Abstract
Background
Direct testing of DBS mechanisms in humans is needed to assess therapy and to understand stimulation effects.
Objective
We developed an innovative paradigm for investigation of deep brain stimulation (DBS) on human movement disorders. Temporary connection to the DBS electrode during implantable pulse generator (IPG) replacement permitted analysis of novel patterns of stimulation on motor symptoms, which could enhance efficacy and improve battery life.
Materials and Methods
Patients enrolled in this prospective, IRB-approved study underwent IPG replacement using local (monitored) anesthesia. Following device explant, the DBS electrode was connected to an external, isolated electrical stimulator using a sterile adapter cable. Different temporal patterns of stimulation were delivered while quantifying upper-extremity tremor (tri-axial accelerometry) or bradykinesia (finger-tapping). Upon experiment completion the new IPG was implanted.
Results
Among 159 IPG replacements from 2005-2011, 56 patients agreed to the research study (16 ET, 31 PD, 5 mixed ET/PD tremor, 3 multiple sclerosis, 1 tremor/myoclonus). Surgical procedures were extended by 42 ± 8.2 minutes in 37 patients completing the study. Motor symptoms varied with stimulation pattern, with some patterns showing improved tremor or bradykinesia control. No post-operative infections or complications were observed in the 159 patients.
Conclusion
IPG replacement occurs when the DBS/brain interface is stable and patients demonstrate symptom reduction with known stimulation parameters. Conducting research at this time point avoids DBS implant issues, including temporary microlesion effects, fluctuating electrode impedances, and technical limitations of contemporary IPGs, providing advantageous conditions to conduct translational DBS research with minimal additional risk to research subjects.
Keywords: Deep Brain Stimulation, Subthalamic Nucleus, Thalamus, Movement Disorders
INTRODUCTION
Although deep brain stimulation (DBS) is an effective treatment for movement disorders including essential tremor (ET), Parkinson’s disease (PD), and dystonia, mechanisms underlying the effects of stimulation are only slowly being revealed (1,2). One factor limiting the advancement and understanding of DBS is the limited capability of clinically available implantable pulse generators (IPGs). These devices allow selection of stimulation pulse duration, voltage, and frequency over a pre-specified range (3), and can be used to test the effects of stimulation parameter variation on motor symptoms and physiologic function (4-9). However, present clinical devices lack the ability to control the temporal pattern of stimulation or to record from implanted electrodes, although newer devices will incorporate biopotential recording (10,11). Here we describe an innovative paradigm for intraoperative research on DBS mechanisms, conducted following direct, temporary connection of an external stimulator to the DBS electrode during IPG replacement.
There are few opportunities to study DBS mechanisms in patients with implanted electrodes. During the initial implantation surgery, microelectrodes are commonly used for stimulation and recording, prior to placement of the DBS electrode (12-14). However, once the DBS electrode is placed the effects of stimulation may be occluded by the “microlesion” effect, limiting research on specific DBS parameters (15). Short-term experiments can be carried out with percutaneous extensions attached to the DBS electrodes for up to 2-3 weeks after the initial implant, prior to placement of the IPG (16,17). However, it may be difficult to evaluate effects of stimulation when appropriate electrode contacts and stimulation parameters for symptom relief are neither established nor stable. Further, although recordings are possible during this period (17), the amplitude and character of local field potentials change in the immediate post-operative period (18).
Our novel intraoperative approach is conducted at a stable time, well after the DBS electrode implant (i.e., > 6-12 months), during the elective IPG replacement procedure; this paradigm overcomes several of the limitations associated with testing in the period immediately following electrode implantation. This approach enables early translation of new developments to human subjects, and may prove useful for exploring future extensions of DBS technology into “smart” neuroprosthetic development (19), such as recording evoked responses from the DBS electrode during or between stimulation pulses (20) for the possibility of automatic, internal DBS adjustment (21).
MATERIALS AND METHODS
Following review and approval by the Duke University Institutional Review Board (IRB), patients with DBS for treatment of movement disorders undergoing elective IPG replacement were recruited to participate in the research study. All subjects participated on a volunteer basis and gave written informed consent prior to enrollment. DBS mechanisms of action were explored through intraoperative quantification of motor symptoms in response to novel temporal patterns of stimulation.
