TBC).
Methods
We measured Ca2+ signaling in human TBC, transfected with small interfering RNAs (siRNAs) against mRNAs encoding CD36 and GPR120 (or control siRNAs). We also studied Ca2+ signaling in TBC from CD36−/− mice and from wild-type lean and obese mice. Additional studies were conducted with mouse enteroendocrine cell line STC-1 that express GPR120 and stably transfected with human CD36. We measured release of serotonin and GLP-1 from human and mice TBC in response to CD36 and GPR120 activation.
Results
High concentrations of linoleic acid induced Ca2+ signaling via CD36 and GPR120 in human and mice TBC as well as in STC-1 cells, whereas low concentrations induced Ca2+ signaling via only CD36. Incubation of human and mice fungiform TBC with lineoleic acid downregulated CD36 and upregulated GPR120 in membrane lipid rafts. Obese mice had decreased spontaneous preference for fat. Fungiform TBC from obese mice had reduced Ca2+ and serotonin responses but increased release of GLP1, along with reduced levels of CD36 and increased levels of GPR120 in lipid rafts.
Conclusions
CD36 and GPR120 have non-overlapping roles in TBC signaling during oro-gustatory perception of dietary lipids; these are differentially regulated by obesity.
Keywords: Serotonin, Linoleic acid, GLP-1, Lipids
Introduction
Oral perception of dietary fat was until recently thought to involve mainly texture and olfactory cues; however, accumulating evidence strongly suggests existence of a taste modality, devoted to the detection of long-chain fatty acids (LCFA).1 Mice can recognize dietary fat and FA solutions in the oral cavity in the absence of olfactory or textural cues2. Humans can taste LCFA even when textural properties are masked and olfaction is eliminated using a nose clip2.
CD36 is highly expressed apically on lingual gustatory epithelium in rats and mice3,4 and CD36 deletion completely abolishes the spontaneous preference of wild-type mice for LCFA4. In mouse circumvallate taste bud cells (TBC), LCFA-mediated activation of CD36 released Ca2+ from the endoplasmic reticulum (ER) via a phospholipase-C (PLC)-dependent mechanism.5 Furthermore, LCFA via CD36 induced the phosphorylation of Src kinases, that regulate the opening of store-operated Ca2+ (SOC) channels in TBC. The SOC channels in the mouse TBC are composed of orai1/3 proteins, and are under the control of stromal interaction molecule-1 (STIM1) which orchestrates ER Ca2+ sensing and release.6 STIM1 may play a key role in fat perception as its deletion eliminates the mice's spontaneous preference for fat.6
Similar to CD36, the GPR120 and GPR40 members of G protein-coupled receptors (GPCRs) were shown to mediate the taste responses to fatty acids7. Both GPR120 and GPR40 knock-out mice showed a diminished preference for linoleic and oleic acids with reduced taste nerve responses. GPR40 expression was undetectable in rat8 and human TBC9 and only GPR120, is expressed in type II cells, the true taste receptor cells of the lingual epithelium.7
The findings to date, thus suggest that TBC CD36 and GPR120 are the potential candidates for lipid taste perception.1,10 However, information on their relative roles remains largely unavailable. Why are there two receptor candidates for one taste? 11 There is evidence in mice that TBC CD36 and GPR120 might respond differently to dietary fat. During a meal, lingual CD36 expression is downregulated which is paralleled by reduction of fat preference12. In contrast, no changes are observed in GPR120 expression in circumvallate papillae during the meal12, suggesting that this receptor plays an alternative role distinct from that of CD36. The present study was undertaken to test the hypothesis that CD36, which has high affinity for LCFA, might function as the primary receptor for fat taste detection while GPR120 would operate to amplify signaling mainly under excess FA supply. For this we elucidated Ca2+ signaling pathways, triggered by LCFA interaction with CD36 or GPR120 versus a specific agonist of GPR120 in human fungiform TBC. Selective siRNA silencing of CD36 and GPR120 was used to dissect their respective roles. The data were further validated using TBC from CD36-/- mice and STC-1 endocrine cells, endogenously expressing GPR120 and transfected with human CD36. The effect of high fat feeding on CD36 and GPR120 expression levels and membrane distribution were examined in fungiform TBC from lean and obese mice. Lipid gustatory preference, TBC Ca2+ signaling and the associated release of serotonin and glucagon-like peptide-1 (GLP-1) were determined.
Materials and Methods
Materials
Human13-15 and mice TBC5,6 were prepared and maintained as previously reported. All studies adhered to protocols approved by the Schulman Associates Institutional Review Board (Cincinnatti, OH) for human TBC, for mice TBC by the Regional Ethical Committee of Dijon (France) and the Animal Studies Committee of Washington University (St Louis, MO). Cell culture media were from Lonza Verviers (Belgium) and Fura-2/AM from Invitrogen (USA) (See supplementary Materials and Methods).
Measurement of Ca2+ signaling
Isolated TBC suspended in fresh IMDM containing 10% fetal bovine serum were seeded (2×105/well) onto a Willico-Dish wells.6 Changes in intracellular Ca2+ ([Ca2+]i) were monitored using a Nikon microscope (TiU) equipped with EM-CCD (Lucas) camera for real time recording of digital images and an S-fluor 40x oil immersion objective. Planes were taken at Z intervals of 0.3 μm and the images analyzed (NIS-Elements). Changes in [Ca2+]i were expressed as ΔRatio, calculated as the difference between the peak F340/F380. The data were averaged for 20–40 individual cells per run from 3–9 experiments with at least 3 cell preparations. For experiments in Ca2+-free medium, CaCl2 was replaced by EGTA (2 mM).
Statistical analysis
Data are presented as means ± SEM. Significance of differences between mean values was determined by one way ANOVA (Statistica, Statsoft, France), followed by a least-significant-difference test.
Results
Human fungiform TBC co-express GPR120 and CD36
Our initial studies sought to determine the signalling events involved in transduction of fat taste in humans with emphasis on the respective roles of CD36 and GPR120. To date there is little information related to these processes in human TBC. Human fungiform TBC sorted for CD36 expression were found to express the markers of taste receptor cells PLC-β2 and α-gustducin (Fig. 1A, B) and all CD36 expressing TBC also co-expressed GPR120 (Fig. 1C).
