Summary
Maternal depression serves as a potent source of stress among offspring, greatly enhancing the risk of numerous adverse outcomes including youth depression. Several factors moderate the transmission of depression from mothers to offspring. However, the role of genetic characteristics in this process merits further exploration. Consistent with an interpersonal perspective on depression, the present study focused on a genetic polymorphism that has been shown to be relevant to social functioning, the rs53576 polymorphism of the oxytocin receptor gene (OXTR). In a community sample of 441 youth, OXTR genotype moderated the association between maternal depression in early childhood and youth depressive symptoms in adolescence, such that youth possessing at least one A allele of OXTR who also had a history of maternal depression exhibited the highest levels of depressive symptoms at age 15. In order to explore possible interpersonal mediators of this effect, conditional process analyses examined the role of youth social functioning in adolescence. Results suggest that OXTR genotype may partially account for the transmission of maternal depression to youth and support the role of dysfunctional social processes as a mechanism through which OXTR influences the development of depressive symptoms.
Keywords: oxytocin receptor gene, OXTR, intergenerational transmission of depression, maternal depression, adolescents, social functioning
Introduction
Maternal depression is strongly associated with numerous adverse outcomes among offspring, with a particularly robust association between maternal and youth depression (Downey & Coyne, 1990; Beardslee et al., 1998). Estimates suggest that offspring of depressed mothers are three times more likely to experience depression than those without a depressed mother (Lieb et al., 2002). A history of maternal depression is also associated with characteristics of youth depression that reflect poorer prognosis, including earlier onset (Hammen et al., 2008), higher rates of recurrence (Lieb et al., 2002), and increased risk of comorbid conditions (Hammen & Brennan, 2001).
However, despite the enhanced risk conferred by maternal depression, half of all youth with a depressed mother remain free of psychopathology (Pilowsky et al., 2006). Various factors have been identified as moderators of the transmission of maternal depression, including youth gender (Davies & Windle, 1997), emotion regulation abilities (Silk et al., 2006), and self-esteem (Pargas et al., 2010). Consistent with an interpersonal perspective on depression (Joiner & Coyne, 1999), social factors such as parent-child relationship quality, interpersonal stress, and peer functioning have also been recognized as moderators (Hammen et al., 2004; Pargas et al., 2010).
Given the moderate heritability of depression (Sullivan et al., 2000) as well as some evidence supporting the interplay of genes and the environment in its development (Caspi et al., 2003), additional research is needed to explore the role of candidate genes in the intergenerational transmission of depression. To date, only two studies have examined offspring genetic characteristics as moderators of maternal depression’s effect on youth (Thompson et al., 2011; Oppenheimer et al., 2013), offering preliminary support for the role of the serotonin transporter and oxytocin receptor genes. These findings suggest that genetic factors may influence individual vulnerability to the adverse effects of maternal depression. However, additional research is needed to explore the longitudinal influence of genetic moderators and establish potential mediators of this process.
Given the importance of interpersonal processes in the etiology of depression (Joiner & Coyne, 1999), identifying genetic polymorphisms that exhibit a strong association with social factors is a logical focus of research. Interpersonal functioning is particularly relevant to the association between maternal and youth depression because depressed mothers often exhibit problematic parenting behavior (e.g., hostility, disengagement) that may influence the development of youth depression (Lovejoy et al., 2000; Brennan et al., 2003). Research linking the oxytocin receptor gene (OXTR) with interpersonal processes in humans suggests that it is a promising candidate as one moderator of the intergenerational transmission of depression (Kumsta & Heinrichs, 2013). Oxytocin is a neurohormone that has been associated with a range of affiliative behaviors in humans including demonstrations of trust (Kosfeld et al., 2005), parental bonding (Gordon et al., 2008), and sensitivity to others’ mental state (Domes et al., 2007).
An emerging body of literature supports an association between the rs53576 single nucleotide polymorphism (SNP) of OXTR and social processes, with most studies suggesting that the GG genotype is associated with prosocial behavior. Individuals who are homozygous for the G allele report higher levels of dispositional empathy and perform more accurately on a theory of mind task than A carriers (i.e., AG or AA genotype; Rodrigues et al., 2009). When interacting with a romantic partner, GG homozygotes exhibited more nonverbal affiliative behaviors (e.g., smiling, head nodding) than A carriers (Kogan et al., 2011). Individuals who are homozygous for the G allele also displayed more trust during an economics task than A carriers, controlling for personality factors and attachment style (Krueger et al., 2012). Parents with the GG genotype exhibited heightened sensitivity to their child’s needs when helping them to complete a problem-solving task (Bakermans-Kranenburg & van IJzendoorn, 2008).
Evidence also suggests that the rs53576 polymorphism of OXTR may be associated with depressive symptoms and affect. Individuals carrying an A allele exhibited higher levels of depressive symptoms than GG homozygotes (Saphire-Bernstein et al., 2011), while males who are homozygous for the A allele demonstrated lower positive affect than G carriers (Lucht et al., 2009). In contrast, Costa et al. (2009) found evidence for an association between the GG genotype of OXTR and depression in a clinical sample. These results indicate that OXTR may be associated with depression, but additional research is needed to elucidate this relationship. Furthermore, the results of a meta-analysis by Bakermans-Kranenburg and van IJzendoorn (in press) suggest that findings purporting main effects of OXTR genotype on biological and psychological outcomes should be interpreted with caution. Thus, the present study explores the rs53576 polymorphism of OXTR as a moderator of the intergenerational transmission of depression in order to examine its interactive effects with environmental characteristics.
