Abstract
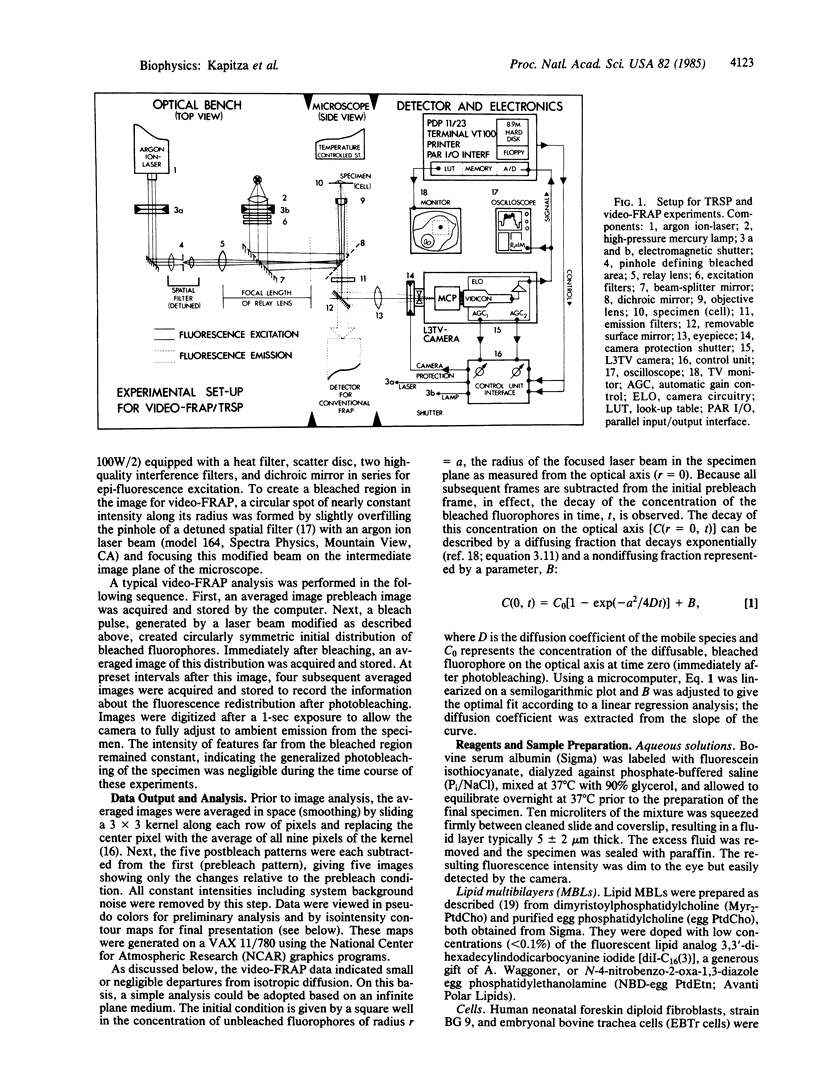
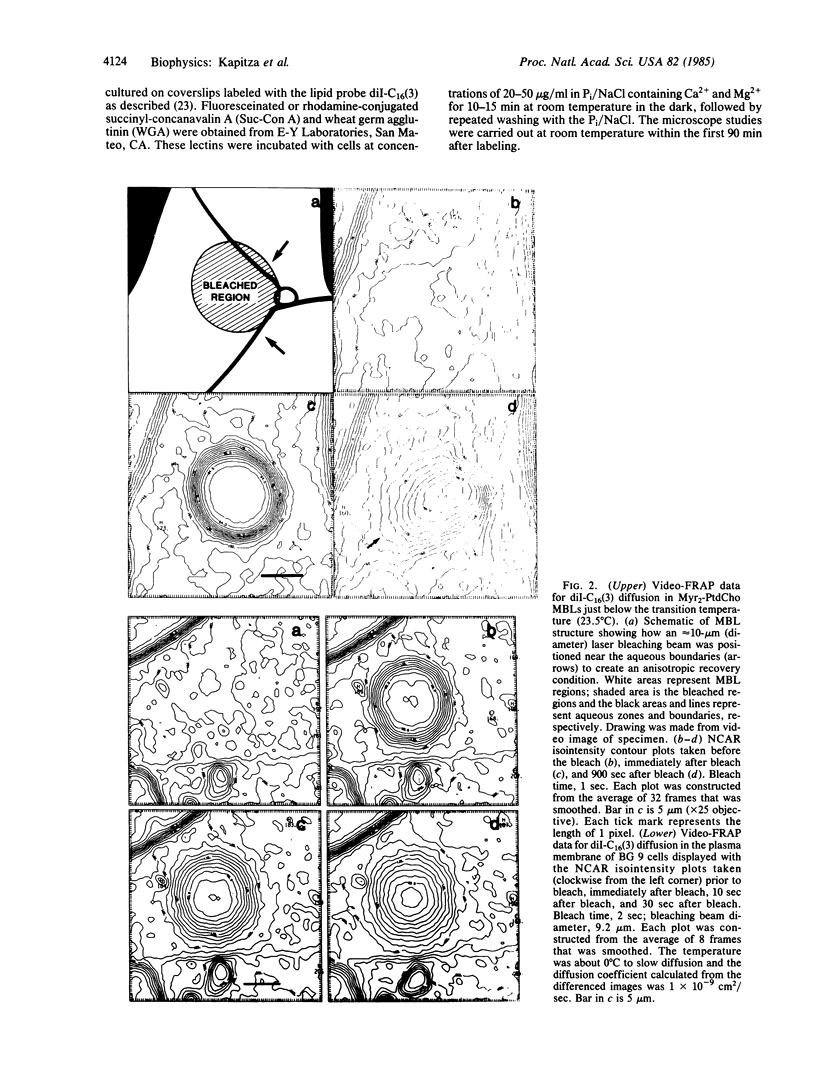
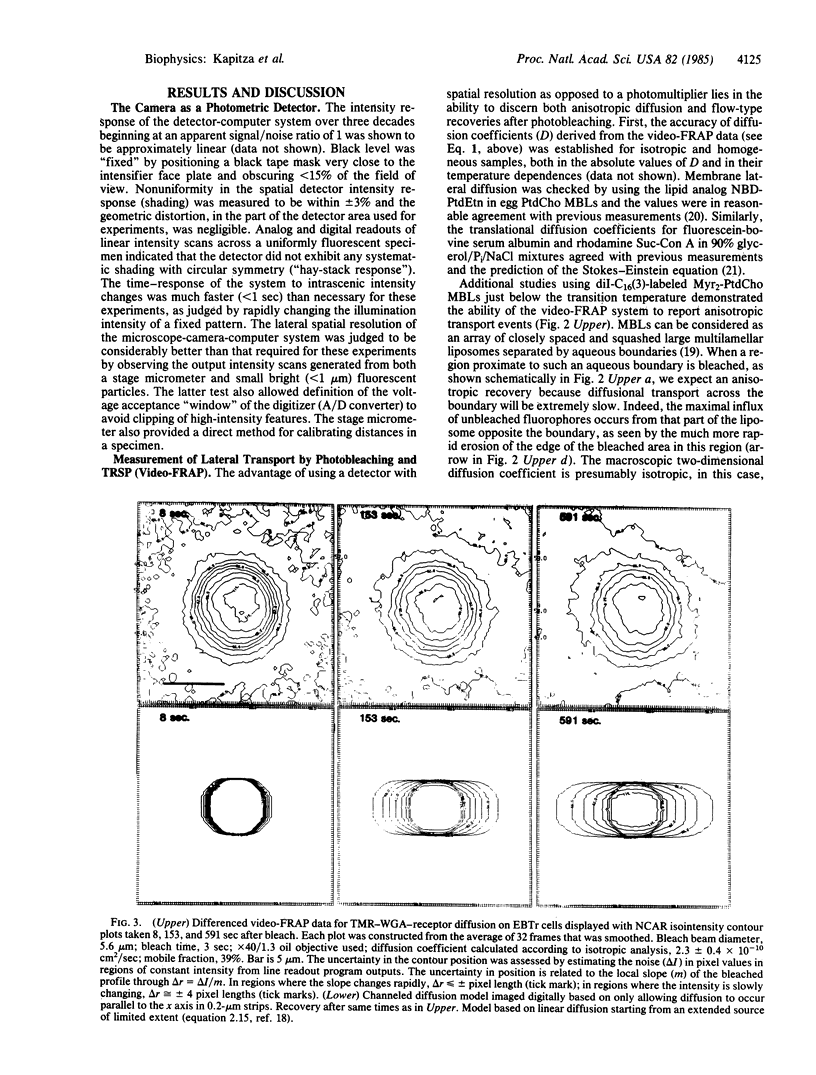
Spatially resolving light detectors allow, with proper calibration, quantitative analysis of the variations in two-dimensional intensity distributions over time. An ultrasensitive microfluorometer was assembled by using as a detector a microchannel plate-intensified video camera. The camera was interfaced with a software-based digital video analysis system to digitize, average, and process images and to directly control the timing of the experiments to minimize exposure of the specimen to light. The detector system has been characterized to allow its use as a photometer. A major application has been to perform fluorescence recovery after photobleaching measurements by using the camera in place of a photomultiplier tube (video-FRAP) with the goal of detecting possible anisotropic diffusion or convective flow. Analysis of the data on macromolecular diffusion in homogenous aqueous glycol solutions yielded diffusion constants in agreement with previous measurements. Results on lipid probe diffusion in dimyristoylphosphatidylcholine multibilayers indicated that at temperatures above the gel-to-liquid crystalline phase transition diffusion is isotropic, and analysis of video-FRAP data yielded diffusion coefficients consistent with those measured previously by using spot photobleaching. However, lipid probes in these multibilayers held just below the main phase transition temperature exhibited markedly anisotropic diffusive fluxes when the bleaching beam was positioned proximate to domain boundaries in the P beta' phase. Lipid probes and lectin receptor complexes diffused isotropically in fibroblast surface membranes with little evidence for diffusion channeled parallel to stress fibers. A