Inclusion and Exclusion Criteria
Candidates for study inclusion were previously diagnosed with essential tremor, Parkinson’s disease (either akinetic or tremor dominant), multiple sclerosis (with ataxia), or myoclonic tremor by a neurologist specializing in movement disorders, and required replacement of one or more DBS IPGs. Subject recruitment occurred at least 6-12 months following DBS electrode implant or revision, once stimulation parameters were clinically effective and stable. Eligible subjects were neurologically stable, capable of executing the intraoperative motor symptom quantification tasks, and demonstrated a reduction of motor symptom severity in response to clinical DBS parameters during preoperative evaluation by the study team. Subjects were asked to forego perioperative sedation and withhold dopaminergic and anti-tremor medications for 12 hours prior to surgery though withholding treatment medications was waived when clinically contraindicated.
Patients were excluded from the study if they exhibited dementia, pregnancy, tremor severity in the DBS off condition that would pose a safety risk during surgery, required general anesthesiaduring IPG replacement (chosen electively or required for extension re-tunneling), or previously underwent other movement disorders surgery (thalamotomy, pallidotomy, etc.).
Intraoperative Methods
Patients undergoing IPG replacement were positioned comfortably supine and the IPG location was prepped, draped, and fully anesthetized with local anesthesia (20-40 ml of 1% lidocaine or 0.5% marcaine) with an anesthesiologist supervising (monitored anesthesia; Fig. 1). The patient’s face and arm contralateral to the IPG were left fully exposed and mobile to facilitate interaction with the experimenters. Following exposure of the IPG, the extension cable was disconnected from the IPG header and connected to an adapter (1×4 Pocket Adaptor for Deep Brain Stimulation, #64001; Medtronic Inc., Minneapolis, MN; Fig. 2F) which was snapped into an adapter box and extension cable (Multi-lead Trailing Cable, #355531; Medtronic Inc.; Fig. 2E), and connected outside the sterile surgical field to an external stimulator (Fig. 2D) using a custom non-sterile extension cable.
Figure 1. Intraoperative Setup and Instrumentation.
During the IPG replacement procedure it is important to maintain the sterile field (since an implant is involved), to allow ready access to the patient by the anesthesiologist for monitoring and assessment of the clinical condition, to allow easy and full control of the extremity being tested by the experimenters, and to maintain a visual path from the patient to the experimenters for instruction and to view the extremity being controlled. The diagram shows how these various goals are achieved, with a partial drape across the patient and complete separation of the sterile surgical field and the non-sterile experimentation and monitoring region.
Figure 2. Research Equipment.
Custom stimulation trains are generated by (A) a laptop-controlled, (B) isolated multifunction data acquisition (DAQ) device. Unilateral stimulation is applied through (C) an optical stimulus isolator using stimulation contacts selected by (D) a custom passive switch box connected to the stimulation electrode via a sterile cable (E) and adaptor (F). Bradykinesia (G) and tremor (H) quantification signals are digitized by the DAQ unit and stored on the laptop.
Unilateral stimulation was applied using an optical stimulus isolator (bp Isolator; FHC Inc., Bowdoinham, ME; Fig. 2C) connected to a laptop-controlled, isolated multifunction data acquisition device (USB-6216 BNC; National Instruments, Austin, TX; Fig. 2A,B). Stimulus train parameters were controlled with custom software (LabVIEW; National Instruments, Austin, TX), using charge-balanced, biphasic, regulated voltage pulses similar to those produced by the IPG. Pulse width and stimulus amplitude were set equal to, or less than values programmed clinically; this prevented charge densities from exceeding the manufacturer’s recommended limit of 30 μC/cm2 per phase (22). Bipolar stimulation contacts were selected for each patient, using clinically programmed combinations for patients with bipolar configurations, and switching stimulation return to a clinically unused contact for patients with monopolar configurations. A custom passive switch box joining the stimulus isolator to the non-sterile extension cable was used to make these connections and high-pass filter any DC offset in the stimulation signal (Fig. 2D). Safety checks of all equipment were conducted by Duke University Clinical Engineering on an annual basis, and the Duke University IRB approved all study equipment prior to use in human subjects.
