Summary
Testicular germ-cell tumours (TGCTs) are the most common cancer in young men; the incidence is increasing worldwide and they have an unusually high rate of metastasis. Despite significant work on TGCTs and their metastases in humans, absence of a mouse model of spontaneous metastasis has greatly limited our understanding of the mechanisms by which metastatic potential is acquired and on their modes of dissemination. We report a new model of spontaneous TGCT metastasis in the 129 family of mice and provide evidence that these are true metastases derived directly from primary testicular cancers rather than independently from ectopic stem cells. These putative metastases (pMETs) occur at similar frequencies among TGCT-affected males in six genetically distinct TGCT-susceptible strains and were largely found in anatomical sites that are consistent with patterns of TGCT metastasis in humans. Various lines of evidence support their pluripotency and germ-cell origin, including presence of multiple endodermal, mesodermal and ectodermal derivatives as well as cells showing OCT4 and SSEA-1 pluripotency markers. In addition, pMETs were never found in males that did not have a TGCT, suggesting that metastases are derived from primary tumours. Finally, pMETS and primary TGCTs shared several DNA copy number variants suggesting a common cellular and developmental origin. Together, these results provide the first evidence for spontaneous TGCT metastasis in mice and show that these metastases originate from primary TGCTs rather than independently from ectopic stem cells.
Keywords: metastasis, mouse model, testicular cancer
Introduction
Testicular germ-cell tumours (TGCTs) are the most common cancer affecting young men between the ages of 15 and 40 years. Although representing only 1.0–1.5% of all cancers (Akbarian et al., 1995), incidence has increased steadily in the last 30 years (Bosl & Motzer, 1997) and currently TGCTs account for nearly 60% of all cancers in men aged 20–40 years (Di et al., 2005). Although the cure rate is high, presence of metastases reduces survival rates. Interestingly, TGCTs have a disproportionately high number of metastases compared with other tumours, averaging 5.8 metastases per primary tumour (Anant & Davidson, 2001; De Giorgi et al., 2008). Approximately 30% of TGCT patients have metastases at the time of diagnosis (Powles et al., 2005), and 15–20% of patients have subclinical metastases in stage 1 seminoma (Benne et al., 1986), which is the most common presentation (Rodriguez et al., 1992).
Metastasis is an important factor in TGCTs, directly affecting treatment modality, tumour surveillance, and survival. Treatment involves surgery and chemotherapy with BEP (bleomycin, etoposide and cisplatin), which together carries a mortality risk of 2.3–4.5% (Fossa et al., 1998; Williams et al., 1987). Resistance to cisplatin occurs in 20% of patients with metastatic disease, further complicating treatment and worsening prognosis (Piulats et al., 2009). After treatment, patients presenting with metastatic disease have a higher incidence of relapse (Holzik et al., 2008) and lower survival rate (Anant & Davidson, 2001).
Testicular cancer metastases target various tissues, including lymph nodes, lung, liver and spleen (De Giorgi et al., 2008). Differentiation of TGCT metastases leads to morphological diversity, which makes it difficult to distinguish between metastases of primary germ-cell tumours and unrelated secondary cancers (Ulbright, 1999). The OCT4 pluripotency marker is present in 100% of tested primary and metastatic seminomas and embryo-nal carcinomas (Cheng, 2004; Jones et al., 2004; Looijenga et al., 2003) and has become an important diagnostic marker for both primary germ-cell tumours and their metastases (Cheng et al., 2007). OCT4 is a transcription factor associated with the maintenance of pluripotency and is expressed in embryonic cells, germ cells and embryonal carcinoma (EC) cells (Okamoto et al., 1990; Rosner et al., 1990). OCT4 expression is dramatically reduced as both primary tumours and their metastases differentiate (Niwa et al., 2000) and may be absent in cells that possess an EC phenotype (Mueller et al., 2010).
Most mouse models of spontaneous TGCTs are limited to a single genetic background, the 129 family of inbred strains (Stevens & Hummel, 1957), with 5–10% of males affected with a TGCT by 3–4 weeks of age (Maris et al., 2005). Several mutations modify this frequency (Di et al., 2005; Heaney & Nadeau, 2008; Di Cristofano et al., 1998; Kimura et al., 2003). For example, the Ter mutation in the Dead-end1 gene increases the incidence of TGCTs to over 94% in Ter/Ter homozygotes (Clark et al., 2004; Matin et al., 1999). In addition, the 129.MOLF-Chr19 chromosome substitution strain, which has chromosome 19 from the MOLF/Ei inbred strain substituted onto the 129/Sv background, has 82–86% of its males affected with a TGCT (Matin et al., 1999).
Developmental features of TGCT stem cells have led to alternative hypotheses about whether extragonadal germ-cell tumours are true metastases of TGCTs in the testis, or whether they originate independently from ectopic stem cells (Fabre et al., 2004; Fine et al., 1962; Hailemariam et al., 1997; Johnson et al., 1973). Primordial germ cells (PGCs), which are the stem cell of many TGCTs, arise early in development, migrate from the base of the allantois to the urogenital ridge, where fetal gonads subsequently develop (Molyneaux et al., 2001; Upadhyay & Zamboni, 1982). In the 129 inbred strains, PGCs in the fetal gonad transform to EC cells and during the next several weeks develop into TGCTs (Stevens, 1962a, 1967). In some cases, PGCs migrate to ectopic locations instead of the urogenital ridge (Anderson et al., 2000; MacLean et al., 2007; Runyan et al., 2006; Upadhyay & Zamboni, 1982; Zamboni & Upadhyay, 1983) and these cells have been proposed to be the origin for extragonadal germ-cell tumours (Dixon & Moore, 1953; Fine et al., 1962; Hail-emariam et al., 1997). Absence of metastasis in these mouse models has until now precluded tests of these alternative hypotheses about the origins of metastasis and extragonadal germ-cell tumours.