second application was to trace the time evolution of cell surface reactions such as patching. The feasibility of following, on the optical scale, the growth of individual receptor clusters induced by the ligand wheat germ agglutinin was demonstrated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Sedat J. W. Three-dimensional architecture of a polytene nucleus. Nature. 1983 Apr 21;302(5910):676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- Benson D. M., Bryan J., Plant A. L., Gotto A. M., Jr, Smith L. C. Digital imaging fluorescence microscopy: spatial heterogeneity of photobleaching rate constants in individual cells. J Cell Biol. 1985 Apr;100(4):1309–1323. doi: 10.1083/jcb.100.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggan J. E., Walter R., Edwards M. S., Borcich J. K., Davis R. L., Koonce M., Berns M. W. Distribution of hematoporphyrin derivative in the rat 9l gliosarcoma brain tumor analyzed by digital video fluorescence microscopy. J Neurosurg. 1984 Dec;61(6):1113–1119. doi: 10.3171/jns.1984.61.6.1113. [DOI] [PubMed] [Google Scholar]
- Burridge K. Changes in cellular glycoproteins after transformation: identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4457–4461. doi: 10.1073/pnas.73.12.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Hou Y., Derzko Z., Wojcieszyn J., Organisciak D. Lipid lateral diffusion in the surface membrane of cells and in multibilayers formed from plasma membrane lipids. Biochemistry. 1981 Sep 1;20(18):5268–5275. doi: 10.1021/bi00521a027. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Kapitza H. G., Sackmann E. Local measurement of lateral motion in erythrocyte membranes by photobleaching technique. Biochim Biophys Acta. 1980;595(1):56–64. doi: 10.1016/0005-2736(80)90247-3. [DOI] [PubMed] [Google Scholar]
