Abstract
There has been controversy regarding the relationship between gastroesophageal reflux, microaspiration, and idiopathic pulmonary fibrosis (IPF). In the last decade, there is increasing evidence supporting a relationship between gastroesophageal reflux, microaspiration, and IPF. Specifically, gastroesophageal reflux is common in IPF, is often asymptomatic in this population, and may impact disease progression and the natural history of IPF. More intriguing are the data suggesting that treatment of gastroesophageal reflux, either medical or surgical, may slow disease progression, as measured by change in forced vital capacity, and improve survival in IPF. Despite the growing evidence, there are still many gaps in our understanding of this relationship. Some of the major gaps include the discrepancy between the prevalence of gastroesophageal reflux in the general population compared to the prevalence of IPF, the unclear causative agent leading to injury, the lack of reliable methods to evaluate for gastroesophageal reflux and microaspiration, and the role of treatment. Further research, including a randomized controlled trial of anti-reflux therapy, needs to be done to clarify the relationship between gastroesophageal reflux, microaspiration, and IPF.
Keywords: idiopathic pulmonary fibrosis, gastroesophageal reflux, microaspiration, pathogenesis, treatment
A CASE
A 60 year-old man presented with progressive dyspnea on exertion and a chronic cough. He is a former smoker. He denies any symptoms to suggest a rheumatologic disorder. He does not have any occupational or environmental exposures. The timeline of events and imaging findings are shown in Figure 1.
Figure 1.
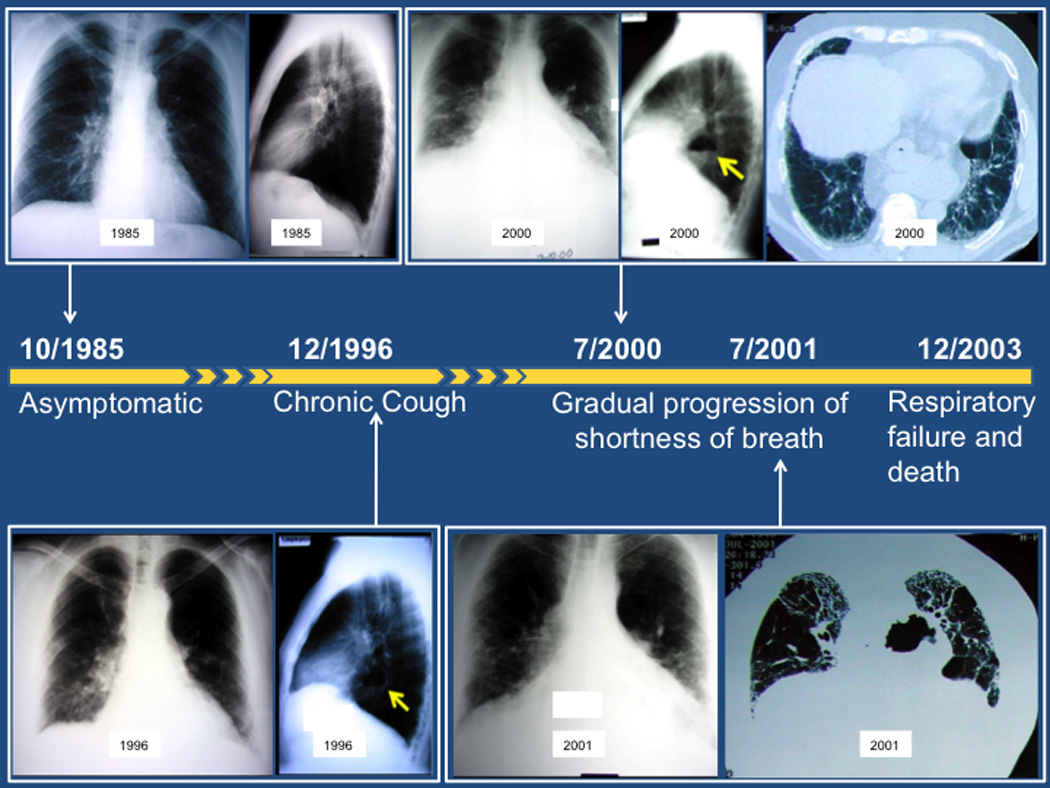
Timeline of symptoms and radiographic studies described in the case. The arrows identify the hiatal hernia.