In addition to examining genetic moderation, the present study tests a potential mechanism of this effect by examining the mediating role of youth social functioning. Maternal depression is associated with youth deficits in interpersonal functioning (Goodman, 2007), with evidence suggesting that impaired social competence is a mediator of the intergenerational transmission of depression (Hammen et al., 2004). Given that OXTR has also been associated with social processes, the present study explores youth social functioning as a mechanism of the relationship between youth OXTR genotype and the intergenerational transmission of depression.
The single previous study that examined youth OXTR genotype and the transmission of maternal depression found that adolescents with the AG genotype of the rs2254298 SNP and a history of maternal depression exhibited the highest levels of depressive symptoms (Thompson et al., 2011). However, the study was limited by its entirely female sample, exclusion of AA homozygotes, and relatively small sample size (N = 92). The present study seeks to extend this research by examining the role of the more-studied OXTR rs53576 polymorphism on the transmission of maternal depression to offspring in a moderately large community sample of adolescents. Additional analyses explore youth social functioning as one possible mechanism of the impact of OXTR on the intergenerational transmission of depression. It is expected that (a) youth OXTR genotype will interact with maternal depression in early childhood to predict youth depressive symptoms at age 15, such that youth with a history of maternal depression who also possess at least one A allele of OXTR will exhibit the highest levels of depressive symptoms and (b) this association will be mediated by indicators of youth social functioning.
Methods
Participants
Participants were drawn from a community sample of women and their children participating in the Mater Misericordiae Mothers’ Hospital-University of Queensland Study of Pregnancy (Keeping et al., 1989), a longitudinal study of a birth cohort in Brisbane, Queensland, Australia between 1981 and 1984. Of the more than 7000 participants in the original study, a subsample of 815 mother-child pairs were selected in order to examine the effects of maternal depression on youth, oversampling for maternal depression as determined by scores on the Delusions-Symptoms-States Inventory (DSSI; Bedford & Foulds, 1978) which was completed by mothers at four points between pregnancy and youth age 5, and confirmed through diagnostic assessment on the Structured Clinical Interview for DSM-IV (SCID-I; First et al., 1995) administered at youth age 15. Mean maternal DSSI score prior to youth age 5 was significantly associated with diagnoses of maternal depression based on the SCID-I (B = 0.03, SE = 0.01, p < .001).
Youth and their mothers completed follow-up interviews and questionnaires at youth age 15 and again at youth age 20. The 705 youth who were available for the age 20 follow-up were asked to provide a DNA sample for genetic analysis between ages 22 and 25. Youth who were genotyped for the rs53576 polymorphism of OXTR and completed all measures relevant to the present study (N = 441) did not differ from the remainder of the full sample (N = 374) in terms of race (χ2 (1,793) < 0.01, p = 0.965) or maternal depression history (χ2 (1,811) = 0.34, p = 0.562). However, youth included in the present study were less likely to be male (χ2 (1,815) = 36.44, p < 0.001), suggesting that the results may be affected by sampling bias.
Of the 441 youth included in the present analyses, a total of 261 participants (59.2%) are female, and most are Caucasian (N = 413, 93.7%). A minority of participants identified as Asian (N = 18, 4.1%), Maori/Pacific Islander (N = 5, 1.1%), Aboriginal (N = 2, 0.5%), and other (N = 1, 0.2%). Data on race/ethnicity were not available for two participants. Race (Caucasian vs. other) was not associated with dichotomous (GG vs. AG/AA) genotype (χ2 (1,440) = 2.72, p = 0.099).
Procedure
At youth age 15, youth and their mothers completed a battery of self-report questionnaires and participated in follow-up interviews. Separate interviewers assessed mothers and their offspring, and youth interviewers were blind to maternal depression status. Between youth ages 22 and 25, participants were re-contacted and asked to provide DNA samples for genetic analysis. Participants were mailed consent forms and blood collection kits. Blood samples were drawn at local medical facilities and subsequently returned by courier to the Genetic Epidemiological Laboratory at the Queensland Institute of Medical Research for storage. Samples were later shipped to UCLA and analyzed at the Social Genomics Core of the USC/UCLA Biodemography Center. All participants gave consent/assent at each data collection point, and all procedures were approved by the Institutional Review Boards of the University of Queensland, Emory University, and the University of California, Los Angeles.
Measures
Maternal depression
History of maternal depression was assessed by the SCID-I administered at youth age 15 (First et al., 1995). Mothers were considered to have a history of depression if they met criteria for a diagnosis of a major depressive episode or dysthymic disorder at any point in the first five years of the child’s life. Research suggests that maternal depression experienced in early childhood exerts a particularly negative effect on youth outcomes, resulting in increased risk of childhood internalizing and externalizing problems (Kiernan & Huerta, 2008) as well as heightened rates of youth emotion dysregulation (Maughan et al., 2007). History of maternal depression was coded as a dichotomous variable (depressed/not depressed). A total of 65 participants (14.7%) met criteria for a history of maternal depression by youth age 5.
Youth depressive symptoms
Youth depressive symptoms at age 15 were measured by the Beck Depression Inventory-II (BDI-II; Beck et al., 1996). The BDI-II has demonstrated high internal consistency and convergent validity in clinical and community samples of adults (Beck et al., 1996), and the excellent psychometric properties of the BDI-II have been replicated in community samples of adolescents (Osman et al., 2008). In the present sample, the mean BDI-II score was 6.20 (SD = 6.19) with scores ranging from 0 to 32. The BDI-II demonstrated high internal consistency in the present sample with a Cronbach’s alpha coefficient of 0.85.
