Abstract
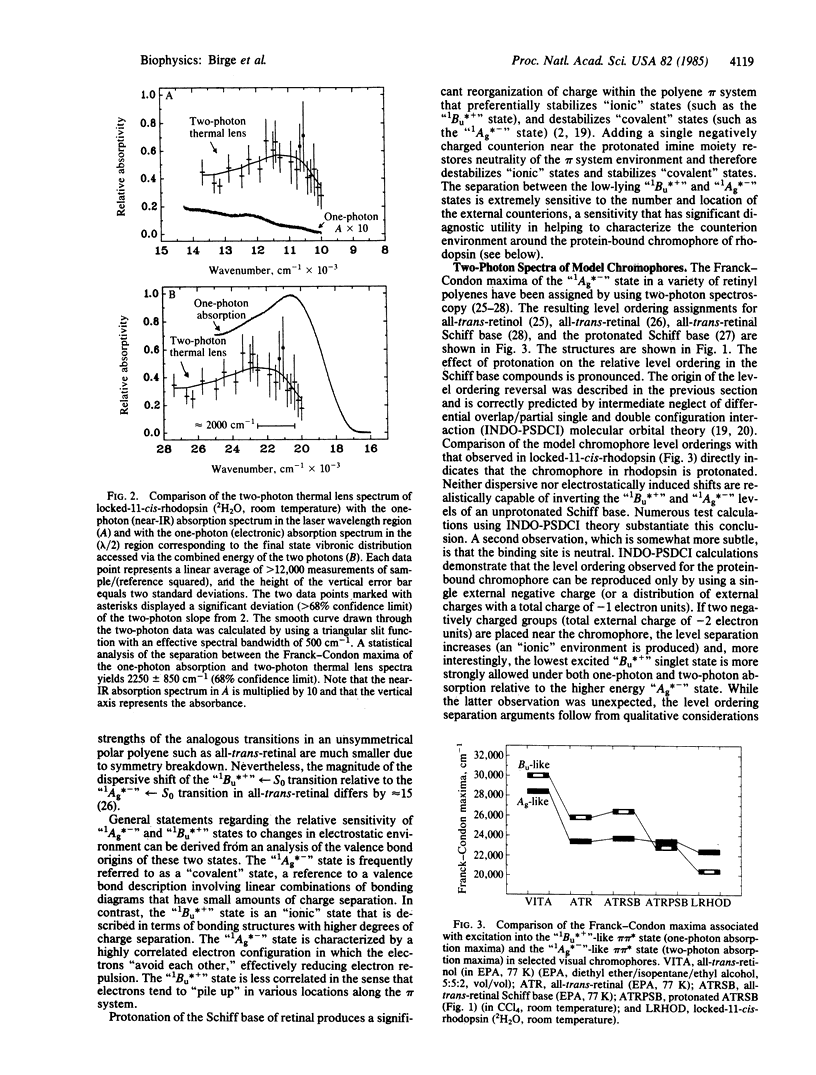
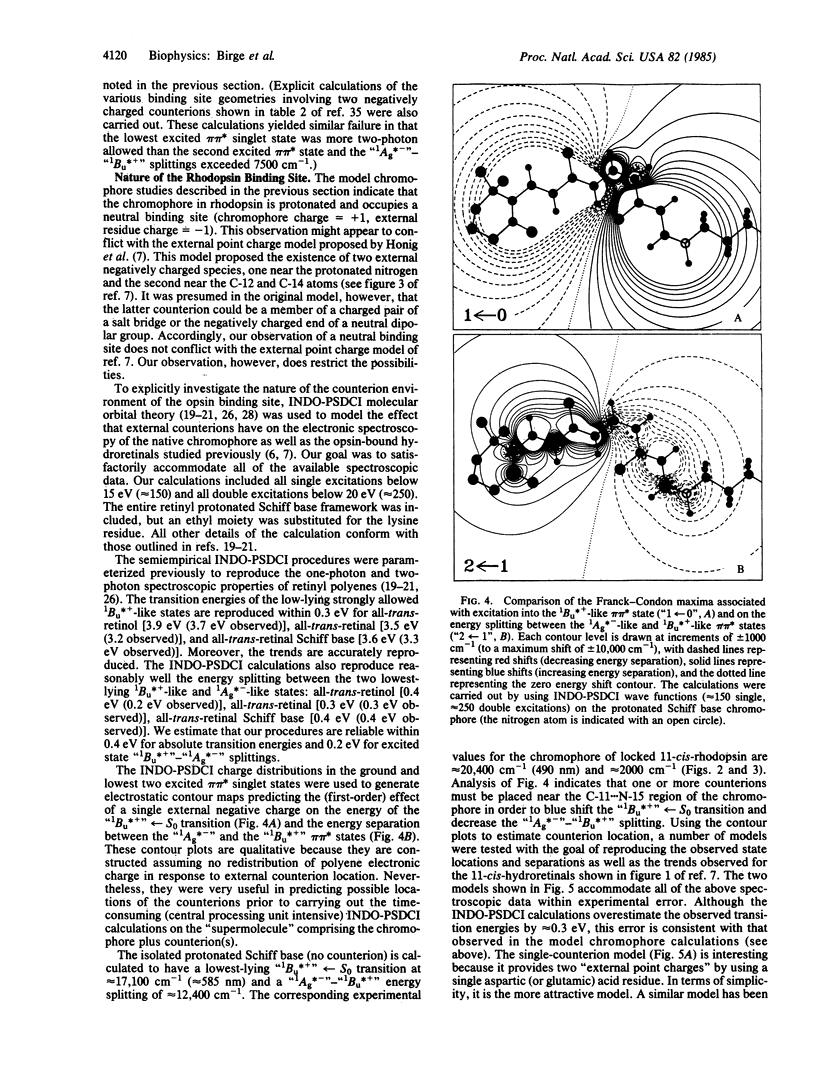
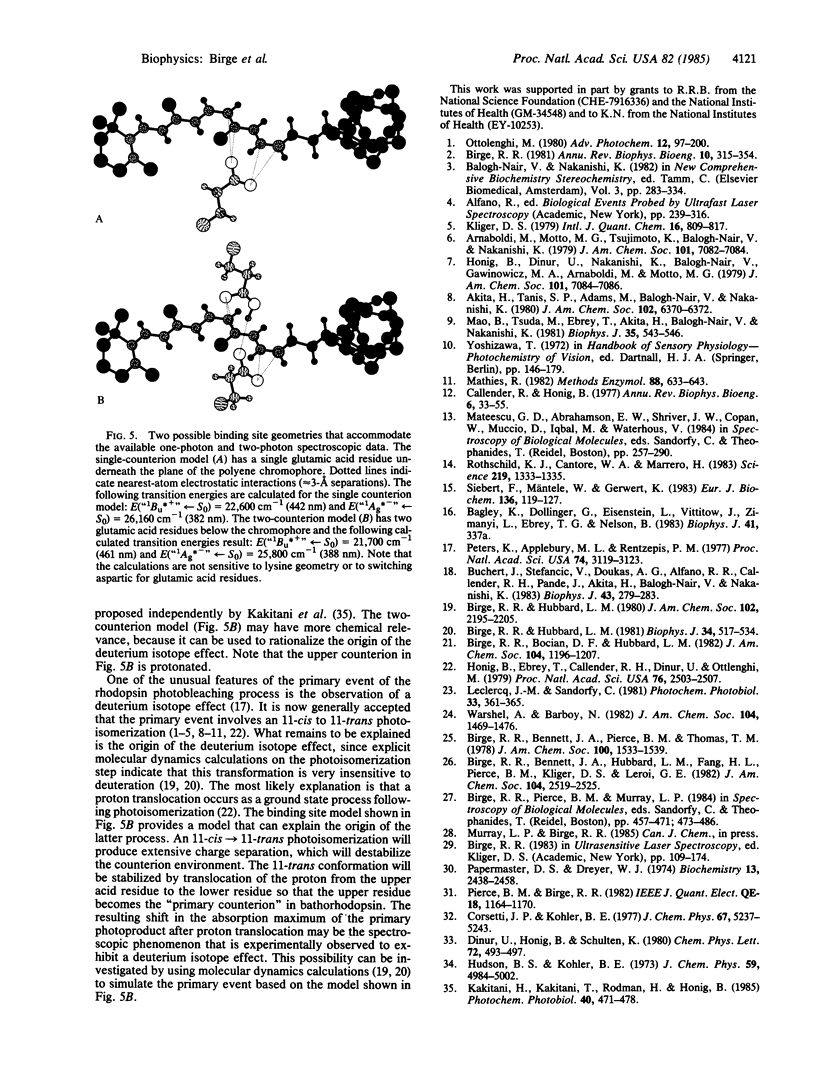
We studied the nature of the protein binding site of rhodopsin, using two-photon spectroscopy to assign the location of the low-lying "covalent" 1Ag*- -like pi pi * state in a model rhodopsin containing a locked-11-cis chromophore. The two-photon thermal lens maximum is observed at 22,800 cm-1, approximately equal to 2000 cm-1 above the one-photon absorption maximum, indicating that the protein environment has induced a level ordering reversal of the low-lying pi pi * states relative to that observed in retinyl Schiff bases in solution. The spectroscopic results clearly indicate that the chromophore is protonated and that the binding site is uncharged. Electrostatic energy contour maps of the binding site are calculated, showing possible locations for the external counterion(s). Two models of the binding site are proposed that accommodate the available spectroscopic data. One model involves a protonated Schiff base chromophore stabilized by a single negatively charged aspartic or glutamic acid residue. A more complicated model involving two residues (one charged, the other neutral) is also proposed. The latter model is interesting because it also accommodates