On review of this patient’s previous studies, a screening chest x-ray was obtained in 1985 and was normal. His pulmonary function tests at this time were also normal. Another chest x-ray was performed in 1996 for work-up of chronic cough. This chest x-ray identified the presence of a hiatal hernia. He was subsequently diagnosed with gastroesophageal reflux disease. In 2000, he was noted to have Barrett’s esophagus during an endoscopy. He also complained of increasing breathlessness. A chest computed tomography scan demonstrated peripheral, basilar predominant reticulation and traction bronchiectasis. Concurrent pulmonary function tests demonstrated evidence of moderate restriction by total lung capacity (59% predicted) and a severely reduced diffusing capacity at 37% predicted. Approximately one year later, he reported increasing dyspnea and a repeat high-resolution computed tomography scan demonstrated a usual interstitial pneumonia pattern, with peripheral, basilar predominant reticulation and traction bronchiectasis with honeycombing. His doctor diagnosed him with idiopathic pulmonary fibrosis. He died from progressive respiratory failure approximately 2 years following this diagnosis.
It is because of cases like this and others, that clinicians have wondered if there is a relationship between gastroesophageal reflux, microaspiration, and pulmonary fibrosis. In this review, we explore the data supporting this relationship and the current gaps in our understanding.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is one of the most common causes of lung fibrosis. This form of fibrosis is characterized by the usual interstitial pneumonia pattern on high-resolution computed tomography scanning and/or surgical lung biopsy.1 The prognosis associated with IPF is poor, with a median survival of approximately 2–3 years following diagnosis.2 In addition, there are currently no approved therapies for IPF in the United States. The etiology of IPF remains unknown, although several factors including smoking, viral infection, and gastroesophageal reflux (GER) with microaspiration have been suggested.1,3
The relationship between GER, microaspiration and IPF is intriguing for several reasons. First, GER is nearly universal in patients with IPF.4–6 Second, there are multiple shared risk factors between IPF and GER, including age, smoking and male gender.1,7 Finally, there are medical and surgical treatments for GER.8 To date, the strategy of most recent clinical trials in IPF have tested agents aimed at slowing the progression of disease through limiting fibroproliferation. Treatment of GER is unique in that it is potentially disease modifying – i.e. it may actually reduce the stimulus for fibroproliferation in the lung by preventing aspiration of gastric refluxate that occurs because of abnormal GER.
PROPOSED HYPOTHESIS AND CONCEPTUAL MODEL
Based on data that are reviewed in this article, GER and microaspiration have been hypothesized to play a role in the pathogenesis and/or natural history of IPF, either through disease progression, acute exacerbation, or survival.3,9
The conceptual model 3 begins with the gastric fluid traveling retrograde through a weakened lower esophageal sphincter (LES). LES weakening can occur for a variety of reasons including the presence of a hiatal hernia, traction from the diaphragm, or medications. In certain instances, the gastric refluxate can travel as high up as the cricopharyngeal region, allowing entrance into the airway. Typically, normal host defenses will clear the refluxate without any clinical sequelae. However, in some people, the gastric refluxate may lead to direct injury of the lung epithelium. This process can lead to repetitive alveolar epithelial cell injury, and in the genetically or otherwise predisposed patient, this may lead to dysregulated wound healing and lung fibrosis. In addition, the pulmonary fibrosis that develops may lead to distortion of the mediastinal structures placing increasing traction on the esophagus. This traction could lead to further weakening of the LES, allowing for more refluxing of gastric contents. This cycle could contribute to the accelerated decline and/or acute exacerbation experienced by patients with IPF.
BIOLOGIC RATIONALE
In addition to the clinical observations noting a relationship between GER, microaspiration and IPF, cell culture and animal models have provided supporting biologic evidence for this relationship. Bile acids have been observed to induce TGF-beta production and enhance fibroblast proliferation in cultured airway epithelial cells.10 Animal models of chronic aspiration have demonstrated parenchymal fibrosis in response to the aspiration,11 and this response appears to be independent of the acidity of the aspirate. Further, a low-mortality acid aspiration lung injury model demonstrated widespread collagen deposition at 2 weeks with loss of normal parenchymal architecture.12
Microaspiration can cause interstitial lung disease
There is also evidence of other interstitial lung diseases caused by microaspiration, such as lipoid pneumonia.13 In this disease, chronic aspiration of mineral oil, used for the treatment of constipation, has been associated with peripheral and basilar predominant alveolar and interstitial abnormalities. On lung biopsy, patients are found to have fat globules present within the alveolar spaces and alveolar macrophages with interstitial edema. In chronic disease, chronic granulomatous pneumonitis with fibroblastic proliferation, fibrosis, foamy macrophages, and foreign-body giant cells are observed.