Youth social functioning
Youth social functioning was assessed using the UCLA Life Stress Interview (LSI; Hammen & Brennan, 2001), which was administered by trained interviewers at youth age 15. This semi-structured interview measures the nature and quality of ongoing conditions in the participant’s life as a measure of chronic stress and adaptive functioning in several relevant domains. Interviewers collect specific details about conditions experienced within the past six months in each of several roles typical of a person of that age (e.g., social life, family relationships, and academic performance). The interviewer then makes objective ratings of functioning using a 1 to 5 scale with factual, behavioral anchors at each level. A score of 5 represents extremely negative conditions/poor functioning, while scores of 1 or 2 are considered relatively high levels of functioning in the specified domain. This measure demonstrates high reliability as well as excellent concurrent validity (Hammen & Brennan, 2001; Hammen et al., 2008).
In the present study, social functioning was measured as a composite of youth functioning in three interpersonal domains of the LSI: close friendships, general peer relationships, and family relationships. Scores in each of the three domains were averaged to create a composite variable representing the level of youth social dysfunction in the six months prior to the age 15 assessment of depressive symptoms. The mean social functioning score was 2.28 (SD = 0.38) and ranged from 1.50 to 3.67. Higher scores represent greater levels of youth social dysfunction.
Genotyping
The oxytocin receptor gene is located on the short arm of the third chromosome at 3p25, and the rs53576 polymorphism of OXTR is found on the third intron (Kumsta & Heinrichs, 2013). OXTR codes for the oxytocin receptor, a G protein-coupled receptor involved in reproductive processes including parturition and lactation as well as affiliative and affective processes as described above (Lee et al., 2009). Due to financial constraints, only one polymorphism of OXTR was genotyped as part of the present study. The rs53576 polymorphism was selected due to the preponderance of literature utilizing this SNP at the time the parent study was conducted as well as evidence suggesting a meaningful association between the rs53576 polymorphism and characteristics of human social functioning. However, the particular physiologic function of the rs53576 polymorphism is unknown.
Genotyping for the rs53576 polymorphism of OXTR was conducted using a commercial TaqMan Genotyping Assay (Applied Biosystems, Foster City, CA) performed on an iCycler real-time PCR instrument (BioRad, Hercules, CA). Test-retest reliability analyses based on duplicated samples yielded a total genotyping error rate of <1%. In the present sample, the genotype frequencies were GG = 192 (43.5%), AG = 196 (44.4%), and AA = 53 (12.0%), and in Hardy-Weinberg equilibrium, χ2 (2,441) = 0.08, p = 0.784. Consistent with the majority of prior OXTR literature reporting more negative social behaviors associated with the A allele (e.g., Thompson et al., 2011; Saphire-Bernstein et al., 2011), genotype was recoded as a dichotomous variable, GG (N = 192) vs. AG/AA (N = 249).
Statistical Analysis
Univariate analysis of variance was used to explore the effect of OXTR genotype on the intergenerational transmission of depression. Due to the higher prevalence of depression among women (Kessler et al., 1993) as well as previous studies that reported interactions between OXTR genotype and gender (e.g., Lucht et al., 2009), gender was included in the analyses. Gender, history of maternal depression, dichotomous OXTR genotype, and their respective interactions were examined as predictors of youth depressive symptoms at age 15 in a single univariate ANOVA. The interaction between history of maternal depression and OXTR genotype served as the crucial test of OXTR as a moderator of the intergenerational transmission of depression. The simple effects of maternal depression history on youth depressive symptoms by OXTR genotype were examined by conducting two additional univariate analyses of variance among GG homozygotes and A carriers, respectively. The alpha level was set at 0.05 for all analyses, unless otherwise noted.
Conditional process analyses were employed in order to explore youth social functioning as a mediator of the effect. Gender was included as a covariate in these analyses. Conditional process analysis techniques are equivalent to mediated moderation analyses, but allow greater flexibility in interpreting the results according to the variable of interest (i.e., the mediator or the moderator). These analyses were conducted using the PROCESS procedure for SPSS (Hayes, 2012), which estimates direct and indirect effects by calculating bias-corrected 95% confidence intervals using bootstrapping techniques (N = 5,000 samples). A significant indirect effect supports the variable of interest as a mediator. Significant effects are those in which the estimated 95% confidence interval does not include zero. Effect sizes were calculated by dividing the indirect effect by the total effect in order to estimate the proportion of the total effect mediated by youth social functioning.
Although race was not associated with genotype (reported above), the analyses of interest were repeated excluding non-Caucasian participants in order to examine the hypothesized associations independent of race/ethnicity.
Results
Descriptive Statistics
Stratified by genotype group and maternal depression history, descriptive statistics for all study variables are presented in Table 1. OXTR genotype did not differ by gender (χ2 (1,441) = 2.07, p = 0.150).
Table 1.
Descriptive statistics for all study variables, stratified by maternal depression history and dichotomous OXTR genotype.
| Variable | GG | A carriers | ||
|---|---|---|---|---|
| No Maternal Depression |
History of Maternal Depression |
No Maternal Depression |
History of Maternal Depression |
|
| N | 168 | 24 | 208 | 41 |
| Female gender (%) | 107 (63.7%) | 14 (58.3%) | 121 (58.2%) | 19 (46.3%) |
| Caucasian race (%)a | 161 (96.4%) | 23 (95.8%) | 190 (91.8%) | 39 (95.1%) |
| Mean (SD) BDI-II score at age 15 |
6.29 (6.32) | 5.25 (5.24) | 5.89 (5.87) | 7.95 (7.53) |
| Mean (SD) social functioning score |
2.25 (0.38) | 2.28 (0.34) | 2.26 (0.37) | 2.51 (0.43) |
Note.