Different temporal patterns of stimulation were delivered to each patient to investigate the effects of variable levels of stimulation pattern irregularity on tremor (23) and bradykinesia (24). Each stimulation train was constructed as a point process where the time between consecutive pulses was a random variable drawn from a gamma probability distribution with a mean of 130 Hz. The standard deviation of this distribution was varied, resulting in high-frequency stimulation trains with different levels of irregularity but the same average frequency. Additional stimulation patterns were designed to test whether long pauses, bursts, or irregularity per se reduced the effectiveness of temporally patterned stimulation on tremor (25). These included three irregular patterns with high entropies, two periodic patterns with low entropy and long pauses or short bursts of pulses, and regular high-frequency stimulation. Motor symptom responses were quantified to determine baseline symptom severity, assess stimulation pattern efficacy, and detect potential carry-over effects influenced by the time course of symptom response, as shown in the outline of the trial design (Fig. 3). Patients were blinded to the order of stimulation train delivery, which was randomized within a repeated measures design.
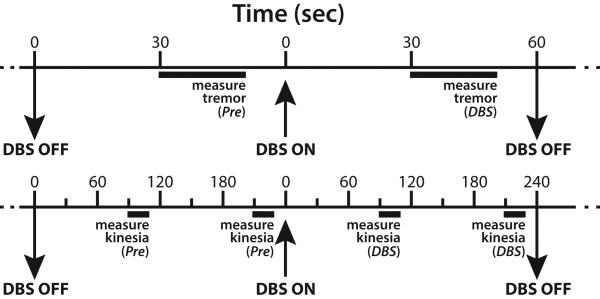
Figure 3. Experimental Trial Design.
Protocols for measuring tremor (above) and bradykinesia (below) include control trials with DBS off (Pre) to establish baseline symptom severity, which may vary with time, followed by trials with DBS on (DBS) to determine the effect of the stimulation treatment. Motor symptoms were quantified during 20 sec trials every 60 sec (tremor) or 120 sec (bradykinesia), and this pattern was repeated for each stimulation treatment.
Tremor was quantified in patients with essential tremor, myoclonic tremor, Parkinson’s disease, and multiple sclerosis using a triaxial accelerometer (CXL04LP3, Crossbow; San Jose, CA) fixed to the dorsum of the hand (Fig. 2H). Accelerometric tremor recordings correlate well with several clinical tremor rating scales (26). Spectral analysis was performed on the recorded x-, y-, and z-axis acceleration signals (power spectral density, Welch’s averaged periodogram, Hanning window, fast Fourier Transform (FFT) length = 5000) in MATLAB (MathWorks; Natick, MA) to quantify tremor severity and frequency (Fig. 4). Each spectrum was integrated from 2-20 Hz, spanning the typical frequency range of tremor (27), and these values were summed to yield the tremor power. Tremor power was averaged for each stimulation pattern across all subjects for statistical analysis (repeated measures ANOVA or linear mixed-modeling) of the patient population.
Figure 4. Typical Results.
Tremor amplitude (A) measured by accelerometric recordings show modulation by DBS. The power spectral density (B) of the recordings was integrated from 1-20 Hz to yield tremor power (C), which decreased with DBS ON. Parkinsonian finger-tapping durations (D) were regularized with DBS ON, decreasing the log-transformed coefficient of variation (CV) of tap duration (E).
In patients with akinetic Parkinson’s disease, bradykinesia was assessed by the rate and regularity of alternate clicking of the two buttons of a mouse with the index and middle fingers of the hand contralateral to stimulation (Fig. 2G). These measures are well correlated with the motor subscore of the UPDRS, and the bradykinesia subscore, in particular (28). The number and duration of the mouse clicks were quantified and converted to a measure of bradykinesia, log CV duration (Figure 4).
Following conclusion of experimental measurements, the DBS extension was disconnected from the adapter, connected to the new IPG, and the replacement procedure continued according to normal care. Patients were followed within 2-3 weeks after IPG replacement to evaluate clinical DBS parameters and monitor for postoperative infection.
Complications Review
We analyzed all DBS IPG replacement procedures conducted at Duke University for comparison to the research subset, also under Duke University IRB approval. Any complications following such procedures are noted or rapidly treated by the movement disorders team, since these patients are followed closely in one clinic. This gives a concurrent denominator for the total population of patients from which the research sample was drawn.