WHAT IS KNOWN ABOUT GER, MICROASPIRATION, AND IPF
Hiatal hernia and GER are common in IPF
Hiatal hernia, a risk factor for GER, has been noted in patients with IPF for decades.14,15 More recently, hiatal hernias identified on chest computed tomography images have been found to be more prevalent in patients with IPF compared to patients with asthma and chronic obstructive pulmonary disease (39% vs. 17% vs. 13%, p-value = 0.01 – <0.001).16 The presence of hiatal hernias using this method also correlated with higher DeMeester scores in the subset that had esophageal function testing available.
Acid GER has also been found to be common in patients with IPF (Table 1). The differences in prevalence across these studies are likely related to methodologic difference in GER measurement (e.g. location of probes) and inconsistent cessation of acid suppressive therapy during the procedure. In the first prospective study of 65 patients with IPF, the prevalence of acid GER was 87% in those not taking anti-reflux therapy.6 In this study, they found no correlation between disease severity, as measured by forced vital capacity (FVC) %predicted and diffusing capacity for carbon monoxide (DLCO) %predicted, and GER severity, as measured by the DeMeester score. In addition, a case-control study of more than 200,000 United States veterans found that individuals with erosive esophagitis had a 1.36 odds ratio of having pulmonary fibrosis.17
Table 1.
| Study | IPF (n) |
Controls (n) | Distal Esophageal Reflux |
Proximal Esophageal Reflux |
|---|---|---|---|---|
| Tobin 1998 4 | 17 | 8 (non-IPF ILD) | 88% | 71% |
| Patti 2005 20 | 18 | n/a | 66% | 33% |
| Salvioli 2006 19 | 65 | 10 (non-IPF ILD) | 67% | - |
| Raghu 2006 6 | 18 | 133 (asthma) | 76% | 63% |
| Sweet 2007 5 | 30 | n/a | 67% | 30% |
| Savarino 2013 18 | 40 | 40 (non-IPF ILD) 40 (healthy volunteers) |
83 % | 66% |
IPF – idiopathic pulmonary fibrosis, ILD – interstitial lung disease
More recently, investigators performed impedence-pH monitoring on patients with IPF.18 They confirmed the higher prevalence of acid GER in patients with IPF compared to 40 non-IPF ILD controls and 40 healthy volunteers. They also found that patients with IPF had a higher number of weakly acidic (i.e. non-acid) reflux events compared to controls.
GER is often asymptomatic in IPF
These studies on GER and IPF also demonstrated that typical symptoms of GER, heartburn, regurgitation and dysphagia, are not present in the majority of patients with IPF. Depending on the study, the presence of typical symptoms of GER ranged from 25–65% with a sensitivity of 65% and specificity of 71% for the presence of pathologic GER on 24-h pH monitoring.4–6,19,20 Therefore, symptoms of GER are not an appropriate screening tool for the presence of abnormal GER in this population.
GER may impact IPF disease progression
There are data to suggest that GER and secondary microaspiration may impact IPF disease progression. In a series of 32 cases of asymmetric IPF cases (asymmetry defined by quantitative high-resolution computed tomography fibrosis score with an asymmetry ratio of >0.2), there was increased reporting of overt GER disease in those with asymmetric IPF compared to symmetric IPF (63% vs. 31%, p = 0.009).21 Interestingly, those with asymmetric IPF reported sleeping on the more affected side, in general. There was no information on the presence of nocturnal cough.
Another study looked at bronchoalveolar lavage pepsin levels in acute exacerbation of IPF patients (n=24) compared to stable IPF patients (n=30).22 In this study, bronchoalveolar lavage pepsin level was associated with acute exacerbation status (p = 0.04) and was primarily driven by a subgroup of 8 cases with very high levels of pepsin in the bronchoalveolar lavage fluid. This study suggested that GER and secondary microaspiration may be involved in the development of acute exacerbation in IPF.