Values do not match the total number of participants due to missing race/ethnicity data (N = 2).
Gene-Environment Correlation
In order to ensure that any observed gene-environment interactions were not confounded by a correlation between genotype and environmental exposure, analyses were conducted to explore the association between OXTR genotype and history of maternal depression. Results revealed that genotype did not differ by maternal depression history (χ2 (1,441) = 1.36, p = 0.244).
OXTR Genotype as a Moderator of the Intergenerational Transmission of Depression
Univariate analysis of variance revealed that consistent with the higher prevalence of depression among women (Kessler et al., 1993), there was a main effect of gender (F(1,433) = 7.78, p = .006, partial η2 = .018), such that females exhibited significantly more depressive symptoms than males at age 15. There were no significant main effects of maternal depression history (F(1,433) = 0.37, p = 0.545, partial η2 = .001) or OXTR genotype (F(1,433) = 2.86, p = 0.092, partial η2 = .007) on adolescent depressive symptoms. Gender did not interact with other study variables, as summarized in Table 2.
Table 2.
Effects of maternal depression history, OXTR genotype, and gender on youth depressive symptoms.
| Predictor | F | p | Partial η2 |
|---|---|---|---|
| Maternal depression history | 0.37 | 0.545 | 0.001 |
| OXTR genotype | 2.86 | 0.092 | 0.007 |
| Gender | 7.78** | 0.006 | 0.018 |
| Maternal depression history * OXTR genotype | 3.92* | 0.048 | 0.009 |
| Maternal depression history * gender | 0.80 | 0.371 | 0.002 |
| OXTR genotype * gender | 1.31 | 0.253 | 0.003 |
| Maternal depression history * OXTR genotype * gender |
0.71 | 0.400 | 0.002 |
Note.
p < .05,
p < .01,
p < .001
As predicted, there was a significant interaction between history of maternal depression and OXTR genotype in the prediction of depressive symptoms at age 15 (F(1,433) = 3.92, p = 0.048, partial η2 = .009). Post-hoc calculations indicated that the power to detect the effect (1- β) was 0.25. Tests of simple effects revealed that youth possessing at least one A allele exhibited a significant association between maternal depression in early childhood and depressive symptoms in adolescence (F(1,245) = 4.44, p = 0.036, partial η2 = .018), such that individuals with a history of maternal depression demonstrated more depressive symptoms at age 15 than individuals without depressed mothers. Among individuals homozygous for the G allele, there was no significant difference between individuals with a history of maternal depression and those without (F(1,188) = 0.76, p = 0.384, partial η2 = .004). In order to account for multiple tests (N = 2), a stringent correction to the alpha level was applied, so that the significance levels attained in the tests of simple effects were compared to an alpha level of 0.025. After applying this correction, the p value of 0.036 obtained in the test of the simple effect among A carriers exceeded the significance threshold. Thus, these results offer preliminary evidence that OXTR genotype and maternal depression history interact to predict youth depressive symptoms at age 15 such that the A allele of the rs53576 polymorphism of OXTR appears to be associated with higher levels of youth depressive symptoms among individuals with a history of maternal depression.
Excluding non-Caucasian participants from the analyses did not alter the direction or significance of these findings. Figure 1 illustrates the significant interaction effect, adjusting for the main effect of gender.
Figure 1.
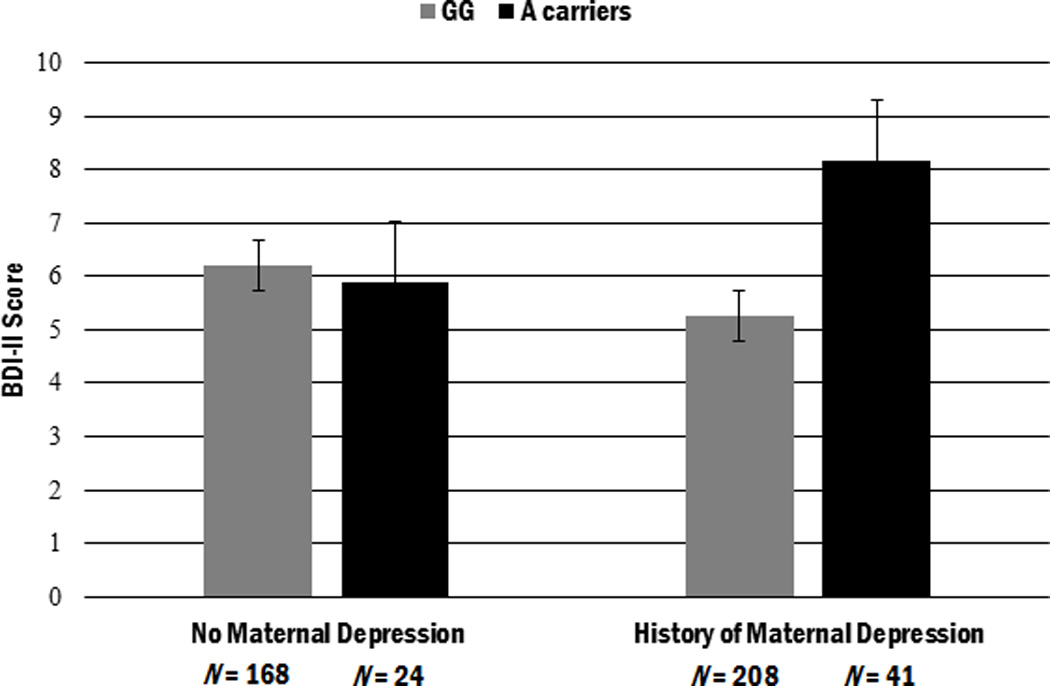
Depressive symptoms at age 15 as a function of maternal depression history and OXTR genotype (adjusted by gender). Individuals carrying at least one A allele of the rs53576 polymorphism of OXTR who also have a history of maternal depression exhibit the highest levels of depressive symptoms at age 15. Error bars represent standard error. N = 441.