During routine necropsies, we discovered putative metastases (pMETs) in several TGCT susceptible mouse strains. In the literature, only a single instance of a spontaneous mouse TGCT pMET has been described, from a large survey of over 7700 129-TerSv males affected with a primary TGCTs (Stevens, 1973); investigation into that pMET was limited to histological analysis. We therefore undertook a systematic study to estimate the rate and anatomical distribution of pMETs in these strains, to test whether they have the same germ-cell origin as TGCTs (Stevens, 1962b, 1967) and if they were true metastases from a primary TGCT in the testis, or alternatively from germ cells that migrated to ectopic locations where they then transformed to extragonadal germ-cell tumours.
Materials and methods
Mice
129S1/SvImJ (JR002448, previously known as 129/SvImJ) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). 129T1/Sv (known previously as 129T1/Sv-p+ Tyrc-ch Dnd1Ter/J) and 129-Chr 19MOLF CSS (CSS-M19, described previously, Matin et al., 1999) were obtained from our research colony. Mice were maintained in the Case Western Reserve University Animal Resource Center on a 12 : 12-h light : dark cycle and fed Lab Diet 5010 (LabDiet, Richmond, IN, USA). All protocols were approved by the Institutional Animal Care and Use Committee.
Genotyping
DNA for PCR genotyping was extracted from tail tissue. The nucleotide substitution that led to the Ter mutation created a Dde1 site that we used for genotyping (Matsumoto et al., 2006).
Tumour surveys
Male mice between the ages of 3 and 15 months were surveyed for TGCTs and pMETs. pMETs were identified macroscopically and confirmed histologically. Tumour incidence was calculated as the percentage of males with either a unilateral or bilateral TGCT; metastasis incidence was calculated both as the percentage of TGCT-affected males with at least one metastasis, and as the percentage of all males with at least one pMET (not shown).
Tissue processing
Testes and macroscopic pMETs were fixed with 10% formalin for at least 48 h. Tissues were then paraffin embedded and sectioned (5 μm) at the Case Comprehensive Cancer Center's Tissue Procurement and Histology Core facility (TPHC). Haematoxylin and eosin staining was performed in the TPHC facility.
Immunohistochemistry
Paraffin sections (5 μm) were rehydrated and blocked with dilution buffer (1X PBS, 3% BSA, 0.1% NaN3, 0.3% Triton X-100), unconjugated goat anti-mouse IgM (Jackson Immunolabs, West Grove, PA, USA) diluted 1 : 1000 and 5% normal goat serum for 1 h at room temperature, then rinsed in PBS. Sections were incubated with rabbit anti-OCT4 (ab19857; Abcam, Cambridge, MA, USA) antibody diluted 1 : 250 and mouse IgM anti-SSEA1 (ab16285; Abcam) diluted 1 : 50 in dilution buffer with 5% normal goat serum overnight at 4 °C. Sections were washed in PBS, and incubated with DyLight 488 anti-mouse IgM and DyLight 594 anti-rabbit IgG (115-486-020 and 711-516-152, respectively, Jackson Immunoresearch) both 1 : 500 in dilution buffer with 5% normal goat serum for 2 h at room temperature. Nuclei were counterstained with DAPI and sections were visualized using a Leica TCS SP2 AOBS filter-free UV/spectral confocal microscope (Leica Microsystems Inc., Bannockburn, IL, USA).
Array-based CGH
DNA was isolated from normal spleen, primary TGCTs and pMETs using Qiagen DNeasy Blood and Tissue kits (Valencia, CA, USA). In cases where primary TGCTs and pMETs were found in conjunction with normal tissue, tumours were visually dissected to minimize the contribution of normal cells to the tumour sample. Samples were sent to Empire Genomics (Buffalo, NY, USA) where hybridizations were performed with the Agilent 244k mouse aCGH array. CGH Microarray images were processed using Feature Extraction Software (v10.5.1.1) (Agilent Technologies, Palo Alto, CA, USA). DNA gains and losses were identified by importing the Feature Extraction-generated CGH.txt files into DNA Analytics (v4.0.76) (Agilent Technologies), and applying the ADM-1 Aberration Algorithm tool with parameters set at: Threshold range 11.0–23.9, Centralization ON, Bin Size 10 and Centralization Threshold 6.0. Regions of significant gain or loss were annotated to the corresponding chromosomes, cytobands and genes using genome build NCBI36/mm8.
Real-time PCR
Putative metastases were selected for validation with real-time PCR based on the number of DNA copy number changes identified within the pMET and the corresponding primary TGCT. Regions of DNA copy number change were used for real-time PCR validation if three or more aCGH probes were targeted to the area and if there was at least one gene in the segment. Genes used for DNA copy number validation were chosen based on their central location within the altered genomic region. Primer sequences are given in supplemental Table S1. Copy number changes were quantified with the Chromo4 real-time PCR system (MJ Research, Watertown, MA, USA) and the PerfeCTa SYBR Green Supermix kit (VWR Scientific, Radnor, PA, USA) using manufacturer-suggested protocols. Copy numbers were normalized to the POMC gene (Hill-Baskin et al., 2009); normal spleen DNA was used to generate standard curves for each primer set. Cut-off values of 1.5 and below were used to define a copy number value of 1, and values of 2.5 and above were used to define copy number values of 3 and above. As described in previous studies, changes in genomic DNA were considered significant when the copy number (n) was below 1.5 or above 2.5 (Braude et al., 2006; Yang et al., 2010).
