Abstract
Risk factors driving sex disparity in esophageal cancer are unclear. Recent molecular evidence suggests hormonal factors. We conducted a national descriptive epidemiological study to assess the hypothesis that estrogen exposure could explain the male predominance in observed esophageal adenocarcinoma incidence. We analyzed the esophageal cancer incidence trends by histology and sex from 1973 to 2008 in nine population-based cancer registries of the Surveillance Epidemiology and End Results (SEER) 9 Registry Database. We used age as a proxy for estrogen exposure in females. The collective age-groups annual percentage change in esophageal adenocarcinoma for females is positive (0.03%; 95% confidence interval: 0.02, 0.03%) during the study period. Interestingly, the esophageal adenocarcinoma annual percentage change in incidence rates for females during the same time period is significantly negative from ages 50–54 to ages 60–64. Even though the incidence of esophageal adenocarcinoma rises in both males and females, the male to female ratio across age peaks in the 50–54 years then decreases. Furthermore, the esophageal adenocarcinoma age-adjusted incidence rate in post-menopausal females age 80 and above increases with age unlike their male counterparts. Taken together, these data support the hypothesis that the endocrine milieu in pre- and peri-menopausal females serves as a protective factor against esophageal adenocarcinoma, and with loss of estrogen or due to the increasing time period away from estrogen exposure, the rate of esophageal adenocarcinoma incidence increases in the older post-menopausal female. Since females comprise the largest portion of the elderly population with esophageal adenocarcinoma, these findings are significant.
Keywords: Esophageal adenocarcinoma, Incidence, Estrogen, Sex disparity
Introduction
Esophageal cancer is among the most deadly malignancies of the gastrointestinal tract (1) with the two most common histologies being esophageal adenocarcinoma and esophageal squamous cell carcinoma. Esophageal adenocarcinoma incidence is 6–10 times higher, and esophageal squamous cell carcinoma incidence is 2–3 times higher, in men than women (2). Esophageal adenocarcinoma incidence has also been increasing in white males more rapidly than any other solid neoplasm in the Western world (3). The factors responsible for the sex disparity in esophageal adenocarcinoma incidence have not been defined, and do not appear to be exclusively associated with changes in known risk factors (4, 5). Recent reports suggest that hormonal factors may play a role in the observed sex disparity in esophageal adenocarcinoma incidence (6–10).
The current literature examining esophageal cancer incidence by age and sex over time is limited. There is a paucity of epidemiologic studies assessing estrogen exposure as a contributor to the sex ratio incidence differences seen in esophageal cancer. We conducted a national descriptive epidemiological study to assess the hypothesis that estrogen exposure could explain the sex disparity in observed esophageal adenocarcinoma incidence.
Materials and Methods
We analyzed esophageal adenocarcinoma incidence trends from 1973 to 2008 in the nine population-based cancer registries, individually and collectively, in the National Cancer Institute Surveillance Epidemiology and End Results (SEER) Research Database. The annual percentage change in incidence rates for esophageal cancer from SEER 9 in 1973 – 2008 is based on rates age-adjusted to the 2000 U.S. standard population. Linear regression models with joinpoint were used when analyzing trend changes of annual percentage change (APC) (NCI Joinpoint Program Version 3.5 and R). Analytic software identified when annual percentage change was significantly different from zero (p<0.05) and the points at which trend changed. The SEER Stat Software was used to calculate incidence rates (and 95% confidence intervals (CI)) per 100,000 person-years for males and females. Age-adjusted incidence rates were tabulated by years, age groups, race, both sex and overall. Delta method was applied when calculating male-to-female ratios in each five year age group for esophageal adenocarcinoma beginning at age 40 until age 84.
SEER 9 registries are located in Atlanta, Detroit, San Francisco, Seattle, Utah, New Mexico, Iowa, Hawaii, and Connecticut. All SEER sites are obliged to meet the Gold Standard Registry Certification from the North American Association of Central Cancer Registries, Inc., for completeness, accuracy, and timeliness of data. To obtain access to the SEER database we completed a data user agreement and the data were maintained on a password protected computer. This study was approved by the Johns Hopkins Institutional Review Board as exempted research.
Esophageal cancer histology in the SEER Research Database is coded according to the International Classification of Disease for Oncology (ICD-O) version 3. The codes 8140–8575 were used to identify esophageal adenocarcinoma.. We used age as a proxy for estrogen exposure in females as endogenous estrogen production decreases with the onset of menopause, which is largely age dependent. Calendar time changes in incidence rates were assessed to confirm secular trends in the occurrence of esophageal adenocarcinoma in men and women.