Mediation by Youth Social Functioning
Conditional process analyses examined whether adolescents’ social functioning serves as a mechanism of the association between OXTR genotype and the intergenerational transmission of depression. We examined a model utilizing the composite measure of social functioning as a mediator of the interaction between maternal depression and OXTR genotype in the prediction of adolescent depressive symptoms (Figure 2). Conditional process analyses revealed that the indirect effect of social functioning was significant, as demonstrated by a 95% bootstrap confidence interval that does not include zero (95% CI: 0.04, 1.92). Thus, social functioning over the past six months mediates the interaction between maternal depression history and OXTR genotype in the prediction of youth depressive symptoms. The ratio of the indirect effect to the total effect was 0.25, indicating that approximately 25% of the total effect was mediated by youth social functioning over the past six months. The r2 value of 0.08 indicates that approximately 8% of the variance was explained by this model. These results are summarized in Table 3.
Figure 2.
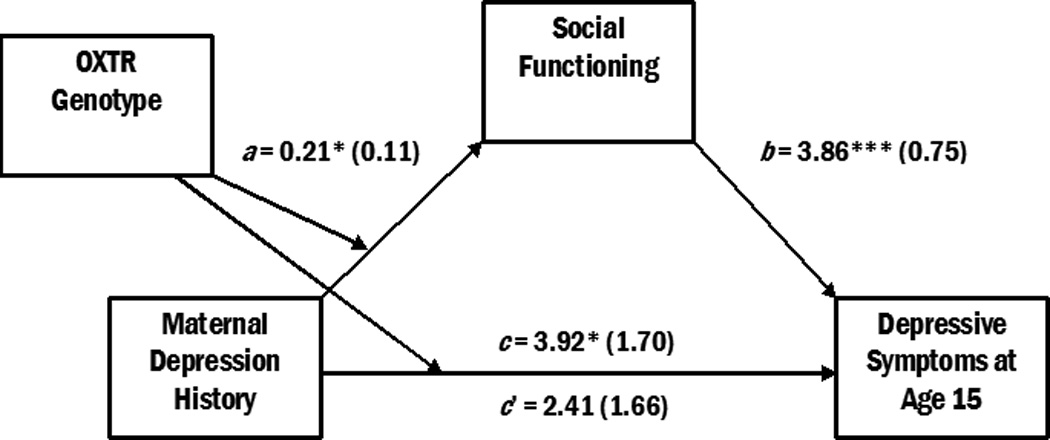
Conditional process model of the association between maternal depression history, OXTR genotype, social functioning, and depressive symptoms. OXTR genotype moderates the association between maternal depression in early childhood and youth depressive symptoms at age 15. This interaction is mediated by youth social functioning in the six months prior to age 15. Unstandardized regression coefficients (with standard errors) are presented. *p < .05, **p < .01, ***p < .001. N = 441.
Table 3.
Indirect effects of maternal depression history and OXTR genotype on youth depressive symptoms via youth social functioning.
| Indirect Effect |
95% CI (lower, upper) |
||
|---|---|---|---|
| Sample | Effect | SE | |
| Overall | 0.82* | 0.48 | (0.04, 1.92) |
| GG | 0.14 | 0.29 | (−0.36, 0.82) |
| A carriers | 0.97* | 0.38 | (0.39, 1.89) |
Note.
Significant indirect effect as evidenced by 95% confidence interval that does not include zero. CI, confidence interval.
Examining the conditional indirect effects of maternal depression history on depressive symptoms by OXTR genotype revealed that youth social functioning significantly mediated this association among A carriers (95% CI: 0.39, 1.89), but not GG homozygotes (95% CI: −0.36, 0.82). The proportion of the total effect that was mediated by youth social functioning among A carriers was 0.41, while the proportion mediated by GG homozygotes was 0.12.
Repeating these analyses after excluding non-Caucasian participants revealed that youth social functioning continued to significantly mediate the association among maternal depression history, OXTR genotype, and youth depressive symptoms among A carriers (95% CI: 0.34, 1.96), but not among GG homozygotes (95% CI: −0.37, 0.93). The difference between the indirect effect among A carriers vs. that of GG homozygotes indicates that OXTR genotype continued to predict the strength of the indirect effect of youth social functioning on the intergenerational transmission of depression when the sample is restricted to Caucasian participants. The omnibus test of the role of OXTR genotype on the indirect effect of youth social functioning was only slightly reduced when excluding non-Caucasian participants (95% CI: −0.04, 1.98). This suggests that there is an effect of OXTR genotype on the indirect effect of youth social functioning that is independent of race/ethnicity, although the relatively small sample size of the present study may have prevented the omnibus test from attaining statistical significance in the Caucasian-only sample.