Statistical analysis
Chi-square contingency tests were used to test for significant differences in TGCT and metastatic rates between strains. Spearman's correlation test was used to estimate the correlation between the incidence of primary TGCTs and pMETs among strains.
Results
Frequency of males affected with a primary TGCT
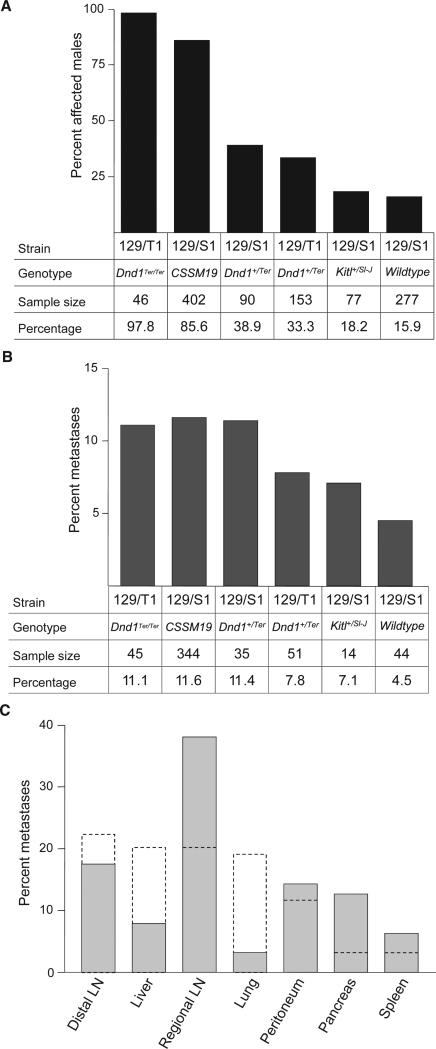
We examined a total of 1045 males, ranging in age from 3 to 15 months, from six genetically distinct members of the 129 family of inbred strains. These six groups included the 129S1/SvImJ inbred strain, Dnd1Ter/+ heterozygotes on two backgrounds (129S1 and 129T1), Dnd1Ter/Ter homozygotes on the 129T1 background, KitlSl-J/+ heterozygotes on the 129S1 background, and the 129-Chr19MOLF Chromosome Substitution Strain (CSS). As expected, the frequency of affected males varied widely (Fig. 1a), with rates that were consistent with previous reports (Lam et al., 2004; Matin et al., 1999; Noguchi & Stevens, 1982; Stevens & Hummel, 1957; Youngren et al., 2005).
Figure 1.
Incidence of TGCTs and pMETs among six TGCT-susceptible strains. (A) Males affected with a primary TGCT. (B) pMETs among males with a TGCT. (C) Anatomical distribution of pMETs in mice and humans.
Frequency of males with a pMET
We found pMETs in each of the six strains surveyed, with the frequency of markers with at least one pMET in TGCT-affected males ranging from 4.5% in the 129/S1 strain to 11.6% in the CSSM19 strain (Fig. 1b). Occur-rence of pMETs did not vary significantly among these strains (χ2 = 2.7, p = 0.7), and there was no correlation between the primary TGCT rate and the metastatic rate (Spearman's r = 0.7, p = 0.23), suggesting that the number of pMETs found in each strain depended only on the absolute number of primary TGCTs found within that strain, and not on the primary TGCT rate in each strain or on the particular genetic attributes of any of the six strains.
Do TGCTs and pMETs arise independently?
An important question concerns whether primary TGCTs and pMETs have independent origins. In particular, if pMETs are derived from primary TGCTs, which would be the case if they are metastases, then pMETs should be found only in males that also have a TGCT. In contrast, independent occurrence would be consistent with separate origins. We tested the hypothesis of independent origins by examining occurrence (numbers) of males with a TGCT or a pMET. Interestingly, pMETs were found only in males that also have a TGCT (χ2 = 61.6, p < 0.0001), suggesting that pMETs are derived from the primary tumour (Table 1).
Table 1.
Test for independent occurrence of TGCTs and pMETs
| No pMET | pMET present | Total | |
|---|---|---|---|
| Primary TGCT present | 477 | 56 | 533 |
| No primary TGCT | 556 | 0 | 556 |
| Total | 1033 | 56 | 1089 |
Distribution of metastases
We categorized pMETs (n = 63) from our surveys according to secondary tissue sites (Fig. 1c). Regional lymph nodes (renal, inguinal and lumbar lymph nodes) accounted for 38.1% of the sites. Other sub-diaphragmatic sites were peritoneal masses (14.3%), pancreas (12.7%), liver (7.9%) and spleen (6.3%). Super-diaphragmatic metastases in distal lymph nodes (cervical, mediastinal and axillary/brachial) accounted for 17.5% and lung (3.2%) of all metastases. Interestingly, pMETs were not found in adrenal glands or kidneys, which are common sites for metastasis in humans. The wide anatomical distribution of TGCT metastases in our survey is consistent with data for humans (Disibio & French, 2008), suggesting that the source cells for mouse and human TGCT metastases have common biological origins and properties.