the observed deuterium isotope effect in the form of a proton translocation between the two residues. The translocation is assumed to be a ground state process, initiated subsequent to the photoisomerization of the chromophore and energetically driven via destabilization of the counterion environment as a result of isomerization-induced charge separation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birge R. R., Hubbard L. M. Molecular dynamics of trans-cis isomerization in bathorhodopsin. Biophys J. 1981 Jun;34(3):517–534. doi: 10.1016/S0006-3495(81)84865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R. Photophysics of light transduction in rhodopsin and bacteriorhodopsin. Annu Rev Biophys Bioeng. 1981;10:315–354. doi: 10.1146/annurev.bb.10.060181.001531. [DOI] [PubMed] [Google Scholar]
- Buchert J., Stefancic V., Doukas A. G., Alfano R. R., Callender R. H., Pande J., Akita H., Balogh-Nair V., Nakanishi K. Picosecond kinetic absorption and fluorescence studies of bovine rhodopsin with a fixed 11-ene. Biophys J. 1983 Sep;43(3):279–283. doi: 10.1016/S0006-3495(83)84351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callender R. Resonance Raman studies of visual pigments. Annu Rev Biophys Bioeng. 1977;6:33–55. doi: 10.1146/annurev.bb.06.060177.000341. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakitani H., Kakitani T., Rodman H., Honig B. On the mechanism of wavelength regulation in visual pigments. Photochem Photobiol. 1985 Apr;41(4):471–479. doi: 10.1111/j.1751-1097.1985.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Leclercq J. M., Sandorfy C. On the possibility of protein-chromophore charge transfer in visual pigments. Photochem Photobiol. 1981 Mar;33(3):361–365. doi: 10.1111/j.1751-1097.1981.tb05430.x. [DOI] [PubMed] [Google Scholar]
- Mao B., Tsuda M., Ebrey T. G., Akita H., Balogh-Nair V., Nakanishi K. Flash photolysis and low temperature photochemistry of bovine rhodopsin with a fixed 11-ene. Biophys J. 1981 Aug;35(2):543–546. doi: 10.1016/S0006-3495(81)84809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Cantore W. A., Marrero H. Fourier transform infrared difference spectra of intermediates in rhodopsin bleaching. Science. 1983 Mar 18;219(4590):1333–1335. doi: 10.1126/science.6828860. [DOI] [PubMed] [Google Scholar]
- Siebert F., Mäntele W., Gerwert K. Fourier-transform infrared spectroscopy applied to rhodopsin. The problem of the protonation state of the retinylidene Schiff base re-investigated. Eur J Biochem. 1983 Oct 17;136(1):119–127. doi: 10.1111/j.1432-1033.1983.tb07714.x. [DOI] [PubMed] [Google Scholar]