Discussion
The present study found that variations in the level of depressive symptoms among adolescent offspring of depressed women were significantly related to OXTR genotype. Youth carrying at least one A allele of the rs53576 polymorphism of OXTR who also have a history of early exposure to maternal depression displayed the highest levels of depressive symptoms in adolescence. Individuals with the GG genotype did not demonstrate an association between maternal depression in early childhood and later depressive symptoms. Thus, there is some evidence that certain youth appear to be protected from the negative influence of maternal depression as a result of OXTR genotype. These findings corroborate the results of the single previous study that explored the role of OXTR genotype in the intergenerational transmission of depression (Thompson et al., 2011), which suggested that the A allele of the rs2254298 polymorphism confers heightened risk for the transmission of maternal depression. The results also contribute to the limited extant literature examining the role of genetic variables in the intergenerational transmission of depression and offer one explanation for the variable transmission of depression from mothers to youth.
The oxytocin receptor gene was hypothesized to be relevant to youth depressive outcomes due to the association between interpersonal behaviors and OXTR genotype (see Kumsta & Heinrichs, 2013 for a review). This is consistent with the association between maternal depression and interpersonal dysfunction among offspring (Goodman, 2007), as well the association between youth interpersonal dysfunction and depression (Hammen et al., 2004). Accordingly, the present study tested one potential mechanism of the association between OXTR and the intergenerational transmission of depression, finding that youth social functioning serves as a mediator of the transmission of maternal depression, as moderated by OXTR genotype. Youth with a history of maternal depression who possess at least one A allele of the rs53576 polymorphism of OXTR appeared to exhibit deficits in peer and family functioning in young adolescence. These difficulties in social relationships predicted increases in depressive symptoms. Thus, the present findings are consistent with a model of intergenerational risk for depression that emphasizes social functioning, a process that is mediated by both psychosocial and genetic factors.
Despite support for a general pattern linking OXTR to depression via interpersonal dysfunction, the specific interpersonal processes that are affected by OXTR in the intergenerational transmission of depression remain to be identified. Existing research assessing the functions of oxytocin and the rs53576 polymorphism of OXTR suggests several possible mechanisms that should be explored further, including empathic processes (Rodrigues et al., 2009), trust (Kosfeld et al., 2005; Krueger et al., 2012), and attachment (Costa et al., 2009). Alternative theories indicate that oxytocin may be associated with differences in the salience of social cues or with the regulation of approach and withdrawal behavior, rather than affiliative processes specifically (Kemp & Guastella, 2011; Olff et al., in press). Thus, additional research is needed to elucidate the function of oxytocin and the oxytocin receptor gene in social interactions and to clarify the specific pathways through which OXTR affects social functioning and the experience of depressive symptoms.
OXTR genotype may also influence the ability of youth to adaptively respond to the stresses associated with having a depressed mother. Maternal depression serves as a potent source of stress in early childhood (Essex et al., 2002) and is also associated with a heightened risk of secondary stressors, including negative parenting practices and marital discord (Lovejoy et al., 2000; Hammen, 2009). Some research suggests that the rs53576 polymorphism of the OXTR genotype may confer sensitivity to the environment, such that individuals with a particular genotype exhibit increased physiological reactivity to environmental stressors (Rodrigues et al., 2009; Norman et al., 2011). Therefore, some youth may be particularly sensitive to the stresses associated with maternal depression, leading to more negative outcomes. OXTR genotype also appears to influence patterns of support seeking in response to stress (Kim et al., 2010) and moderates stress reactivity in the presence of supportive friend (Chen et al., 2011) based on studies utilizing the rs53576 SNP. These findings suggest a variety of possible mechanisms of the association between the intergenerational transmission of depression, OXTR genotype, and social functioning that warrant clarification through future research.
The present study has several strengths, including the use of both moderation and mediation analyses to elaborate on the processes through which genetic factors and social functioning affect the intergenerational transmission of depression, the use of comprehensive, detailed measures of social environmental characteristics, and the inclusion of a community sample of male and female adolescents examined during a critical developmental period. Further, the study expanded on the single previous study on this topic by utilizing the more-studied rs53576 polymorphism and extending the prior finding to males.
Nevertheless, important limitations must be acknowledged. First, although the present study was much larger (N = 441) than the single previous study on this topic (N = 92), future projects should utilize even larger samples in order to conduct a stronger test of the hypothesized associations. Large studies that broadly sample participants across racial and ethnic categories are particularly needed in order to elucidate the role of OXTR in human social functioning and the intergenerational transmission of depression. It is likely that idiosyncrasies of the present findings are largely due to issues of sample size. For example, it is probable that the alteration in the test of the simple effect among A carriers following the application of a stringent correction to the alpha level was strongly affected by limitations of sample size. Although some confidence in this effect may be gained from the strength of the effect among A carriers in the mediation analyses, future research should confirm these findings using larger samples. The present study provides preliminary findings to be explored and replicated in future large-scale studies of diverse participants that are better able to identify the true impact of the rs53576 polymorphism of OXTR on human functioning.
Additionally, although there was not a significant gene-environment correlation between OXTR genotype and maternal depression, it is likely that the effects observed in the present study to some extent reflect associations between maternal genetic characteristics, youth genetic characteristics, and parenting behaviors. Therefore, the possibility remains that the present results may be partially attributable to correlations between genetic characteristics and the environment. Given that maternal OXTR genotype was not measured, it is possible that interpersonal deficits exhibited in the offspring of depressed mothers may be attributable to maternal genetic characteristics, including the effect of candidate genes other than OXTR. Maternal depression was essentially treated as an environmental factor in the present study, although maternal depression’s impact on offspring undoubtedly arises from the combined effects of genetic and environmental processes. Future research should examine both the environmental and genetic influence of maternal depression on youth outcomes as well as the effect of genetic and environmental characteristics that are shared by mothers and offspring.