Figure 1. Characterization of human fungiform TBC.
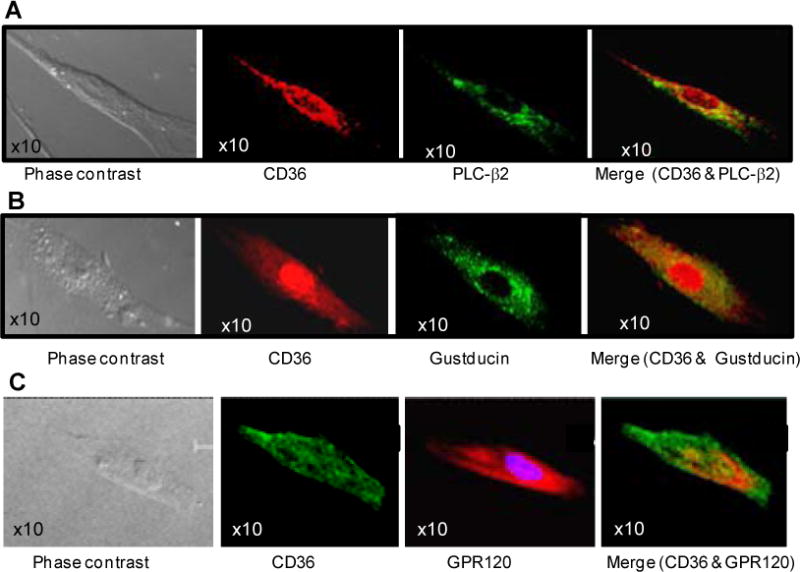
Images were acquired with Leica TCS-SP2 confocal laser scanning microscope (A, B & C). Immunoreactivity for CD36 (red in A, B; green in C), GPR120 (red in C) and PLC-β (green in A) and α-gustducin (green in B) was observed in cultured TBC.
Linoleic acid (LA) and grifolic acid (GA) induce capacitative Ca2+ signaling in human TBC through PTK and G-protein dependent mechanisms
Two taste receptor ligands Linoleic acid (LA) and grifolic acid (GA) were used for the fat taste studies to help determine specificity of agonist responses and sensitivity to various inhibitors. LA used by us and others1,2 is primarily a CD36 ligand that is abundantly present in vegetable oil and thus a representative fatty component of the Western diet. GA, purified by Hara et al.16 from the edible mushroom Albatrellus dispansus is a selective GPR120 ligand based on studies of Ca2+ signaling in enteroendocrine STC-1 cells16.
Linoleic acid addition to human fungiform TBC at a concentration of 20 μM induced a rise in intracellular Ca2+ ([Ca2+]i) (Fig. 2A, B) and a similar effect was observed with 20 μM grifolic acid (GA) (Fig. 2C, D). LA- and GA-induced increases in [Ca2+]i were higher in Ca2+-containing as compared to Ca2+-free medium (Fig. 2B, D). In presence of Ca2+, blockers of store-operated Ca2+ (SOC) channels, i.e., SKF96365, 2-aminoethoxydiphenyl borate (2-ABP) and econazole, significantly diminished LA-induced increase in [Ca2+]i (Fig. 2E, p<0.001) and to a lesser extent that induced by GA (Fig. 2F, p<0.001). Different inhibitors of protein tyrosine kinases (PTKs), i.e., PP2, genistein and SU6656, markedly (60-90%) curtailed the rise of [Ca2+]i after LA (Fig. 2E, p<0.001) and significant but less pronounced (20-40%) inhibition was observed with GA (Fig. 2F, p<0.001).
Figure 2. Effects of LA and GA on Ca2+ signaling in human fungiform TBC.
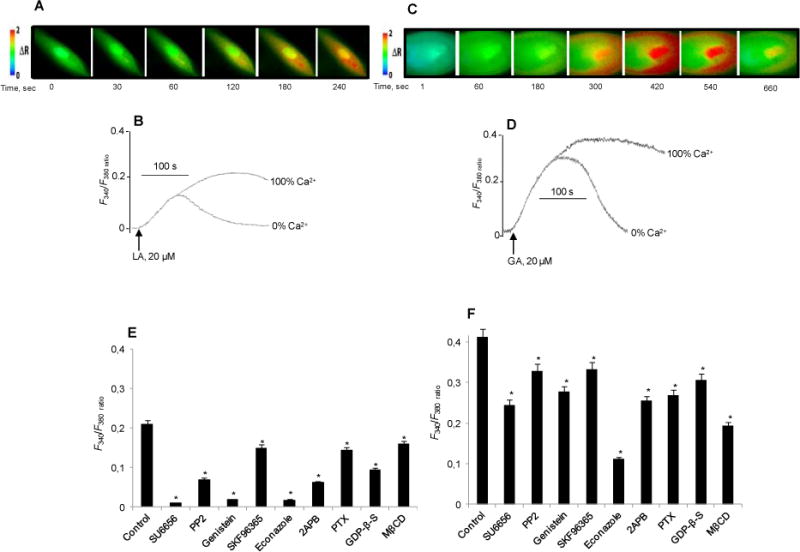
The cultured TBC (2×105 cells/assay) were loaded with Fura-2/AM and the changes in intracellular Ca2+ (F340/F380) were monitored. The experiments were performed in Ca2+-containing (A - F) and in Ca2+-free media (B, D). In A and C, the colored time-lapse images show the changes in intracellular Ca2+ ([Ca2+]i) evoked, respectively by LA and GA. In E and F, TBC before exposure to 20 μM LA (E) or GA (F) were preincubated (20 min) with: SU6656 (5 μM), PP2 (10 μM), genistein (30 μM), SKF96365 (15 μM), econazole (30 μM), 2-APB (30 μM), PTX (10 ng/ml), GDP-β-S (300 μM), or M-βCD (2.5 mM). Data are means ± SEM (n=7). *p<0.001) as compared to control.