RESULTS
Stimulator Device Change Procedures
Between 2005 and 2011 we conducted 56 intraoperative stimulation experiments at Duke University Hospital, out of a total of 159 IPG replacement surgical procedures, and 37/56 subjects completed the experimental protocol. The IPGs being replaced included a mixture of Soletra and Kinetra devices (Medtronic, Inc.), treating a wide variety of movement disorders. The research patient population (n=56) demonstrated various movement disorders: 16 ET, 31 PD, 5 mixed ET/PD tremor, 3 multiple sclerosis/ataxia, and 1 tremor/myoclonus. Device batteries lasted 2.9 ± 1.8 years (n=159 total) before needing replacement, for tremor and Parkinsons patients (29). The surgical procedures for IPG replacement were extended by 42 ± 8.2 minutes in the 37 patients completing the study. No infections or other complications were observed in either the overall group or the smaller, research subset.
Quantifying the Effect of DBS on Tremor
The results of a typical experiment on a patient with ET and thalamic (Vim) DBS are shown in Figure 4 (A-C). The amplitude of accelerometric recordings of tremor varied with DBS on and off (Fig. 4A), as did the spectral power of these recordings at low frequencies (Fig. 4B). Mean log-transformed tremor power decreased with DBS on (Fig. 4C), showing a significant reduction in tremor. Comparable results were used to determine the effects of specific characteristics of the temporal pattern of stimulation on tremor. Tremor reduction in response to gamma-distributed pulse trains decreased as the irregularity of stimulation increased (23). Additional investigation showed that there were no significant differences in tremor between trains with or without bursts or between trains that were irregular or periodic, but stimulus trains with pauses were significantly less effective than trains without them. These results suggest that reduced efficacy of random stimulation was due to pauses in stimulus trains, rather than temporal irregularity per se (25).
Quantifying the Effect of DBS on Bradykinesia
The results of a typical experiment on a patient with PD and STN DSB are shown in Figure 4 (D,E). Alternating clicking of the two buttons of a computer mouse was used to quantify the effects of different temporal patterns of DBS on bradykinesia (24). The mouse clicks can be performed in a short period of time (Fig. 4D), and the log CV duration demonstrates a significant effect of DBS (Fig. 4E). Temporally irregular stimulus trains also reduced tapping variability, but increasing stimulation pattern irregularity reduced efficacy, and these results support the hypothesis that effective DBS regularizes neuronal firing patterns (25).
DISCUSSION
This type of novel, translational research procedure provides a unique and powerful approach to explore mechanisms of DBS function, since direct access to the intracranial electrodes is very limited during the clinical treatment of these patients. Although it is routine to test electrodes for clinical function during the initial implant procedure, the brain-electrode interface is not yet fully established, and symptom severity commonly changes during the procedure (15,18). For example, it is common that tremor may dissipate over time with the electrode in place, due to a “microthalamotomy” effect; the tremor may not return postoperatively for days to weeks due to the mild edema that develops around the electrode (15). Similarly, there is a significant improvement in bradykinesia intraoperatively following STN electrode implants, and even over the short intraoperative time this may evolve, precluding careful distinction of DBS parameter effects. After several months the interface with the DBS electrode stabilizes and the clinical parameters for treatment become more consistent (9). Thus, at the time of the IPG replacement surgery, the parameters are well defined for optimal improvement in symptoms, and the electrode characteristics are stable. Therefore, this is an excellent time to test the functioning and mechanisms of the DBS. This approach may also be suitable for recording biopotentials from the implanted electrode (10,11,21), which can be used to explore paradigms for closed-loop control of DBS (19).
The disadvantages to such intraoperative research include the extra time required, the need to have an awake patient who can cooperate with experimenter instructions, and the possible risks of the additional intraoperative time, particularly infection. However, infection with DBS battery replacement appears to be a very uncommon complication (30,31), and the rate of infection is sufficiently low that we cannot estimate the true rate for our current sample. Intraoperative stimulation was limited to bipolar contact configurations for all patients in our cohort, including those that typically used monopolar stimulation, and this may have influenced the symptom response to stimulation.
However, our paradigm could be modified to deliver monopolar stimulation using a temporary infraclavicular return electrode near the IPG pocket. Common reasons for patients declining participation in the research include possible anxiety during the awake procedure and particularly severe, uncomfortable symptom recurrence, more than the remote risk of infection. For example, some patients with exceptionally severe tremor are intensely uncomfortable when their DBS is off, hence they may request general anesthesia for the IPG replacement procedure.
In conclusion, this novel translational research platform takes advantage of stable clinical stimulation parameters and DBS/brain interface, shows very low risk, and enables direct temporary connection to the implanted brain electrode. These conditions make IPG replacement surgery an advantageous, heretofore underutilized setting for DBS mechanisms research.