Histological composition
To determine whether these pMETs contained cell and tissue types derived from multiple germ layers, which is characteristic of TGCTs and their metastases, we examined their composition histologically. Consistent with the hypothesis that these pMETs originate from germ cells, many contained cells and tissues from multiple germ layers. For example, glandular structures were found in a mediastinal lymph node pMET mixed with less differentiated cells and lined with malignant cells (Fig. 2a,b). A pMET found in the lung showed both epithelioid and papillary cells (Fig. 2c). Although these papillary structures can arise from either ectodermal or endodermal cells, the structures in this pMET suggest an endodermal origin. Epithelioid cells and undifferentiated malignant cells are also found in the pMET from a diaphragmatic nodule (Fig. 2d). Other pMETs display tissues consistent with a mesodermal origin (bone and cartilage, Supplemental Figure S1). Overall, the composition and histological phenotype of these pMETs are consistent with a pluripotent germ-cell origin.
Figure 2.
Histology of pMETs. (A) Lymph node. At low power, much of the node is effaced with a neoplasm composed of glandular structures intermingled with sheets of poorly differentiated malignant cells. (B) High power view of a portion of the lymph node shown in (A). The glands are irregular in shape and size, and are lined with cytologically malignant cells. A mitotic figure is noted in one of the lining cells. (C) Lung involved with metastatic malignant neoplasm, part of which is composed of densely packed epithelioid cells, and part of which shows papillary architecture. The inset shows the neoplasm at high power. The malignant cells have an appearance suggestive of closely packed papillary structures. (D) Section from a diaphragmatic nodule that consists of undifferentiated cells. The larger cells with more abundant cytoplasm (left) have an epithelioid appearance. A large part of the neoplasm (right) consists of small dark immature-appearing cells that are remarkably proliferative. The arrows indicate abundant mitotic figures.
Immunohistochemistry
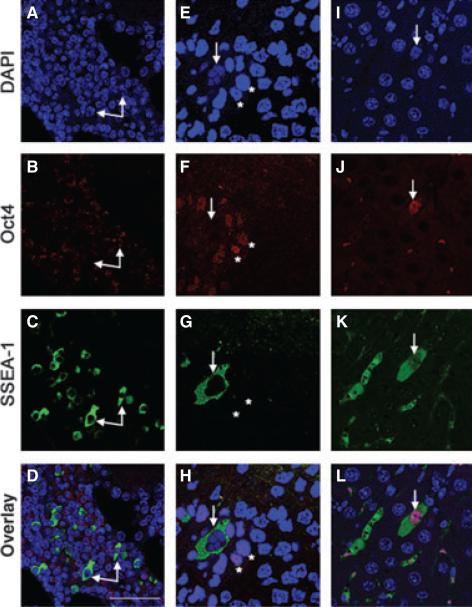
To test the germ-cell origin hypothesis for pMETs more rigorously, immunohistochemistry was used to examine OCT4 expression. In humans, OCT4 positive cells in metastases are diagnostic for germ-cell tumours that exhibit pluripotency (Cheng, 2004; Cheng et al., 2007; Looijenga et al., 2003). As a secondary marker for pluripotent cells, we used SSEA-1, which is a specific marker for EC cells, embryonic stem (ES) cells and PGCs in the mouse (Fenderson et al., 1987).
We evaluated pMETs from three different target tissues (lymph node, peritoneum and pancreas) for the presence of OCT4 and SSEA-1 (Fig. 3). In keeping with its role as a transcription factor, OCT4 is normally found in nuclei (Rosner et al., 1990), whereas antibodies to SSEA-1 react with a lactoseries oligosaccharide antigen on the cell surface (Solter & Knowles, 1978). In this analysis, a small number of cells in a pMET from a diaphragmatic nodule were positive for nuclear OCT4 staining in the absence of SSEA-1 (Fig. 3f and h, asterisks), pMETs from a mediastinal lymph node and the diaphragmatic nodule showed discrete cells that stained positively for SSEA-1 (Fig. 3c and g respectively, arrows) without OCT4 (Fig. 3d and h respectively, arrows), and a minority of cells from a pancreatic pMET stained positively for both (Fig. 3l, arrow). Normal lymph nodes were negative for both OCT4 and SSEA-1 staining (data not shown).
Figure 3.
Immunohistochemistry of OCT4 and SSEA-1 in pMETs. OCT4 positive cells were present in F (asterisks) and J (arrow), but absent in (B). SSEA-1 positive cells (arrows) were observed in each metastasis (C, G, and K) and co-localized with OCT4 in one sample (L, arrow). Nuclei were stained with DAPI (A, E and I). Scale bar: 44 μm.
DNA copy number changes in metastases and primary TGCTs
Finally, we sought genetic evidence that pMETs are derived from TGCTs. This evidence was based on tests for shared genomic alterations between pairs of pMETs and primary TGCTs from individual mice. We expected that pMETs that have a primary TGCT origin should share specific genomic alterations with primary tumour cells in testes from the same mouse. Array-CGH (comparative genomic hybridization) was used to identify DNA copy number changes in metastases, with genomic DNA sequence from the same strain as the wild-type reference. We then used real-time PCR to verify these changes in pMETs as well as test for the same changes in the corresponding primary TGCTs. Although numerous small gains and losses were found in all pMETs compared with normal mouse DNA, no DNA copy number changes were common to all metastases (Table 2). Whenever possible, we examined pMETs from males affected with bilateral TGCTs because DNA copy number changes in metastases originating from a primary TGCT should match changes found in one, but not both, of the primary tumours.
Table 2.