Pertusis toxin (PTX), a G-protein inhibitor, and guanosine-5′-O-2-thiodiphosphate (GDP-β-S), a non-hydrolysable GDP analogue, significantly diminished both LA- (30 and 60%) and GA-evoked (40 and 30%) increases of [Ca2+]i in human TBC (Fig. 2E, F). Disruption of lipid rafts by methyl-β-cyclodextrin (M-βCD) also decreased Ca2+ signaling in the presence of either LA (30%) (Fig. 2E) or GA (50%) (Fig. 2F). The sulfo-N-succinimidyl derivative of oleate (SSO), which binds lysine-164 in CD36 and blunts LCFA-induced Ca2+ signaling,17 curtailed (75%) LA-induced increase in [Ca2+]i (F340/F380 ratio, 0.21±0.016 vs. 0.06±0.003, p<0.001).
LA and GA induce serotonin and GLP-1 release in human TBC
We previously showed that mice TBC respond to LCFA by releasing serotonin (5-hydroxytryptamine, 5-HT), and this release is dependent on the rise of [Ca2+]i.5 In human fungiform TBC, LA (20 μM) and GA (20 μM) both triggered release of serotonin with GA (61.3±2.48% above control, p<0.001) being more potent than LA (36.4±1.39% above control, p<0.001).
Glucagon-like peptide-1 (GLP-1) and its receptor (GLP-1R) have been identified in mouse TBC, suggesting their involvement in taste perception.18 Addition of 20 μM LA or GA to human TBC induced a small and equivalent release of GLP-1 (LA, 103.06±1.61 vs GA, 102.93±1.45 pmol per 1×106 cells) that significantly (p<0.001) exceeded the GLP-1 released by control TBC (plus 0.1% v/v ethanol vehicle) (90.29±1.20 pmol of GLP-1 per 1×106 cells).
LA and GA induce additive effects on Ca2+ signaling and IP3 production in human TBC
LA and GA exerted an additive response on Ca2+ signaling (Supplementary Fig. 1A, B, C). GA-induced rise of [Ca2+]i exceeded that triggered by LA both at 20 μM or 100 μM (Supplementary Fig. 1B, C). Consistent with this, production of IP3 which triggers release of ER Ca2+ and subsequent SOC Ca2+ influx was higher in GA-treated TBC as compared to LA-treated cells (Supplementary Fig. 2). In addition, IP3 release in response to LA and GA combined exceeded that observed with each alone (Supplementary Fig. 2).
Knockdown of CD36 and GPR120 exert additive suppression of Ca2+ signaling in human TBC
To dissect the CD36 versus GPR120 specific signalling responses, we used (Figure 3A) siRNA knockdown to selectively diminish the expression of these proteins in human fungiform TBC. CD36 siRNA-transfected TBC had a reduced (∼45%) Ca2+ response to 20 μM LA with no influence on that induced by 20 μM GA (Fig. 3B). TBC transfected with GPR120 siRNA had a small but significant decrease (∼18%) in the LA-induced Ca2+ rise with a much stronger reduction (∼75%) of the GA-induced response (Fig. 3B). Combined transfection with CD36 and GPR120 siRNA suppressed LA-induced Ca2+ signaling by about 80% but caused no further reductions in the response to GA as compared to cells transfected with GPR120 siRNA alone (Fig. 3B).
Figure 3. Effects of siRNA targeting CD36 or GPR120 on LA- and GA-evoked Ca2+ signaling in human fungiform TBC.
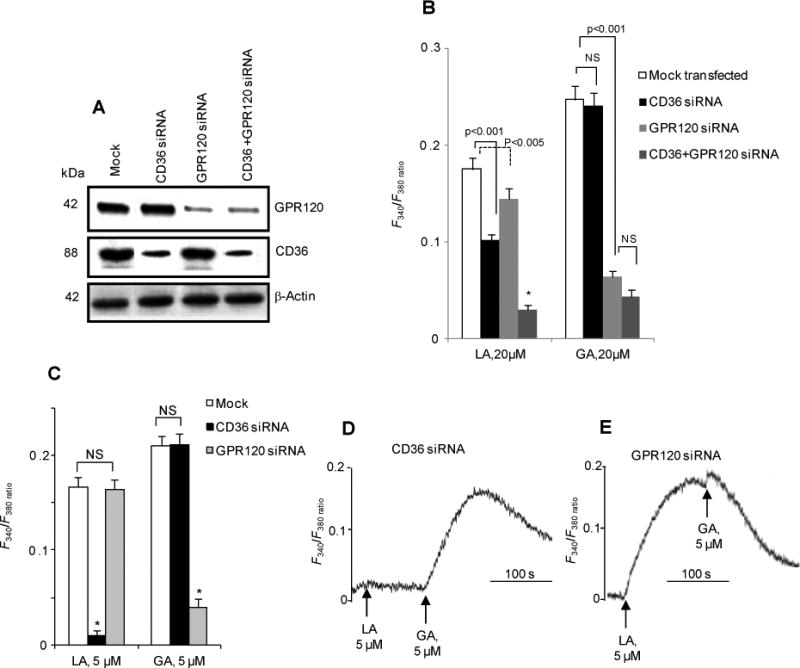
A: Western blots showing selective downregulation of CD36 and GPR120 by siRNA targeting CD36 and/or GPR120. Mock: non-targeting siRNA. β-actin is the loading control. B-E: Increases in [Ca2+]i induced by either LA or GA in TBC transfected by the various siRNA. Data are means ± SEM (n=4) conducted in triplicates.
LA at low concentrations induces Ca2+ signaling only via CD36 in human and mouse TBC
LA at 5 μM failed to raise [Ca2+]i in human TBC, transfected with CD36 siRNA (Fig. 3C, D). In TBC transfected with GPR120 siRNA, LA (5 μM), but not GA (5 μM), induced a significant rise of [Ca2+]i (Fig. 3C, E).
To strengthen these observations, we conducted experiments on mice fungiform TBC, isolated from CD36 null mice (CD36-/-). LA at 5 μM increased [Ca2+]i in wild-type (WT) mice TBC, whereas no rise was observed in TBC from CD36-/- mice (Fig. 4A). LA at 20 μM evoked in CD36-/- TBC a small rise in [Ca2+]i, which was substantially less (70%) than that observed in TBC from WT mice (Fig. 4B). There was no difference in GA signaling to [Ca2+]i between TBC from WT or CD36-/- mice (Fig. 4C). Addition of 20 μM GA following that of 20 μM LA failed to induce an additive rise of [Ca2+]i in TBC from CD36-/- mice (Fig. 4D) indicating that LA is an effective competitor of GA for GPR120 activation of Ca2+ signaling.16 In TBC from WT mice, addition of LA and then GA triggered an additive response (Fig. 4E) in line with LA primarily interacting with CD36 (Fig. 4B) while GA only activates GPR120 (Fig. 4C).