Acknowledgments
Financial Support: NIH Grants R21 NS066115 and R01 NS40894
Footnotes
Authorship Statement: All authors contributed to the design, conduct, analysis, and manuscript preparation of this study. Each reviewed and approved the final manuscript. Funding was provided by Drs. Turner and Grill through NIH R21 NS066115 and R01 NS40894.
Conflict of Interest Statement: There are no personal or institutional financial interests in this research by any author.
REFERENCES
- 1.Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17(Suppl 3):S69–72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- 2.Birdno MJ, Grill WM. Mechanisms of deep brain stimulation in movement disorders as revealed by changes in stimulus frequency. Neurotherapeutics. 2008;5:14–25. doi: 10.1016/j.nurt.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clin Neurophysiol. 2004;115(11):2431–41. doi: 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Beuter A, Titcombe MS. Modulation of tremor amplitude during deep brain stimulation at different frequencies. Brain Cogn. 2003;53:190–2. doi: 10.1016/s0278-2626(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 5.Heming EA, Choo R, Davies JN, Kiss ZH. Designing a thalamic somatosensory neural prosthesis: consistency and persistence of percepts evoked by electrical stimulation. IEEE Trans Neural Syst Rehabil Eng. 2011;19:477–82. doi: 10.1109/TNSRE.2011.2152858. [DOI] [PubMed] [Google Scholar]
- 6.Herzog J, Hamel W, Wenzelburger R, Pötter M, Pinsker MO, Bartussek J, et al. Kinematic analysis of thalamic versus subthalamic neurostimulation in postural and intention tremor. Brain. 2007;130:1608–25. doi: 10.1093/brain/awm077. [DOI] [PubMed] [Google Scholar]
- 7.Kuncel AM, Cooper SE, Wolgamuth BR, Clyde MA, Snyder SA, Montgomery EB, Jr, et al. Clinical response to varying the stimulus parameters in deep brain stimulation for essential tremor. Mov Disord. 2006;21:1920–8. doi: 10.1002/mds.21087. [DOI] [PubMed] [Google Scholar]
- 8.O’Suilleabhain PE, Frawley W, Giller C, Dewey RB., Jr. Tremor response to polarity, voltage, pulsewidth and frequency of thalamic stimulation. Neurology. 2003;60:786–90. doi: 10.1212/01.wnl.0000044156.56643.74. [DOI] [PubMed] [Google Scholar]
- 9.Waldau B, Clayton DA, Gasperson LB, Turner DA. Analysis of the time course of the effect of subthalamic nucleus stimulation upon hand function in Parkinson’s patients. Stereotact Funct Neurosurg. 2011;89:48–55. doi: 10.1159/000323340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanslaski S, Cong P, Carlson D, Santa W, Jensen R, Molnar G, et al. An implantable bi-directional brain-machine interface system for chronic neuroprosthesis research. Conf Proc IEEE Eng Med Biol Soc. 2009:5494–7. doi: 10.1109/IEMBS.2009.5334562. [DOI] [PubMed] [Google Scholar]
- 11.Stanslaski S, Afshar P, Cong P, Giftakis J, Stypulkowski P, Carlson D, et al. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation. IEEE Trans Neural Syst Rehabil Eng. 2012;20:410–21. doi: 10.1109/TNSRE.2012.2183617. [DOI] [PubMed] [Google Scholar]
- 12.Filali M, Hutchison WD, Palter VN, Lozano AM, Dostrovsky JO. Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp Brain Res. 2004;156:274–81. doi: 10.1007/s00221-003-1784-y. [DOI] [PubMed] [Google Scholar]
- 13.Hanson TL, Fuller AM, Lebedev MA, Turner DA, Nicolelis MA. Subcortical neuronal ensembles: an analysis of motor task association, tremor, oscillations, and synchrony in human patients. J Neurosci. 2012;32:8620–32. doi: 10.1523/JNEUROSCI.0750-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil PG, Carmena JM, Nicolelis MA, Turner DA. Ensemble recordings of human subcortical neurons as a source of motor control signals for a brain-machine interface. Neurosurgery. 2004;55:27–38. [PubMed] [Google Scholar]
- 15.Kondziolka D, Lee JY. Long-lasting microthalamotomy effect after temporary placement of a thalamic stimulating electrode. Stereotact Funct Neurosurg. 2004;82:127–30. doi: 10.1159/000079844. [DOI] [PubMed] [Google Scholar]