Treatment of GER may slow disease progression and improve survival in IPF
An initial case series of patients with IPF suggested stabilization and possible improvement in pulmonary physiology in four patients treated with medical and/or surgical therapy for gastroesophageal reflux.23 A slightly larger case series of pre-transplant IPF patients demonstrated stabilization in oxygen needs in those who had undergone a pre-transplant nissen fundoplication (a surgical therapy for GER).24 More recently, an abstract described a case series of 14 patients with progressive IPF who underwent Nissen fundoplication. Over an average of 7 months, the mean FVC increased by 0.08L when comparing pre and post FVC values.25
Larger cohort studies have investigated the relationship between medical therapy for GER (e.g. proton pump inhibitors and histamine 2 receptor blockers) and outcome, including disease progression and survival, in patients with IPF. The study that investigated disease progression (as measured by change in FVC) as the primary endpoint utilized the placebo arms of the three IPFnet randomized controlled trials.26–28 This study found that those reporting use of medical therapy for GER had a slower decline in FVC compared to those who did not report use of medical therapy for GER (-0.06L, 95% CI −0.11 to −0.01 vs. −0.12, 95% CI −0.17 to −0.08, p-value = 0.05) (Figure 2).29 In addition, there were fewer acute exacerbations in the group taking medical therapy for GER compared to those not taking medical therapy for GER (0 events vs. 9 events, p-value = 0.0017).
Figure 2.
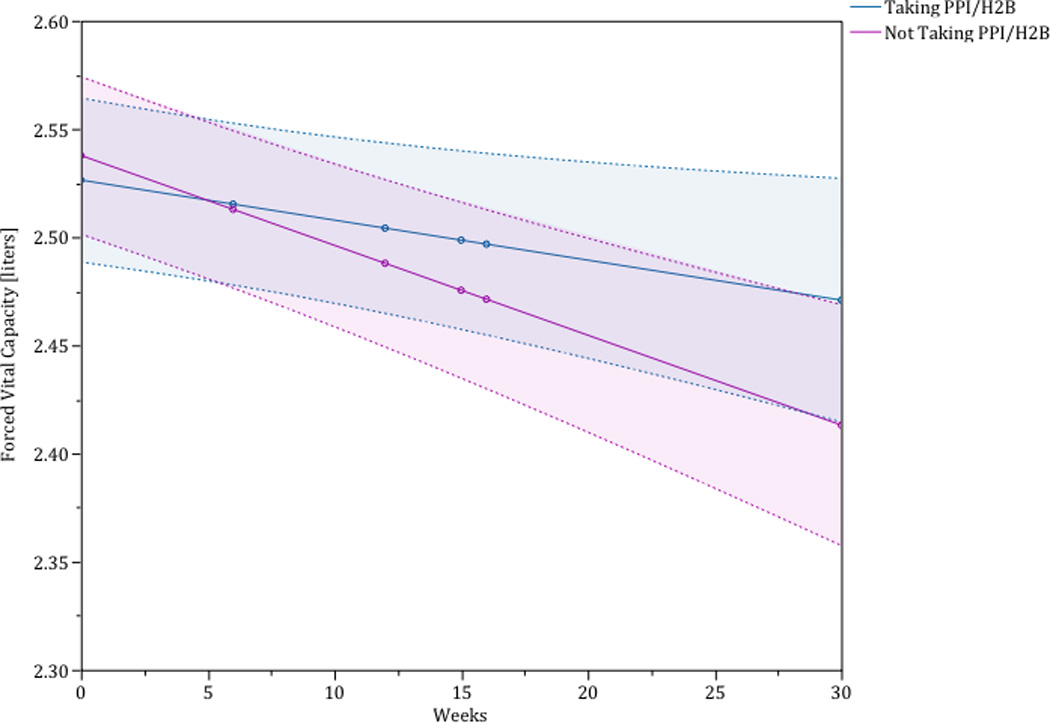
The blue line depicts the change in forced vital capacity of those patients taking a proton pump inhibitor or histamine-2 blocker. The red line depicts the change in forced vital capacity of those patients not taking a proton pump inhibitor or histamine-2 blocker. The shaded region around each line is the 95% confidence interval. This analysis is adjusted for sex, baseline forced vital capacity %predicted, and baseline diffusing capacity for carbon monoxide %predicted.