DNA copy number variation in pMETs and corresponding primary TGCTs. Values are given as copy number where n = 2 is normal diploid. Probed genes were chosen based on their central location in the altered DNA segment identified with aCGH. Highlighted values represent shared changes between pMETs and primary TGCTs. pMETs in Samples 1 and 2 originated from males with bilateral primary TGCTs, Sample 3 originated from a male with a fused bilateral left and right TGCT (a), and Sample 4 is from a male with a unilateral left TGCT
| Sample | Strain and metastasis | Gene (Chr) | Left TGCT | Right TGCT | Metastasis |
|---|---|---|---|---|---|
| 1 | CSSM19 | Map2k2 (Chr10) | 2.44 | 2.3 | 1.04 |
| Peritoneal mass | Olfr77 (Chr9) | 2.42 | 2.28 | 1.14 | |
| D230025D16Rik (Chr8) | 2.14 | 1.24 | 1.42 | ||
| Igf1r (Chr7) | 2.28 | 1.38 | 0.84 | ||
| Tmem48 (Chr4) | 1.84 | 1.36 | 1.42 | ||
| 2 | CSSM19 | Xiap (ChrX) | 2.04 | 1.16 | 1.62 |
| Pancreatic mass | Map2k2 (Chr10) | 2.52 | 2.74 | 1.54 | |
| Nudt3 (Chr17) | 2.94 | 2.46 | 2.56 | ||
| Adam6b (Chr12) | 3.86 | 2.4 | 3.58 | ||
| Ywhag (Chr5) | 3.24 | 4.1 | 3.1 | ||
| Pou2f2 (Chr7) | 2.46 | 3.56 | 2.26 | ||
| 3 | CSSM19 Lumbar lymph node | Slc34a2 (Chr5) | 0.97a | 1.14 | |
| 4 | 129/SvDnd1+/Ter Peritoneal mass | Skint3 (Chr4) | 6.62 | n/a | 0.4 |
We found consistent evidence with derivation of pMETs from primary TGCTs (Table 2). For example, the peritoneal mass in the CSS-M19 mouse shared 3 of 5 DNA changes (Chrs. 4, 7 and 8) with the right primary TGCT, all of which were deletions (copy number <1.5), which is consistent with derivation of the pMET from the right primary TGCT. The remaining two DNA copy losses, on Chrs. 9 and 10, were found in the pMET, but not in either the right or the left primary TGCT from the same mouse. With the exception of those changes identified in the pMET and the right primary TGCT, the probed genes did not show other detectable copy number variation.
The changes identified in Sample 2 that passed our cut-off threshold were all duplications (copy number >2.5). These DNA changes were most closely shared with the changes found in the left primary TGCT. In particular, the changes on chromosomes 5 and 12 in the pMET were most similar to changes found in the left TGCT because the right TGCT duplications on chromosome 5 were larger, and no copy number changes were found on chromosome 12. The change on chromosome 17 was found in both the pMET and the left TGCT, although a similar change in the right TGCT approached the cut-off threshold. Unlike Sample 1, Sample 2 showed changes in the primary right TGCT that were not found in the contra-lateral TGCT or the pMET itself (Chrs. 7, 10 and X).
Only one DNA copy number change was observed in the pMETs in Samples 3 and 4. Although the bilateral primary TGCTs from the source male in Sample 3 were fused (a rare occurrence in cases of bilateral TGCTs), the DNA copy number loss on chromosome 5 found in the pMET from Sample 3 was shared with the primary TGCT fusion. In contrast, the loss of genomic DNA on chromo-some 4 in Sample 4 found in the pMET was not shared with the unilateral left TGCT, but instead, the left primary TGCT showed a significant gain in this chromosomal region.
Discussion
Metastasis is a prevalent feature of human TGCTs, directly affecting treatment and survival. Although many TGCTs and their metastases respond readily to chemo-therapy, 10–25% of patients with metastases are resistant to treatment (Piulats et al., 2009) and 5–10% of patients relapse after initial treatment (Loehrer et al., 1998). Understanding the mechanisms of metastatic transformation and dissemination is therefore essential to reduce the morbidity and mortality associated with resistant and recurrent TGCT metastases.
Several lines of evidence point to a germ-cell origin for pMETs. pMETs are composed of cell and tissue types derived from all three germ lineages with ectodermal and endodermal derivatives predominating (Fig. 2). In addition, undifferentiated embryonal cells were evident histologically and confirmed with two markers of pluripotency, namely OCT4 and SSEA-1 (Fig. 3), and these cells are highly mitotic (Fig. 2d). These results are consistent with a seminoma or EC component in these metastases (Cheng, 2004; Jones et al., 2004). OCT4 is used to diagnose metastatic germ-cell tumours in humans (Cheng, 2004) and SSEA-1 is a marker for EC cells in mice (Solter & Knowles, 1978). Although OCT4 and SSEA-1 are both markers of EC cells in mice, co-localization of OCT4 and SSEA1 was uncommon in our sample of metastases. This finding of OCT4+/SSEA-1– and OCT4–)/SSEA-1+ cells is not completely unexpected, as EC cell variants lacking OCT4 and SSEA-1 have been described (Mueller et al., 2010; Rosenstraus, 1983).
Assays for DNA copy number changes strongly suggest that pMETs are derived directly from primary TGCTs in the testis rather independently from ectopic stem cells. Among the four pMETs analysed with CGH, seven significant DNA copy number changes were found, the majority of which were deletions (54%). Three of the four samples with validated changes showed shared genomic DNA changes between the primary TGCT and the pMET. Importantly, in the two samples from mice with bilateral primary TGCTs, shared DNA changes were found between the metastasis and one, but not both, of the primary tumours. Together, these results suggest that the pMETs are metastases that originate from a specific primary TGCT and do not represent extragonadal germ-cell tumours (EGCTs).