Figure 4. Effects of CD36 deficiency on LA- and GA-evoked Ca2+ signaling in mouse fungiform TBC.
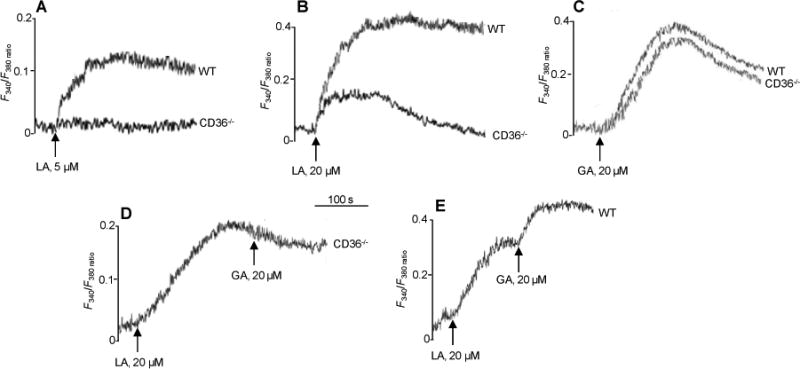
Ca2+ imaging studies were performed on fungiform TBC from wild-type (WT) or CD36−/− mice and the changes in [Ca2+]i (F340/F380) were monitored as for Figure 2. The data are representative of at 4-5 experiments conducted in triplicates.
LA and GA evoke differential Ca2+ signaling in enteroendocrine STC-1 cells
To confirm the above observations, we analyzed the changes in [Ca2+]i in STC-1 cells. STC-1 cells express GPR120 but not CD36 and a line stably expressing human CD36 was generated19 (Supplementary Fig. 3A) to dissect the relative roles of the two receptors.
In cells lacking CD36, LA at 5 μM did not increase [Ca2+]i (Fig. 5A), whereas a response was observed in CD36-expressing cells (Fig. 5B), which was not altered by GPR120 knock-down (Fig. 5C). LA at 20 μM evoked a small rise in [Ca2+]i in STC-1 cells expressing GPR120, but lacking CD36 (Fig. 5D). A strong rise in [Ca2+]i was observed in cells expressing CD36 (Fig. 5E), which was not affected by GPR120 knock-down (Fig. 5F). Pretreatment with the CD36 inhibitor SSO curtailed LA-induced increases in [Ca2+]i in STC-1 cells, expressing CD36 (Supplementary Fig. 3B).
Figure 5. Effects of LA and GA on Ca2+ signaling in STC-1 cells.
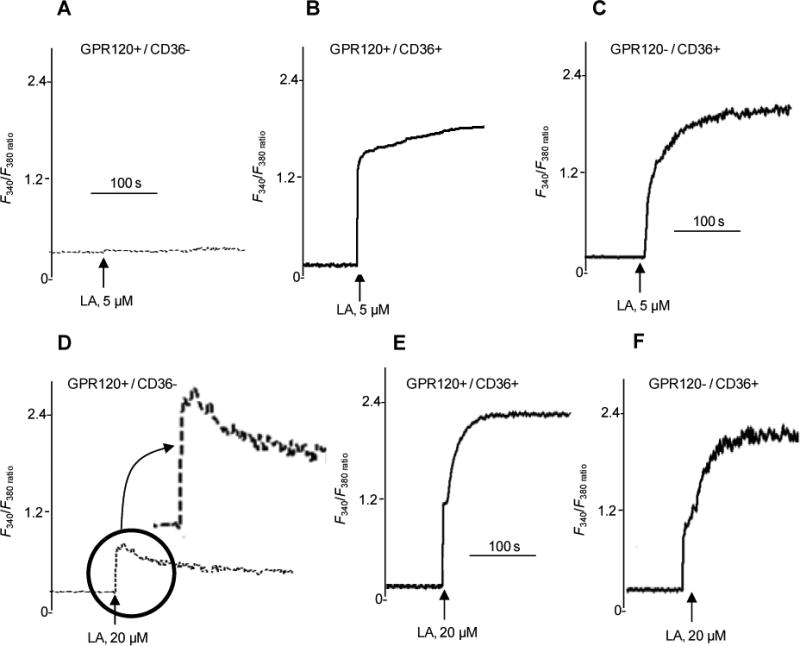
The studies were performed on STC-1 cells that endogenously express GPR120 but lack CD36 (GPR120+/CD36-), that stably express human CD36 (GPR120+/CD36+) or that express CD36 and are transfected with GPR120 siRNA (GPR120-/CD36+). The changes in intracellular Ca2+ (F340/F380) are shown in response to 5 or 20 μM LA. The data are representative of at 4-5 experiments conducted in triplicates.
LA alters distribution of CD36 and GPR120 in membrane rafts of human and mouse TBC
CD36 is localized in membrane microdomains known as rafts20 while less is known about membrane distribution of GPR120. Since localization in lipid rafts can impact Ca2+ signaling by influencing G-protein activation and IP3 production,21,22 the effect of LA on CD36 and GPR120 membrane distribution was explored. Treatment of human or mice fungiform TBC for 20 min with LA did not alter protein levels of CD36 and GPR120 (not shown) but did cause changes in membrane distribution. In human fungiform TBC, CD36 and GPR120 were present in both raft (fractions 3-6) and non-raft (fractions 7-10) triton-soluble membranes (Fig. 6A-C). LA-treatment of human fungiform TBC decreased CD36 level in the rafts without affecting level in the soluble fractions (Fig. 6A-C). In contrast, LA exposure increased GPR120 levels in the raft fractions (Fig. 6A, B).
Figure 6. Effects of LA-exposure on the distribution of CD36 and GPR120 in raft fractions in human (A, B & C) and mouse (D, E & F) fungiform TBC.