A retrospective study of 204 patients with IPF enrolled prospectively in longitudinal cohort studies was used to examine the relationship between medical therapy for GER and survival. In this study, use of medical therapy for GER was an independent predictor of longer survival time (HR 0.47, 95% CI 0.24 to 0.93, p-value = 0.03).30 Patients who reported a history of Nissen fundoplication also appeared to do better than those who did not have a Nissen fundoplication (unadjusted HR 0.29, p-value = 0.04), but the numbers were too small to determine if this relationship was confounded.
CURRENT GAPS IN OUR UNDERSTANDING
There is increasing evidence that GER, microaspiration and IPF are related and that treatment of GER and/or microaspiration may be beneficial to patients with IPF. However, there are still many unanswered questions.
Why are there so many people with GER disease and so few people with IPF?
There is an obvious discordance between the prevalence of GER disease in the general population and the prevalence of IPF.31,32 This may be due to several reasons. One possibility is that patients with GER disease have variable degrees and duration of aspiration events. A more likely hypothesis is that there is some other co-factor that pre-disposes some patients to the development of IPF (e.g. smoking, short telomere length, genetic predisposition). This hypothesis would suggest that IPF would only develop as a result of chronic lung injury from repetitive aspiration events in a genetically or otherwise predisposed individual. However, there are no data at this time to support this hypothesis.
What is causing the injury?
Assuming that microaspiration of gastric contents plays some role in the natural history or pathogenesis of IPF, it remains unclear what is causing the injury. There are several components in gastric contents (e.g. food, bile, pepsin, acid) and animal models suggest that injury of the lung parenchyma may be independent of the acidity of the microaspirate.11 Clinical studies have primarily focused on the role of medical therapy for reflux in IPF, however, there is some suggestion that surgical therapy for reflux in IPF may add additional benefit.30
How should we evaluate for GER and microaspiration?
Current diagnostic testing for GER and microaspiration are not sensitive or specific. As discussed previously, symptoms are absent in the majority of IPF patients with pathologic GER.4–6 Measurement of GER using 24-hour pH monitoring and/or impedence monitoring can only identify the presence or absence of GER and does not provide any information on microaspiration.33–35 Barium swallow studies can identify gross aspiration events, but the patient has to aspirate at the time of the study and is less useful in identifying those that chronically microaspirate.36 Pulmonary scintigraphy is more sensitive than barium swallow studies.37,38 However, this procedure is not widely available, it is expensive, and there is an associated radiation exposure. Biomarkers have also been investigated as a test for GER and microaspiration. The two most commonly studied biomarkers are bronchoalveolar lavage levels of pepsin and bile acids.39–42 Although these biomarkers are likely very specific for the presence of microaspiration secondary to GER, several factors make clinical use of these biomarkers potentially problematic. These include the need for a semi-invasive procedure (i.e. bronchoscopy), problems with dilution, and lack of standardization in measurement. In addition, there is no information on how the concentrations of these biomarkers change over time and with aspiration events.
How should we manage GER and microaspiration in patients with IPF?
Although there is increasing evidence to suggest that treatment of GER in IPF may be beneficial, there has not been a randomized controlled trial to assess the true efficacy of GER treatment in patients with IPF. In addition, it also is unknown if surgical therapy would be superior to medical therapy of GER in this patient population. Until these studies are performed, the management of GER and microaspiration in IPF will remain uncertain.
Currently, the management of GER and microaspiration in IPF are physician dependent. The IPF guideline recognizes that the data to support treatment of asymptomatic GER in patients with IPF are weak.1 Nonetheless, they suggest that “asymptomatic GER should be treated in the majority of patients with IPF, but not treating asymptomatic GER may be a reasonable choice in a minority.”1 In my practice, patients are screened for typical and atypical symptoms of GER. If typical symptoms are present, I begin empiric treatment with medical acid suppressive therapy. If atypical symptoms are present, I generally evaluate for abnormal GER with 24-hour pH monitoring. If more aggressive therapy is indicated due to initial severity of disease or failure of standard medical treatment, I collaborate with gastroenterology and surgery to determine the best treatment course.
CONCLUSION
Gastroesophageal reflux is common in patients with IPF. There are growing data to support a role for gastroesophageal reflux and microaspiration in the natural history of this disease. More importantly, there are now data to suggest that treatment of gastroesophageal reflux and microaspiration may be beneficial to patients with IPF. A randomized controlled trial of surgical and/or medical therapy for gastroesophageal reflux is needed.