In humans, extragonadal germ-cell tumours (EGCTs) are rare, occurring in 3–5% of all germ-cell tumours (Richie & Steele, 2007). These tumours have been proposed to originate either from embryonic germ cells that fail to migrate towards the gonads (Dixon & Moore, 1953; Fine et al., 1962; Hailemariam et al., 1997), more primitive totipotent cells (Johnson et al., 1973), or a clinically undetected primary TGCT (Fabre et al., 2004). Recent case reports suggest that most retroperitoneal EGCTs can be considered to be silent or ‘burned out’ primary TGCTs (Fabre et al., 2004). Even in many cases where a primary diagnosis of an EGCT was made, further investigation found that a primary TGCT was present (Angulo et al., 2009). Our data suggest that this is also the case in the mouse model of TGCTs, because metastases were never observed in the absence of a primary TGCT, even in the 129/S1Kitl+/Sl-J line, which has a known PGC migration defect (Runyan et al., 2006).
Management of metastasis plays a pivotal role in treatment of TGCT patients. In addition to the initial effects of chemotherapy and surgical interventions, several late physical effects occur, including an increased risk of cardiovascular disease (Huddart et al., 2003), the development of metabolic syndrome (Huddart & Norman, 2003), secondary cancers (Travis et al., 2005) and reduced fertility (Brydoy et al., 2005). Identification of genetic changes that are associated with TGCT metastasis would enable targeted treatment for at-risk individuals. In our study, however, consistent genomic DNA changes were not found, even in the small number of metastases that were examined. The limited human aCGH data available for TGCT metastases have not, to date, revealed consistent genomic changes (Korkola et al., 2008). The diversity of incidental chromosomal changes in our metastases reflects the variety of changes found in the primary tumours, and is consistent with results from human aCGH surveys (Korkola et al., 2008; Skotheim et al., 2006). These results suggest that the changes driving metastasis may be unrelated to chromosomal rearrangements, and may instead result from epigenetic or miRNA alterations. Recent evidence suggests that DNA methylation patterns distinguish invasive from non-invasive urothelial cancers (Wolff et al., 2010), and that miRNAs play a role in breast cancer [reviewed in (Shi et al., 2010)]. The mouse model reported here may help identify the kinds of genetic and epigenetic changes that are associated with, and perhaps responsible for, TGCT metastasis.
Identification of a mouse model of spontaneous TGCT metastasis presents a unique opportunity to study the mechanisms involved in the acquisition of metastatic potential and the modes of dissemination. The fixed genetic background in this model helps to discriminate between driving and incidental changes in found in metastasis, including miRNA expression and epigenetic states. The nature of this model also allows for genetic manipulation and may serve as a basis for drug discovery in TGCT metastasis treatment. Finding the alterations that lead to metastasis may lead to the identification of metastatic signatures in human TGCTs and the development of treatment strategies based on metastatic risk.
Supplementary Material
Acknowledgements
We thank Sabine Schafer for helpful comments that improved the manuscript. NIH grants NCI CA75056 and NCRR RR12305 supported this work.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Akbarian S, Smith MA, Jones EG. Editing for an AMPA receptor subunit RNA in prefrontal cortex and striatum in Alzheimer's disease, Huntington's disease and schizophrenia. Brain Res. 1995;699:297–304. doi: 10.1016/0006-8993(95)00922-d. [DOI] [PubMed] [Google Scholar]
- Anant S, Davidson NO. Molecular mechanisms of apolipo-protein B mRNA editing. Curr Opin Lipidol. 2001;12:159–165. doi: 10.1097/00041433-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Anderson R, Copeland TK, Scholer H, Heasman J, Wylie C. The onset of germ cell migration in the mouse embryo. Mech Dev. 2000;91:61–68. doi: 10.1016/s0925-4773(99)00271-3. [DOI] [PubMed] [Google Scholar]
- Angulo JC, Gonzalez J, Rodriguez N, Hernandez E, Nunez C, Rodriguez-Barbero JM, et al. Clinicopathological study of regressed testicular tumors (apparent extragonadal germ cell neoplasms). J Urol. 2009;182:2303–2310. doi: 10.1016/j.juro.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Benne R, Van den BJ, Brakenhoff JP, Sloof P, Van Boom JH, Tromp MC. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986;46:819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242–253. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- Braude I, Vukovic B, Prasad M, Marrano P, Turley S, Barber D, et al. Large scale copy number variation (CNV) at 14q12 is associated with the presence of genomic abnormalities in neoplasia. BMC Genomics. 2006;7:138. doi: 10.1186/1471-2164-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydoy M, Fossa SD, Klepp O, Bremnes RM, Wist EA, Wentzel-Larsen T, Dahl O. Paternity following treatment for testicular cancer. J Natl Cancer Inst. 2005;97:1580–1588. doi: 10.1093/jnci/dji339. [DOI] [PubMed] [Google Scholar]
- Cheng L. Establishing a germ cell origin for metastatic tumors using OCT4 immunohistochemistry. Cancer. 2004;101:2006–2010. doi: 10.1002/cncr.20566. [DOI] [PubMed] [Google Scholar]
- Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De JJ, et al. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 2007;211:1–9. doi: 10.1002/path.2105. [DOI] [PubMed] [Google Scholar]
- Clark AT, Rodriguez RT, Bodnar MS, Abeyta MJ, Cedars MI, Turek PJ, et al. Human STELLAR, NANOG, and GDF3 genes are expressed in pluripotent cells and map to chromosome 12p13, a hotspot for teratocarcinoma. Stem Cells. 2004;22:169–179. doi: 10.1634/stemcells.22-2-169. [DOI] [PubMed] [Google Scholar]
- De Giorgi U, Nicolai N, Tana S, Tavolini IM, Palazzi S, Bracarda S, et al. IGG practice guidelines on germ cell tumor in adult male patients. Tumori. 2008;94:96–109. doi: 10.1177/030089160809400118. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Di PA, Vries EG, Gietema JA, Spierings DC, de JS. Testicular germ cell tumours: the paradigm of chemo-sensitive solid tumours. Int J Biochem Cell Biol. 2005;37:2437–2456. doi: 10.1016/j.biocel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132:931–939. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- Dixon FJ, Moore RA. Testicular tumors; a clinicopathological study. Cancer. 1953;6:427–454. doi: 10.1002/1097-0142(195305)6:3<427::aid-cncr2820060302>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fabre E, Jira H, Izard V, Ferlicot S, Hammoudi Y, Theodore C, et al. ‘Burned-out’ primary testicular cancer. BJU Int. 2004;94:74–78. doi: 10.1111/j.1464-410X.2004.04904.x. [DOI] [PubMed] [Google Scholar]
- Fenderson BA, Andrews PW, Nudelman E, Clausen H, Hakomori S. Glycolipid core structure switching from globo- to lacto- and ganglio-series during retinoic acid-induced differentiation of TERA-2-derived human embryonal carcinoma cells. Dev Biol. 1987;122:21–34. doi: 10.1016/0012-1606(87)90328-9. [DOI] [PubMed] [Google Scholar]
- Fine G, Smith RW, Jr, Pachter MR. Primary extragenital choriocarcinoma in the male subject. Case report and review of the literature. Am J Med. 1962;32:776–794. doi: 10.1016/0002-9343(62)90167-5. [DOI] [PubMed] [Google Scholar]
- Fossa SD, Kaye SB, Mead GM, Cullen M, de WR, Bodrogi I, et al. J Clin Oncol. Vol. 16. European Organization for Research and Treatment of Cancer, Genito-Urinary Group, and the Medical Research Council Testicular Cancer Working Party; Cambridge, United Kingdom: 1998. Filgrastim during combination chemotherapy of patients with poor-prognosis metastatic germ cell malignancy. pp. 716–724. [DOI] [PubMed] [Google Scholar]
- Hailemariam S, Engeler DS, Bannwart F, Amin MB. Primary mediastinal germ cell tumor with intratubular germ cell neoplasia of the testis–further support for germ cell origin of these tumors: a case report. Cancer. 1997;79:1031–1036. [PubMed] [Google Scholar]
- Heaney JD, Nadeau JH. Testicular germ cell tumors in mice: new ways to study a genetically complex trait. Methods Mol Biol. 2008;450:211–231. doi: 10.1007/978-1-60327-214-8_15. [DOI] [PubMed] [Google Scholar]
- Hill-Baskin AE, Markiewski MM, Buchner DA, Shao H, DeSantis D, Hsiao G, et al. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet. 2009;18:2975–2988. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzik MF, Sijmons RH, Hoestra-Weebers J, Sleijfer DT, Hoekstra HJ. Clinical and genetic aspects of testicular germ cell tumours. Hered Cancer Clin Pract. 2008;6:3–14. doi: 10.1186/1897-4287-6-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddart RA, Norman A. Changes in BMI after treatment of testicular cancer are due to age and hormonal function and not chemotherapy. Br J Cancer. 2003;89:1143–1144. doi: 10.1038/sj.bjc.6601178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, Dearnaley DP. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21:1513–1523. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Laneri JP, Mountain CF, Luna M. Extragonadal germ cell tumors. Surgery. 1973;73:85–90. [PubMed] [Google Scholar]
- Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol. 2004;28:935–940. doi: 10.1097/00000478-200407000-00014. [DOI] [PubMed] [Google Scholar]
- Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130:1691–1700. doi: 10.1242/dev.00392. [DOI] [PubMed] [Google Scholar]
- Korkola JE, Heck S, Olshen AB, Reuter VE, Bosl GJ, Houldsworth J, Chaganti RS. In vivo differentiation and genomic evolution in adult male germ cell tumors. Genes Chromosom Cancer. 2008;47:43–55. doi: 10.1002/gcc.20504. [DOI] [PubMed] [Google Scholar]
- Lam MY, Youngren KK, Nadeau JH. Enhancers and suppressors of testicular cancer susceptibility in single- and double-mutant mice. Genetics. 2004;166:925–933. doi: 10.1093/genetics/166.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehrer PJ, Sr, Gonin R, Nichols CR, Weathers T, Einhorn LH. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol. 1998;16:2500–2504. doi: 10.1200/JCO.1998.16.7.2500. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology. 2007;148:4560–4567. doi: 10.1210/en.2007-0492. [DOI] [PubMed] [Google Scholar]
- Maris C, Dominguez C, Allain FH. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 2005;272:2118–2131. doi: 10.1111/j.1742-4658.2005.04653.x. [DOI] [PubMed] [Google Scholar]