Furthermore, the association between depressive symptoms and social functioning is likely bidirectional, and the choice to examine youth social functioning as a mediator in the present study does not preclude the possibility that the results could also be accounted for by a model in which youth depressive symptoms predict subsequent social functioning. The present study sought to reduce the probability of this alternate model by utilizing a measure of youth social functioning designed to capture functioning throughout the previous six months, while the measure of youth depressive symptoms evaluated symptoms during the previous two weeks. However, both measures were administered at the same assessment point. Therefore, the present findings should not be interpreted as offering a purely causal explanation for the association between the variables of interest. Future studies should seek to measure youth depressive symptoms and social functioning repeatedly across childhood and early adolescence in order to elucidate the timing and relative influence of these variables on one another.
The present analyses examined a relatively limited outcome (i.e., depressive symptoms among adolescents). Future research should attempt to explore the specificity of the observed results to depressive outcomes by incorporating broadband outcome measures of youth psychopathology and functioning (Seeley et al., 2011). Future studies should also extend this research to include more distal outcomes in adulthood and should examine other polymorphisms of OXTR. Due to financial constraints, the present study examined a single, commonly studied SNP of OXTR that had previously been associated with depressive symptoms and social functioning in humans, but additional research using a variety of polymorphisms is needed.
Despite growing interest in the role of oxytocin and OXTR, much additional research is needed in order to elucidate the specific functions associated with oxytocin and the gene that codes for its receptor. The present study contributes to greater understanding of the role of OXTR in the development of depression among offspring of depressed mothers and identifies social functioning as a mediator of this association, but future research should continue to explicate mechanisms of this effect using large, ethnically diverse samples. By identifying the means through which OXTR genotype differentially contributes to mental health outcomes and social processes, the transmission of depression from mothers to youth can be better understood.
Acknowledgements
Special thanks to the research teams and project coordinators Robyne Le Brocque, Cheri Dalton Comber, and Sascha Hardwicke, and to the parents and youth in the Mater Cohort for their participation in this study. The cooperation of Patricia Brennan, Emory University, and Jake Najman of the University of Queensland and head of the MUSP program are gratefully acknowledged. We are also grateful for the assistance of the Social Genomics Core of the USC/UCLA Biodemography Center.
Role of the Funding Source
This research was supported by NIMH R01MH052239, NSF DGE-0707424, and a grant from the UCLA Cousins Center for Psychoneuroimmunology. Study sponsors were not involved in any aspect of study design; collection, analysis, or interpretation of data; preparation of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Contributors
Sarah M. Thompson designed the present study, completed the literature review, conducted the statistical analyses, and prepared the manuscript. Constance Hammen designed the parent study and assisted in the preparation of the manuscript. Lisa R. Starr contributed techniques for statistical analyses and assisted in the preparation of the manuscript. Jake M. Najman designed the parent study and was responsible for data collection. All authors contributed to and have approved the final manuscript.
References
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc. Cogn. Affect. Neurosci. 2008;3(2):128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatr. Genet. in press doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- Beardslee WR, Versage EM, Gladstone TRG. Children of affectively ill parents: A review of the past 10 years. J. Am. Acad. Child. Psy. 1998;37(11):1134–1141. doi: 10.1097/00004583-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Bedford A, Foulds G. Delusions-Symptoms-States Inventory of Anxiety and Depression. Windsor, UK: National Foundation for Educational Research; 1978. [Google Scholar]
- Brennan PA, Le Brocque R, Hammen C. Maternal depression, parent-child relationships, and resilient outcomes in adolescence. J. Am. Acad. Child. Psy. 2003;42(12):1469–1477. doi: 10.1097/00004583-200312000-00014. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc. Natl. Acad. Sci. USA. 2011;108(50):19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Pini S, Gabelloni P, Abelli M, Lari L, Cardini A, Muti M, Gesi C, Landi S, Galderisi S, Mucci A, Lucacchini A, Cassano GB, Martini C. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34:1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Davies PT, Windle M. Gender-specific pathways between maternal depressive symptoms, family discord, and adolescent adjustment. Dev. Psychol. 1997;33(4):657–668. doi: 10.1037//0012-1649.33.4.657. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol. Psychiat. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Downey G, Coyne JC. Children of depressed parents: An integrative review. Psychol. Bull. 1990;108(1):50–76. doi: 10.1037/0033-2909.108.1.50. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biol. Psychiat. 2002;52(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, D.C.: American Psychiatric Press; 1995. [Google Scholar]
- Goodman SH. Depression in mothers. Annu. Rev. Clin. Psychol. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: Associations with bonding and psychological distress. Psychophysiology. 2008;45(3):349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Hammen CL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. 2nd ed. New York, NY: Guilford Press; 2009. pp. 275–297. [Google Scholar]