Acknowledgements
Talmadge E. King, Jr., MD for providing the case.
Funding: NIH/NCRR/OD UCSF-CTSI KL2 RR024130
Footnotes
Disclosure/Conflicts: none
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 3.Lee JS, Collard HR, Raghu G, et al. Does chronic microaspiration cause idiopathic pulmonary fibrosis? Am J Med. 2010;123:304–311. doi: 10.1016/j.amjmed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobin RW, Pope CE, 2nd, Pellegrini CA, et al. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158:1804–1808. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 5.Sweet MP, Patti MG, Leard LE, et al. Gastroesophageal reflux in patients with idiopathic pulmonary fibrosis referred for lung transplantation. J Thorac Cardiovasc Surg. 2007;133:1078–1084. doi: 10.1016/j.jtcvs.2006.09.085. [DOI] [PubMed] [Google Scholar]
- 6.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenberg A. Effects of environment and lifestyle on gastroesophageal reflux disease. Dig Dis. 2011;29:229–234. doi: 10.1159/000323927. [DOI] [PubMed] [Google Scholar]
- 8.Bredenoord AJ, Pandolfino JE, Smout AJ. Gastro-oesophageal reflux disease. Lancet. 2013;381:1933–1942. doi: 10.1016/S0140-6736(12)62171-0. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G. Idiopathic pulmonary fibrosis: increased survival with "gastroesophageal reflux therapy": fact or fallacy? Am J Respir Crit Care Med. 2011;184:1330–1332. doi: 10.1164/rccm.201110-1842ED. [DOI] [PubMed] [Google Scholar]
- 10.Perng DW, Chang KT, Su KC, et al. Exposure of airway epithelium to bile acids associated with gastroesophageal reflux symptoms: a relation to transforming growth factor-beta1 production and fibroblast proliferation. Chest. 2007;132:1548–1556. doi: 10.1378/chest.07-1373. [DOI] [PubMed] [Google Scholar]
- 11.Appel JZ, 3rd, Lee SM, Hartwig MG, et al. Characterization of the innate immune response to chronic aspiration in a novel rodent model. Respir Res. 2007;8:87. doi: 10.1186/1465-9921-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amigoni M, Bellani G, Scanziani M, et al. Lung injury and recovery in a murine model of unilateral acid aspiration: functional, biochemical, and morphologic characterization. Anesthesiology. 2008;108:1037–1046. doi: 10.1097/ALN.0b013e318173f64f. [DOI] [PubMed] [Google Scholar]
- 13.Travis WD, Colby TV, Koss MN, Rosado-de-Crhistenson ML, Muller NL, King TE., Jr . Lipoid Pneumonia and Chronic Fibrosis. In: King DW, editor. Non-Neoplastic Disorders of the Lower Respiratory Tract. Washington, DC: American Registry of Pathology and the Armed Forces Institute of Pathology; 2002. [Google Scholar]
- 14.Pearson JE, Wilson RS. Diffuse pulmonary fibrosis and hiatus hernia. Thorax. 1971;26:300–305. doi: 10.1136/thx.26.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mays EE, Dubois JJ, Hamilton GB. Pulmonary fibrosis associated with tracheobronchial aspiration. A study of the frequency of hiatal hernia and gastroesophageal reflux in interstitial pulmonary fibrosis of obscure etiology. Chest. 1976;69:512–515. doi: 10.1378/chest.69.4.512. [DOI] [PubMed] [Google Scholar]
- 16.Noth I, Zangan SM, Soares RV, et al. Prevalence of hiatal hernia by blinded multidetector CT in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:344–351. doi: 10.1183/09031936.00099910. [DOI] [PubMed] [Google Scholar]
- 17.el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology. 1997;113:755–760. doi: 10.1016/s0016-5085(97)70168-9. [DOI] [PubMed] [Google Scholar]
- 18.Savarino E, Carbone R, Marabotto E, et al. Gastro-oesophageal reflux and gastric aspiration in idiopathic pulmonary fibrosis patients. Eur Respir J. 2013 doi: 10.1183/09031936.00101212. [DOI] [PubMed] [Google Scholar]
- 19.Salvioli B, Belmonte G, Stanghellini V, et al. Gastro-oesophageal reflux and interstitial lung disease. Dig Liver Dis. 2006;38:879–884. doi: 10.1016/j.dld.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Patti MG, Tedesco P, Golden J, et al. Idiopathic pulmonary fibrosis: how often is it really idiopathic? J Gastrointest Surg. 2005;9:1053–1056. doi: 10.1016/j.gassur.2005.06.027. discussion 1056–1058. [DOI] [PubMed] [Google Scholar]