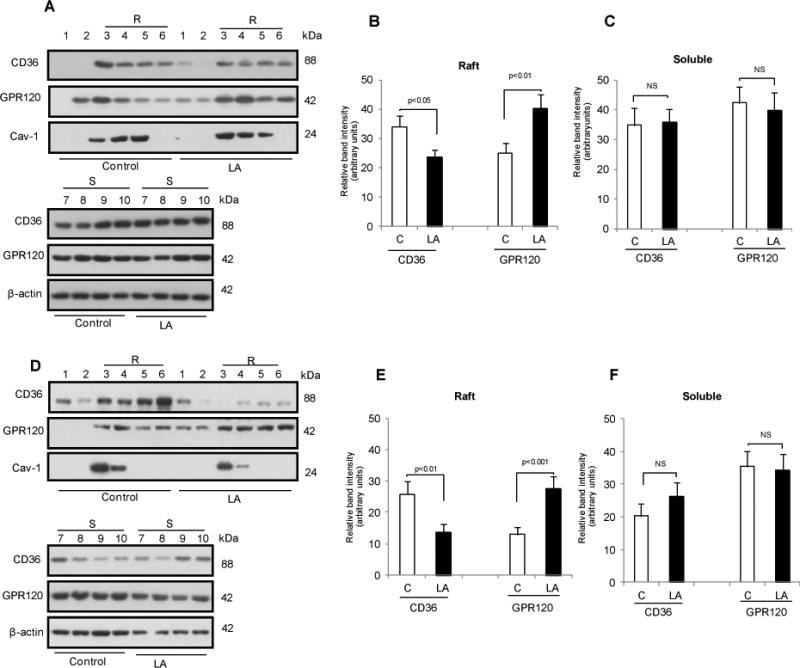
The TBC were treated or not (control) with 20 μM LA for 20 min A & D: Cells were homogenized in 1% Triton X-100 at 4°C, and the lysates were subjected to discontinuous 5–40% sucrose gradient centrifugation. Different fractions were collected from the top of the gradient, and equal volumes of aliquots from each fraction were subjected to western blot. Histograms show the relative band intensity (arbitrary units) measured by densitometry of protein content in raft (3-6) and soluble fractions (7-10). The data were normalized with respect to band intensity of caveolin-1 (Cav-1) for raft fractions and β-actin for soluble fractions, measured under similar conditions. B & C are derived from A and E & F are derived from D. Data are means ± SEM conducted in triplicates.
Comparable results were obtained with mouse fungiform TBC (Fig. 6D-F), as LA-treatment was associated with less CD36 and more GPR120 (Fig. 6D, E) in the raft fractions.
High fat fed obese mice have reduced fat preference with abnormalities of TBC Ca2+ signaling, release of serotonin and GLP-1 and altered CD36 and GPR120 membrane raft distribution
To gain insight into the physiological role of FA-induced Ca2+ signaling, we explored whether it is altered during obesity. Studies were conducted with mice fed a high-fat diet (HFD) for two months. This regimen resulted in 20% more body weight gain as compared to controls fed standard chow and in hyperglycemia (glucose levels: 1.1±0.8 g/l in control mice vs 1.75±0.05 g/l in HFD mice). GA and LA increased [Ca2+]i in the TBC of mice fed the standard diet as expected (Fig. 7A). The HFD reduced the Ca2+ response to LA whereas it increased that induced by GA (Fig. 7A). Expression of mRNA encoding CD36, GPR120 and α-gustducin was not altered by the HFD (not shown). However, CD36 protein level in both raft and non-raft fractions decreased (Fig. 7 C-F) while more GPR120 distributed to lipid rafts in the TBC of HFD fed mice (Fig. 7 C, E).
Figure 7. High fat diet-induced obesity modulates Ca2+ signaling, gustatory fat preference and CD36 and GPR120 raft distribution in mouse fungiform TBC.
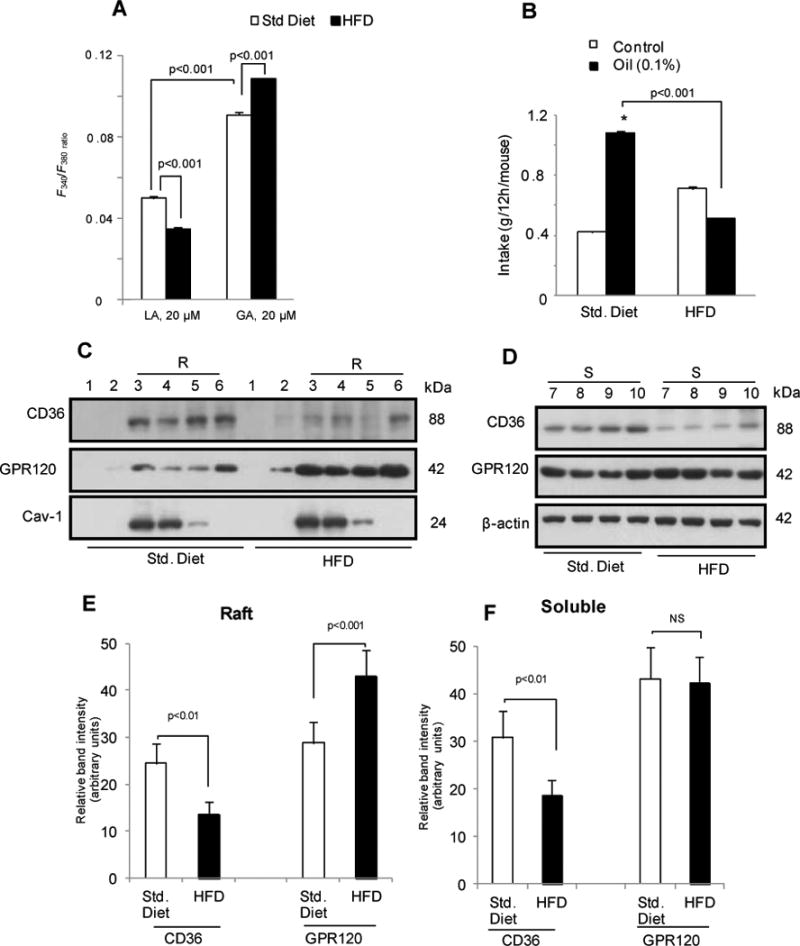
The fungiform TBC were isolated from mice fed a standard chow diet (Std. Diet) or a high fat diet (HFD) for two months. A: Changes in [Ca2+]i. B: Spontaneous fat preference of mice fed standard or HF diets. Values are means ± SEM (n=6). * (p<0.001) Oil as compared to control solution. C & D: Distribution of CD36 and GPR120 in raft (R, 3-6) and soluble (S, 7-10) fractions from mice fed Std. or HF diets. Caveolin-1 (cav-1) is a raft marker. E & F: Relative densitometry quantifying data (arbitrary units) derived from C and D after normalization for band intensity of caveolin-1 (Cav-1) for raft fractions and β-actin for soluble fractions. NS=non-significantly different. Data are means ± SEM conducted in triplicates.