- Hammen C, Brennan PA. Depressed adolescents of depressed and nondepressed mothers: Tests of an interpersonal impairment hypothesis. J. Consult. Clin. Psych. 2001;69(2):284–294. doi: 10.1037//0022-006x.69.2.284. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D. Patterns of adolescent depression to age 20: The role of maternal depression and youth interpersonal dysfunction. J. Abnorm. Child Psych. 2008;36:1189–1198. doi: 10.1007/s10802-008-9241-9. [DOI] [PubMed] [Google Scholar]
- Hammen C, Shih JH, Brennan PA. Intergenerational transmission of depression: Test of an interpersonal stress model in a community sample. J. Consult. Clin. Psych. 2004;72(3):511–522. doi: 10.1037/0022-006X.72.3.511. [DOI] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper] 2012 Retrieved from http://www.afhayes.com/
- Joiner TE, Coyne JC, editors. The Interactional Nature of Depression: Advances in Interpersonal Approaches. Washington, D.C.: American Psychological Association; 1999. [Google Scholar]
- Keeping JD, Najman JM, Morrison J, Western JS, Andersen MJ, Williams GM. A prospective longitudinal study of social, psychological and obstetric factors in pregnancy: Response rates and demographic characteristics of the 8556 respondents. Brit. J. Obstet. Gynaec. 1989;96(3):289–297. doi: 10.1111/j.1471-0528.1989.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. The role of oxytocin in human affect: A novel hypothesis. Curr. Dir. Psychol. Sci. 2011;20(4):222–231. [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity and recurrence. J. Affect. Disorders. 1993;29(2–3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kiernan KE, Huerta MC. Economic deprivation, maternal depression, parenting and children’s cognitive and emotional development in early childhood. Brit. J. Sociol. 2008;59(4):783–806. doi: 10.1111/j.1468-4446.2008.00219.x. [DOI] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, Suh EM, Graham K, Taylor SE. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proc. Natl. Acad. Sci. USA. 2010;107(36):15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan A, Saslow LR, Impett EA, Oveis C, Keltner D, Rorigues Saturn S. Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. Proc. Natl. Acad. Sci. USA. 2011;108(48):19189–19192. doi: 10.1073/pnas.1112658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Krueger F, Parasuraman R, Iyengar V, Thornburg M, Weel J, Lin M, Clarke E, McCabe K, Lipsky RH. Oxytocin receptor genetic variation promotes human trust behavior. Front. Hum. Neurosci. 2012;6(4):1–9. doi: 10.3389/fnhum.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: Neurogenetics of the human oxytocin system. Curr. Opin. Neurobiol. 2013;23:11–16. doi: 10.1016/j.conb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Lee H, Macbeth AH, Pagani JH, Young WS. Oxytocin: The great facilitator of life. Prog. Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb R, Isensee B, Hofler M, Pfister H, Wittchen HU. Parental major depression and the risk of depression and other mental disorders in offspring: A prospective-longitudinal community study. Arch. Gen. Psychiat. 2002;59(4):365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clin. Psychol. Rev. 2000;20(5):561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Rosenberger A, Grabe HJ, Schroeder W, Volzke H, Freyberger HJ, Herrmann FH, Kroemer H, Rosskopf D. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:860–866. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Maughan A, Cicchetti D, Toth SL, Rogosch FA. Early-occurring maternal depression and maternal negativity in predicting young children’s emotion regulation and socioemotional difficulties. J. Abnorm. Child Psych. 2007;35(5):685–703. doi: 10.1007/s10802-007-9129-0. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Hawkley L, Luhmann M, Ball AB, Cole SW, Berntson GG, Cacioppo JT. Variation in the oxytocin receptor gene influences neurocardiac reactivity to social stress and HPA function: A population based study. Horm. Behav. 2011;61:134–139. doi: 10.1016/j.yhbeh.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Oppenheimer CW, Hankin BL, Young JF, Smolen A. Youth genetic vulnerability to maternal depressive symptoms: 5-HTTLPR as moderator of intergenerational transmission effects in a multiwave prospective study. Depress. Anxiety. 2013;30:190–196. doi: 10.1002/da.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman A, Barrios FX, Gutierrez PM, Williams JE, Bailey J. Psychometric properties of the Beck Depression Inventory-II in nonclinical adolescent samples. J. Clin. Psychol. 2008;64(1):83–102. doi: 10.1002/jclp.20433. [DOI] [PubMed] [Google Scholar]
- Pargas RCM, Brennan PA, Hammen C, Le Brocque R. Resilience to maternal depression in young adulthood. Dev. Psychol. 2010;46(4):805–814. doi: 10.1037/a0019817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky DJ, Wickramaratne PJ, Rush AJ, Hughes CW, Garber J, Malloy E, King CA, Cerda G, Sood AB, Alpert JE, Wisniewski SR, Trivedi MH, Talati A, Carlson MM, Liu HF, Fava M, Weissman MM. Children of currently depressed mothers: A STAR*D ancillary study. J. Clin. Psychiat. 2006;67(1):126–136. doi: 10.4088/jcp.v67n0119. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc. Natl. Acad. Sci. USA. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire-Bernstein S, Way BM, Kim HS, Sherman DK, Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proc. Natl. Acad. Sci. USA. 2011;108(37):15118–15122. doi: 10.1073/pnas.1113137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley JR, Kosty DB, Farmer RF, Lewinsohn PM. The modeling of internalizing disorders on the basis of patterns of lifetime comorbidity: Associations with psychosocial functioning and psychiatric disorders among first-degree relatives. J. Abnorm. Psychol. 2011;120(2):308–321. doi: 10.1037/a0022621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Shaw DS, Forbes EE, Lane TL, Kovacs M. Maternal depression and child internalizing: The moderating role of child emotion regulation. J. Clin. Child Adolesc. 2006;35(1):116–126. doi: 10.1207/s15374424jccp3501_10. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiat. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36:144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