- 16.Birdno MJ, Cooper SE, Rezai AR, Grill WM. Pulse-to-pulse changes in the frequency of deep brain stimulation affect tremor and modeled neuronal activity. J Neurophysiol. 2007;98:1675–84. doi: 10.1152/jn.00547.2007. [DOI] [PubMed] [Google Scholar]
- 17.Ince NF, Gupte A, Wichmann T, Ashe J, Henry T, Bebler M, et al. Selection of optimal programming contacts based on local field potential recordings from subthalamic nucleus in patients with Parkinson’s disease. Neurosurgery. 2010;67:390–7. doi: 10.1227/01.NEU.0000372091.64824.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa M, Marceglia S, Servello D, Foffani G, Rossi L, Sassi M, et al. Time dependent subthalamic local field potential changes after DBS surgery in Parkinson’s disease. Exp Neurol. 2010 Apr;222:184–90. doi: 10.1016/j.expneurol.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Riley J, Raore B, Boulis N. Chained lightning: advances in neuromodulation. Clin Neurosurg. 2009;56:9–17. [PubMed] [Google Scholar]
- 20.Kent AR, Grill WM. Recording evoked potentials during deep brain stimulation: development and validation of instrumentation to suppress the stimulus artefact. J Neural Eng. 2012;9:036004. doi: 10.1088/1741-2560/9/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouse AG, Stanslaski SR, Cong P, Jensen RM, Afshar P, Ullestad D, et al. A chronic generalized bi-directional brain-machine interface. J Neural Eng. 2011;8:036018. doi: 10.1088/1741-2560/8/3/036018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medtronic, Inc. N’Vision Clinician Programmer Guide for Activa RC, Activa PC, and Activa SC. Medtronic, Inc.; Minneapolis, MN: 2010. [Google Scholar]
- 23.Birdno MJ, Kuncel AM, Dorval AD, Turner DA, Grill WM. Tremor varies as a function of the temporal regularity of deep brain stimulation. Neuroreport. 2008;19:599–602. doi: 10.1097/WNR.0b013e3282f9e45e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorval AD, Kuncel AM, Birdno MJ, Turner DA, Grill WM. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol. 2010;104:911–21. doi: 10.1152/jn.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birdno MJ, Kuncel AM, Dorval AD, Turner DA, Gross RE, Grill WM. Stimulus features underlying reduced tremor suppression with temporally patterned deep brain stimulation. J Neurophysiol. 2012;107:364–83. doi: 10.1152/jn.00906.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elble RJ, Pullman SL, Matsumoto JY, Raethjen J, Deuschl G, Tintner R, et al. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain. 2006;129:2660–6. doi: 10.1093/brain/awl190. [DOI] [PubMed] [Google Scholar]
- 27.Pahwa R, Lyons KE. Essential tremor: differential diagnosis and current therapy. Am J Med. 2003;115:134–42. doi: 10.1016/s0002-9343(03)00259-6. [DOI] [PubMed] [Google Scholar]
- 28.Taylor Tavares AL, Jefferis GS, Koop M, Hill BC, Hastie T, Heit G, et al. Quantitative measurements of alternating finger tapping in Parkinson’s disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation. Mov Disord. 2005;20:1286–98. doi: 10.1002/mds.20556. [DOI] [PubMed] [Google Scholar]
- 29.Halpern CH, McGill KR, Baltuch GH, Jaggi JL. Longevity analysis of currently available deep brain stimulation devices. Stereotact Funct Neurosurg. 2011;89:1–5. doi: 10.1159/000321710. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia R, Dalton A, Richards M, Hopkins C, Aziz T, Nandi D. The incidence of deep brain stimulator hardware infection: the effect of change in antibiotic prophylaxis regimen and review of the literature. Br J Neurosurg. 2011;25:625–31. doi: 10.3109/02688697.2011.566384. [DOI] [PubMed] [Google Scholar]
- 31.Fenoy AJ, Simpson RK., Jr. Management of device-related wound complications in deep brain stimulation surgery. J Neurosurg. 2012;116:1324–32. doi: 10.3171/2012.1.JNS111798. [DOI] [PubMed] [Google Scholar]