Control mice fed the standard diet exhibited a strong preference for 0.1% canola oil as compared with a control solution (Fig. 7B) and this preference was significantly diminished in HFD fed obese mice (Fig. 7B).
LA-induced serotonin release was reduced in TBC from HFD as compared to standard diet fed mice (Supplementary Fig. 4A). However, GA activated release of serotonin was not altered (Supplementary Fig. 4A). Interestingly, GLP-1 release triggered by GA was enhanced in TBC from HFD as compared to standard diet fed mice (Supplementary Fig. 4B) while LA-stimulated GLP-1 release was unchanged (Supplementary Fig. 4B). This suggested that GLP-1 release is mediated by GPR120 and it is facilitated by recruitment of GPR120 to lipid rafts.
Discussion
The present study showed that human fungiform TBC sorted for CD36 expression also express GPR120 together with the markers of taste receptor cells α-gustducin and PLC-β. Both CD36 and GPR120 are implicated in oro-gustatory perception of dietary lipids.1,4,6,7 Expression, mRNA and protein, of CD363,4 and GPR1208,12 has been reported in circumvallate, foliate and fungiform papillae of rodents. However, this is the first documentation of their co-expression in individual human TBC.
Our study evaluated Ca2+ signaling, an early event downstream of taste receptor activation, in response to a long-chain fatty acid, LA, a ligand of CD36 and presumably of GPR120, as compared to GA, a selective GPR120 agonist.16 Both LA and GA induced increases in IP3 production and [Ca2+]i suggesting they acted to mobilize Ca2+ from the ER via a PLC-dependent mechanism, possibly via PLC-β present in human fungiform TBC. LA-evoked Ca2+ signaling was significantly curtailed in the presence of SSO, indicating that this FA mobilized Ca2+, in large part, by binding to CD36.5,23 Like in mouse TBC,5 CD36 in human fungiform TBC appears coupled to PTK and Ca2+ signaling.
As reported in the mouse,5,6 the present study confirms involvement of SOC channels in the Ca2+ response to LCFA by human fungiform TBC. Disruption of G-proteins also diminished the Ca2+ signaling responses to both LA and GA. GPR120 has been documented to elevate [Ca2+]i via interacting with the Gaq/11 family of G proteins24 but the link between CD36 and G-proteins is less clear.24 CD36 signaling might induce IP3 generation directly via phosphorylation of PLC by the CD36 interacting src kinase, which has been reported to phosphorylate the PLCγ isoforms.25
Treatment of human or mice fungiform TBC for 20 min with LA did not significantly downregulate total CD36 protein, which has been shown to become ubiquitinated and degraded after FA treatments19. Indeed, Chevrot et al26 reported lipid-mediated downregulation of CD36 in mouse TBC following a 60 min exposure and Tran et al27 reported its downregulation in the small intestine 2h after lipid intake. Thus, CD36 downregulation would represent a feedback mechanism aiming to reduce FA activation of CD36 signaling. While this downregulation was not measurable after a short 20 min FA treatment, it is a likely contributor to the reduction of TBC CD36 level that we observed in HFD fed obese mice.
Cholesterol depletion of plasma membrane rafts curtailed LA- and GA-evoked increases in [Ca2+]i in human fungiform TBC, suggesting that raft integrity plays an important role in the regulation of Ca2+ signaling. Membrane rafts are domains, rich in cholesterol and sphingolipids that provide a platform for the assembly of signaling complexes.28 In mouse TBC, clustering of sweet taste receptors into the rafts is thought to enhance sweetener action by facilitating tastant-triggered G-protein-coupled receptor signaling.29 Studies, where lipid rafts were disrupted or reconstituted, showed these domains to be indispensable for sweet receptor responsiveness to sucralose.29 Similarly, presence of CD36 in lipid rafts, has been reported to be important for CD36-mediated downstream signaling, as recently reviewed.30
Our findings support the interpretation that both CD36 and GPR120 are functional taste receptors in human fungiform TBC. LA and GA both induced the release of serotonin as previously shown for LA with mice TBC.5,6 GA-induced serotonin release by human TBC exceeded that triggered by LA and it paralleled the higher GA induced rise in [Ca2+]i, consistent with the direct coupling of Ca2+ signaling to serotonin secretion.31 In this context, the higher relative potency of 20μM GA versus 20μM LA in human TBC and not in mouse TBC, might reflect a dietary effect on TBC CD36 in humans. Human intake of dietary fat often exceeds 40% as compared to less that 6% for mice fed standard diets, which would be expected to downregulate CD36 level and function in human TBC. Future studies will explore this possibility.