- Matin A, Collin GB, Asada Y, Varnum D, Nadeau JH. Susceptibility to testicular germ-cell tumours in a 129. MOLF-Chr 19 chromosome substitution strain. Nat Genet. 1999;23:237–240. doi: 10.1038/13874. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Marusawa H, Endo Y, Ueda Y, Matsumoto Y, Chiba T. Expression of APOBEC2 is transcriptionally regulated by NF-kappaB in human hepatocytes. FEBS Lett. 2006;580:731–735. doi: 10.1016/j.febslet.2005.12.081. [DOI] [PubMed] [Google Scholar]
- Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240:488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- Mueller T, Mueller LP, Holzhausen HJ, Witthuhn R, Albers P, Schmoll HJ. Histological evidence for the existence of germ cell tumor cells showing embryonal carcinoma morphology but lacking OCT4 expression and cisplatin sensitivity. Histochem Cell Biol. 2010;134:197–204. doi: 10.1007/s00418-010-0710-1. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3 ⁄ 4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Stevens LC. Primordial germ cell proliferation in fetal testes in mouse strains with high and low incidences of congenital testicular teratomas. J Natl Cancer Inst. 1982;69:907–913. [PubMed] [Google Scholar]
- Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990;60:461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Piulats JM, Jimenez L, Garcia d, Villanueva A, Vinals F, Germa-Lluch JR. Molecular mechanisms behind the resistance of cisplatin in germ cell tumours. Clin Transl Oncol. 2009;11:780–786. doi: 10.1007/s12094-009-0446-3. [DOI] [PubMed] [Google Scholar]
- Powles TB, Bhardwa J, Shamash J, Mandalia S, Oliver T. The changing presentation of germ cell tumours of the testis between 1983 and 2002. BJU Int. 2005;95:1197–1200. doi: 10.1111/j.1464-410X.2005.05504.x. [DOI] [PubMed] [Google Scholar]
- Richie JP, Steele GS. Neoplasms of the testis. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. Saunders Elsevier; Philadelphia: 2007. pp. 893–935. [Google Scholar]
- Rodriguez E, Mathew S, Reuter V, Ilson DH, Bosl GJ, Chaganti RS. Cytogenetic analysis of 124 prospectively ascertained male germ cell tumors. Cancer Res. 1992;52:2285–2291. [PubMed] [Google Scholar]
- Rosenstraus MJ. Isolation and characterization of an embryonal carcinoma cell line lacking SSEA-1 antigen. Dev Biol. 1983;99:318–323. doi: 10.1016/0012-1606(83)90281-6. [DOI] [PubMed] [Google Scholar]
- Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Runyan C, Schaible K, Molyneaux K, Wang Z, Levin L, Wylie C. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development. 2006;133:4861–4869. doi: 10.1242/dev.02688. [DOI] [PubMed] [Google Scholar]
- Shi M, Liu D, Duan H, Shen B, Guo N. Metastasis-related miRNAs, active players in breast cancer invasion, and metastasis. Cancer Metastasis Rev. 2010;29:785–799. doi: 10.1007/s10555-010-9265-9. [DOI] [PubMed] [Google Scholar]
- Skotheim RI, Autio R, Lind GE, Kraggerud SM, Andrews PW, et al. Novel genomic aberrations in testicular germ cell tumors by array-CGH, and associated gene expression changes. Cell Oncol. 2006;28:315–326. doi: 10.1155/2006/219786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci USA. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LC. Testicular teratomas in fetal mice. J Natl Cancer Inst. 1962a;28:247–267. [PubMed] [Google Scholar]
- Stevens LC. The biology of teratomas including evidence indicating their origin form primordial germ cells. Annee Biol. 1962b;1:585–610. [PubMed] [Google Scholar]
- Stevens LC. Origin of testicular teratomas from primordial germ cells in mice. J Natl Cancer Inst. 1967;38:549–552. [PubMed] [Google Scholar]
- Stevens LC. A new inbred subline of mice (129-terSv) with a high incidence of spontaneous congenital testicular teratomas. J Natl Cancer Inst. 1973;50:235–242. doi: 10.1093/jnci/50.1.235. [DOI] [PubMed] [Google Scholar]
- Stevens LC, Hummel KP. A description of spontaneous congenital testicular teratomas in strain 129 mice. J Natl Cancer Inst. 1957;18:719–747. [PubMed] [Google Scholar]
- Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- Ulbright TM. Testis risk and prognostic factors. The patholo-gist's perspective. Urol Clin North Am. 1999;26:611–626. doi: 10.1016/s0094-0143(05)70202-0. [DOI] [PubMed] [Google Scholar]
- Upadhyay S, Zamboni L. Ectopic germ cells: natural model for the study of germ cell sexual differentiation. Proc Natl Acad Sci USA. 1982;79:6584–6588. doi: 10.1073/pnas.79.21.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435–1440. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- Wolff EM, Chihara Y, Pan F, Weisenberger DJ, Siegmund KD, Sugano K, et al. Unique DNA methylation patterns distinguish noninvasive and invasive urothelial cancers and establish an epigenetic field defect in premalignant tissue. Cancer Res. 2010;70:8169–8178. doi: 10.1158/0008-5472.CAN-10-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang C, Zhao X, Wu Q, Fu X, Yu B, et al. Analysis of copy number variations of BS69 in multiple types of hematological malignancies. Ann Hematol. 2010;89:959–964. doi: 10.1007/s00277-010-0966-5. [DOI] [PubMed] [Google Scholar]
- Youngren KK, Coveney D, Peng X, Bhattacharya C, Schmidt LS, Nickerson ML, et al. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature. 2005;435:360–364. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni L, Upadhyay S. Germ cell differentiation in mouse adrenal glands. J Exp Zool. 1983;228:173–193. doi: 10.1002/jez.1402280204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.