- 21.Tcherakian C, Cottin V, Brillet PY, et al. Progression of idiopathic pulmonary fibrosis: lessons from asymmetrical disease. Thorax. 2011;66:226–231. doi: 10.1136/thx.2010.137190. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Song JW, Wolters PJ, et al. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2011 doi: 10.1183/09031936.00050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghu G, Yang ST, Spada C, et al. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest. 2006;129:794–800. doi: 10.1378/chest.129.3.794. [DOI] [PubMed] [Google Scholar]
- 24.Linden PA, Gilbert RJ, Yeap BY, et al. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg. 2006;131:438–446. doi: 10.1016/j.jtcvs.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Raghu G, Mart D, Anstrom KJ, et al. Treatment Of Idiopathic Pulmonary Fibrosis (IPF) With Laparoscopic Anti-Reflux Surgery (LARS) Is Associated With Improvement In Forced Vital Capacity (FVC) Am J Respir Crit Care Med. 2013:A5711. [Google Scholar]
- 26.Zisman DA, Schwarz M, Anstrom KJ, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghu G, Anstrom KJ, King TE, Jr, et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noth I, Anstrom KJ, Calvert SB, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:88–95. doi: 10.1164/rccm.201202-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Collard HR, Anstrom KJ, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomized controlled trials. Lancet Respiratory Medicine. 2013;1:369–376. doi: 10.1016/S2213-2600(13)70105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JS, Ryu JH, Elicker BM, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locke GR., 3rd Current medical management of gastroesophageal reflux disease. Thorac Surg Clin. 2005;15:369–375. doi: 10.1016/j.thorsurg.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Raghu G, Weycker D, Edelsberg J, et al. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 33.Tutuian R. Update in the diagnosis of gastroesophageal reflux disease. J Gastrointestin Liver Dis. 2006;15:243–247. [PubMed] [Google Scholar]
- 34.Oelschlager BK, Chang L, Pope CE, 2nd, et al. Typical GERD symptoms and esophageal pH monitoring are not enough to diagnose pharyngeal reflux. J Surg Res. 2005;128:55–60. doi: 10.1016/j.jss.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Emerenziani S, Sifrim D. New developments in detection of gastroesophageal reflux. Curr Opin Gastroenterol. 2005;21:450–453. [PubMed] [Google Scholar]
- 36.Stoeckli SJ, Huisman TA, Seifert B, et al. Interrater reliability of videofluoroscopic swallow evaluation. Dysphagia. 2003;18:53–57. doi: 10.1007/s00455-002-0085-0. [DOI] [PubMed] [Google Scholar]
- 37.Ravelli AM, Panarotto MB, Verdoni L, et al. Pulmonary aspiration shown by scintigraphy in gastroesophageal reflux-related respiratory disease. Chest. 2006;130:1520–1526. doi: 10.1378/chest.130.5.1520. [DOI] [PubMed] [Google Scholar]
- 38.Crausaz FM, Favez G. Aspiration of solid food particles into lungs of patients with gastroesophageal reflux and chronic bronchial disease. Chest. 1988;93:376–378. doi: 10.1378/chest.93.2.376. [DOI] [PubMed] [Google Scholar]
- 39.Ufberg JW, Bushra JS, Patel D, et al. A new pepsin assay to detect pulmonary aspiration of gastric contents among newly intubated patients. Am J Emerg Med. 2004;22:612–614. doi: 10.1016/j.ajem.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Stovold R, Forrest IA, Corris PA, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: a putative association with rejection. Am J Respir Crit Care Med. 2007;175:1298–1303. doi: 10.1164/rccm.200610-1485OC. [DOI] [PubMed] [Google Scholar]
- 41.Potluri S, Friedenberg F, Parkman HP, et al. Comparison of a salivary/sputum pepsin assay with 24-hour esophageal pH monitoring for detection of gastric reflux into the proximal esophagus, oropharynx, and lung. Dig Dis Sci. 2003;48:1813–1817. doi: 10.1023/a:1025467600662. [DOI] [PubMed] [Google Scholar]
- 42.D'Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1144–1152. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]