CD36 and GPR120 are structurally different glycoproteins predicted to have two and seven transmembrane segments, respectively. Although both recognize LCFA, they markedly differ in affinity for the FA, with CD36 exhibiting much higher affinity28 than GPR120.32 Our data indicate that CD36 is the primary LCFA receptor in TBC. Selective knock-down of either CD36 or GPR120 in human fungiform TBC showed that LA at low concentration induces Ca2+ signaling via CD36 and not through GPR120. LA at a low concentration also failed to increase [Ca2+]i in TBC obtained from CD36-/- mice. Although a high concentration of LA triggered a rise in [Ca2+]i, the response was much smaller than that obtained in WT mice. Thus, in contrast to CD36 which recognizes low FA levels, GPR120 only responds to high FA concentrations and the response obtained is modest. This differential sensitivity was also observed in STC-1 cells expressing GPR120 but not CD3633 which were unresponsive to 5 μM LA (or oleic acid or docosahexaenoic acid, data not shown). STC-1 responsiveness to LCFA was acquired with CD36 expression. High concentrations of LA (20 μM) evoked only a slight rise of [Ca2+]i in CD36 negative STC-1 cells and while the LA response was increased 4 fold by CD36 expression, it was minimally reduced by knockdown of GPR120. These data support the interpretation that CD36 is necessary for fat taste detection at physiological FA concentrations. This would be consistent with the finding that CD36-/- mice lose their fat preference4 and that downregulation of CD36 in circumvallate papillae eliminates fat preference in the absence of changes in GPR120.34 Together our observations suggest that GPR120, being poorly activated by LCFA concentrations, might act downstream of LCFA receptors such as CD36 (and possibly others) to amplify the response to high concentrations of tastants, including dietary FA. This interpretation would be consistent with GPR120 expression in a variety of taste cells responsive to sweet, bitter and umami stimuli.35 Interestingly, a recent study in humans showed that non-fatty acid agonists of GPR120 (such as GA in our study) do not elicit a taste response similar to that of LA despite the finding that they activate the glossopharyngeal nerve in mice.36 Thus, while CD36 would associate with oral LCFA recognition and “taste detection”, GPR120 might function in signal amplification for the “sustained sensing” of food.
Our data with both human and mice fungiform TBC show that acute exposure to LCFA decreases membrane lipid raft localization of CD36 while it increases that of GPR120. This effect is greatly exaggerated by consumption of a HFD, which also decreases total CD36, probably reflecting the effect of chronic exposure to excess FA. Localization in membrane rafts as compared to non raft detergent-soluble domains would modulate IP3 release and Ca2+ signaling,21,22 events integral to TBC CD36 and GPR120 function. In addition to modulating CD36 membrane localization, FA exposure would regulate CD36 internalization and ubiquitination30 possibly involving the activation of CD36 associated src kinases.37
Our data indicate that HFD by resulting in low CD36 expression and distribution to lipid rafts diminishes LCFA-induced Ca2+ signaling and serotonin release. The released serotonin in mice TBC has been shown to act downstream of Ca2+ signaling to transmit the fat taste response to afferent nerve fibers.5,6 Thus diminished release of this neurotransmitter toward synaptic clefts would contribute to the reduced oral sensitivity to FA in obesity. Consistent with this, subjects with attenuated expression of CD36, as a result of genetic variants, exhibit decreased sensitivity for oro-gustatory perception of dietary lipids.38
How the HFD induced changes in CD36 and GPR120 level and signaling might influence the etiology or progression of obesity remains to be determined. Stewart et al39 have reported that the ability to detect oleic acid both orally and within the gastrointestinal tract is compromised in obese men suggesting that the decreased sensitivity might promote more fat intake to reach the same taste perception. However, direct proof for this is lacking and awaits further investigation.
In contrast to its effect on CD36, the HFD increased raft localization of GPR120, which might constitute a compensatory event serving to enhance signal amplification as shown for other GPCR proteins.40 This amplification could involve at least in part the enhanced release of GLP-1 shown in obese mice. GLP-1 receptor knockout mice have reduced taste responses to sweeteners18 and dietary fat34 in behavioral tests. GLP-1 signaling is involved in normal diurnal downregulation of CD36 by dietary fat in the mouse circumvallate papillae,34 contributing to progressive reduction of fat taste sensitivity during a meal and possibly modifying feeding behavior. 34
In summary our data provided new information on the respective signaling functions of CD36 and GPR120 in oro-gustatory fat perception. It would be of interest to extend the studies to the highly innervated circumvallate papillae. Our data documented the mechanistic changes underlying the hyposensitivity to lipid taste observed in obesity. They should guide future studies into the mechanisms responsible for the hedonic preference for fat that associates with high fat consumption that has been reported in obese subjects.41
Supplementary Material
Acknowledgments
Grant support: This work was supported by grants from the French Ministry of Higher Education and Research, Région Bourgogne and ANR SensoFat-2 (ANR-12-BSV1-0027-01), and by grants (DK033301 and DK060022) from the US National Institute of Health (NAA).
Abbreviations
- [Ca2+]i
intracellular calcium
- GPCRs
G protein-coupled receptors
- GA
grifolic acid
- GLP-1
glucagon-like pepetide-1
- GPR120
G protein coupled receptor 120
- GPR40
G protein coupled receptor 40
- HFD
High-fat diet
- IP3
inositol-tris-phosphate
- LA
Linoleic acid
- LCFA
long-chain fatty acid
- PTKs
Protein tyrosine kinases
- SOC
Store-operated Ca2+
- STIM1
stromal interaction molecule-1
- SSO
sulfo-N-succinimidyl oleate
- TBC
taste bud cells
Footnotes
Disclosure: The authors have nothing to disclose.
Author Contributions: Study concept and design (NAK, NAA, MHO); acquisition of data (SSub, SSun); analysis and interpretation of data (NAK, NAA, SSub, SSun); drafting of the manuscript (NAK, NAA, SSub); critical review (PB), statistical analysis (OS); material support (TH, YA), study supervision (NAK, NAA).