- Kohen E., Thorell B., Kohen C., Salmon J. M. Studies on metabolic events in localized compartments of the living cell by rapid microspectrofluorometry. Adv Biol Med Phys. 1974 Jun;15(0):271–297. doi: 10.1016/b978-0-12-005215-8.50013-9. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Berlin R. D. Distribution of receptors and functions on cell surfaces: quantitation of ligand-receptor mobility and a new model for the control of plasma membrane topography. Philos Trans R Soc Lond B Biol Sci. 1982 Nov 4;299(1095):215–235. doi: 10.1098/rstb.1982.0128. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Calcium ion distribution in cytoplasm visualised by aequorin: diffusion in cytosol restricted by energized sequestering. Science. 1975 Dec 19;190(4220):1204–1206. doi: 10.1126/science.1198106. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Saxton W. M., Leslie R. J., Karow M. L., McIntosh J. R. Diffusion coefficient of fluorescein-labeled tubulin in the cytoplasm of embryonic cells of a sea urchin: video image analysis of fluorescence redistribution after photobleaching. J Cell Biol. 1984 Dec;99(6):2157–2164. doi: 10.1083/jcb.99.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., Clark W. R., McConnell H. M. Anisotropic molecular motion on cell surfaces. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5641–5644. doi: 10.1073/pnas.76.11.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasugarn L., McNeil P., Reynolds G. T., Taylor D. L. Microspectrofluorometry by digital image processing: measurement of cytoplasmic pH. J Cell Biol. 1984 Feb;98(2):717–724. doi: 10.1083/jcb.98.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The visualization of fluorescent proteins in living cells by video intensification microscopy (VIM). Cell. 1978 Mar;13(3):501–507. doi: 10.1016/0092-8674(78)90323-9. [DOI] [PubMed] [Google Scholar]