Authors' name shown in bold designate shared first authorship
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan NA, Besnard P. Oro-sensory perception of dietary lipids: New insights into the fat taste transduction. Biochim Biophys Acta. 2009;1791:149–155. doi: 10.1016/j.bbalip.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Mattes RD. Is There a Fatty Acid Taste? Annu Rev Nutr. 2009;29:305–327. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuwatari T, Kawada T, Tsuruta M, et al. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 1997;414:461–464. doi: 10.1016/s0014-5793(97)01055-7. [DOI] [PubMed] [Google Scholar]
- 4.Laugerette F, Passilly-Degrace P, Patris B, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Yassimi A, Hichami A, Besnard P, et al. Linoleic acid induces calcium signaling, SRC-kinase phosphorylation and neurotransmitters release in mouse CD36-positive gustatory cells. J Biol Chem. 2008;283:12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 6.Dramane G, Abdoul-Azize S, Hichami A, et al. STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. J Clin Invest. 2012;122:2267–2282. doi: 10.1172/JCI59953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartoni C, Yasumatsu K, Ohkuri T, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura S, Mizushige T, Yoneda T, et al. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28:49–55. doi: 10.2220/biomedres.28.49. [DOI] [PubMed] [Google Scholar]
- 9.Galindo MM, Voigt N, Stein J, et al. G protein-coupled receptors in human fat taste perception. Chem Senses. 2012;37:123–139. doi: 10.1093/chemse/bjr069. [DOI] [PubMed] [Google Scholar]
- 10.Abumrad NA. CD36 may determine our desire for dietary fats. J Clin Invest. 2005;115:2965–2967. doi: 10.1172/JCI26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdoul-Azize S, Selvakumar S, Sadou H, et al. Ca2+signaling in taste bud cells and spontaneous preference for fat: Unresolved roles of CD36 and GPR120. Biochimie. 2013 Jun 15; doi: 10.1016/j.biochi.2013.06.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Martin C, Passilly-Degrace P, Gaillard D, et al. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One. 2011;6:e24014. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozdener MH, Brand JG, Spielman AI, et al. Characterization of human fungiform papillae cells in culture. Chem Senses. 2011;36:601–612. doi: 10.1093/chemse/bjr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozdener MH, Rawson NE. Primary culture of mammalian taste epithelium. Methods Mol Biol. 2013;945:95–107. doi: 10.1007/978-1-62703-125-7_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozdener H, Spielman AI, Rawson NE. Isolation and culture of human fungiform taste papillae cells. J Vis Exp. 2012;(63):e3730. doi: 10.3791/3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara T, Hirasawa A, Sun Q, et al. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:247–255. doi: 10.1007/s00210-009-0425-9. [DOI] [PubMed] [Google Scholar]
- 17.Kuda O, Pietka TA, Demianova Z, et al. Sulfo-N-succinimidyl Oleate (SSO) Inhibits Fatty Acid Uptake and Signaling for Intracellular Calcium via Binding CD36 Lysine 164. SSO also Inhibits oxLDL Uptake by Macrophages. J Biol Chem. 2013;288:15547–15555. doi: 10.1074/jbc.M113.473298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin YK, Martin B, Golden E, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith J, Su X, El-Maghrabi R, et al. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J Biol Chem. 2008;283:13578–13585. doi: 10.1074/jbc.M800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pohl J, Ring A, Korkmaz U, et al. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell. 2005;16:24–31. doi: 10.1091/mbc.E04-07-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tovey SC, Taylor CW. Cyclic AMP directs inositol (1,4,5)-trisphosphate evoked Ca2+ signalling to different intracellular Ca2+ stores. J Cell Sci. 2013;126:2305–2313. doi: 10.1242/jcs.126144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao Y, Plummer NW, George MD, et al. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci. 2009;106:3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaillard D, Laugerette F, Darcel N, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 24.Park YM, Drazba JA, Vasanji A, et al. Oxidized LDL/CD36 interaction induces loss of cell polarity and inhibits macrophage locomotion. Mol Biol Cell. 2012;23:3057–3068. doi: 10.1091/mbc.E11-12-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao F, Shin HS, Rhee SG. In vitro tyrosine phosphorylation of PLC-gamma 1 and PLC-gamma 2 by src-family protein tyrosine kinases. Biochem Biophys Res Commun. 1993;191:1028–1033. doi: 10.1006/bbrc.1993.1320. [DOI] [PubMed] [Google Scholar]
- 26.Chevrot M, Bernard A, Ancel D, et al. Obesity alters the gustatory perception of lipids in the mouse: plausible involvement of lingual CD36. J Lipid Res. 2013;54:2485–2494. doi: 10.1194/jlr.M039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran TT, Poirier H, Clément L, et al. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem. 2011;286:25201–25210. doi: 10.1074/jbc.M111.233551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnett-Norris J, Lynch D, Reggio PH. Lipids, lipid rafts and caveolae: their importance for GPCR signaling and their centrality to the endocannabinoid system. Life Sci. 2005;77:1625–1639. doi: 10.1016/j.lfs.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Llegems E, Iwatsuki K, Kokrashvili Z, et al. REEP2 enhances sweet receptor function by recruitment to lipid rafts. J Neurosci. 2010;30:13774–13783. doi: 10.1523/JNEUROSCI.0091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrlich D, Humpel C. Effects of ethanol on aggregation, serotonin release, and amyloid precursor protein processing in rat and human platelets. Platelets. 2013 Feb 12; doi: 10.3109/09537104.2013.764979. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith NJ. Low Affinity GPCRs for Metabolic Intermediates: Challenges for Pharmacologists. Front Endocrinol. 2012;3:1. doi: 10.3389/fendo.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundaresan S, Shahid R, Riehl TE, et al. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 2013;27:1191–1202. doi: 10.1096/fj.12-217703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin C, Passilly-Degrace P, Chevrot M, et al. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J Lipid Res. 2012;53:2256–2265. doi: 10.1194/jlr.M025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damak S, Le-Coutre J, Bezencon C, et al. Fat taste receptors and their methods of use. International application published under the patent cooperation treaty. WO2007/014824 A1 2007
- 36.Godinot N, Yasumatsu K, Barcos ME, et al. Activation of tongue-expressed GPR40 and GPR120 by non caloric agonists is not sufficient to drive preference in mice. Neuroscience. 2013;250:20–30. doi: 10.1016/j.neuroscience.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 37.Heit B, Kim H, Cosío G, et al. Multimolecular signaling complexes enable Syk-mediated signaling of CD36 internalization. Dev Cell. 2013;24:372–383. doi: 10.1016/j.devcel.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepino MY, Love-Gregory L, Klein S, et al. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 2012;53:561–566. doi: 10.1194/jlr.M021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart JE, Seimon RV, Otto B, et al. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr. 2011;93:703–711. doi: 10.3945/ajcn.110.007583. [DOI] [PubMed] [Google Scholar]
- 40.Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart JE, Feinle-Bisset C, Golding M, et al. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104:145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