Results
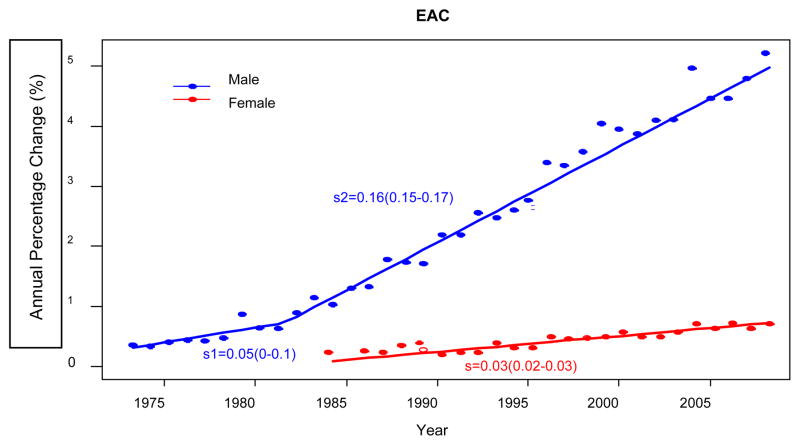
The annual percentage change in the collective (all age groups together) age-adjusted incidence rates for esophageal adenocarcinoma by sex reveals a positive and statistically significant increasing rate after 1983 for both females (0.03%; 95% CI 0.02–0.03%, 1983–2008) and males (0.05%; 95% CI: 0–0.1%) (Figure 1). Annual positive percentage change is significantly higher in males than in females during this period. Males also increased significantly, though moderately, during the period 1973–1980 (0.05%; 95% CI: 0–0.1%) (Figure 1).
Figure 1.
Esophageal adenocarcinoma (EAC) annual percentage change (APC) versus year by sex. Male sex presents one break point at year 1982. Female does not present break points.
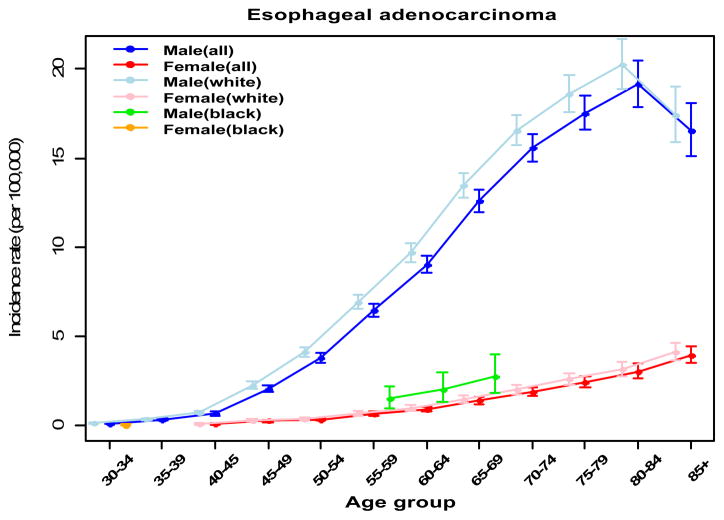
In contrast to males, the annual percentage change of incidence in esophageal adenocarcinoma across ages 50–54 to 60–64 years was negative and significantly below zero in the females (Table 1). In males across ages 40–44 to 70–74 years, the annual percentage change of incidence in esophageal adenocarcinoma increases and the annual percentage change across age is significantly above zero. Otherwise, the annual percentage change is comparable to zero for women in ages 65–69 and older and for men ages 75–79 or older (Table 1). By ages 80–84 the annual percentage change is negative and significantly below zero for males (Table 1). When esophageal adenocarcinoma incidence rates are viewed across the age continuum, overall incidence trends differ by histology and sex. Esophageal adenocarcinoma incidence in men is higher, increases faster at all ages and begins increasing at an earlier age compared to females (50–54 versus 55–59) (Figure 2). The graph of females shows more slowly increasing rate at all ages while the females aged 40–49 to 60–64 experienced a relatively flat trajectory (Figure2). Age-adjusted esophageal adenocarcinoma incidence rate in the post-menopausal females did not peak at 80–84 like their male counterparts, but instead continuously increased into the oldest age group (Figure 2). This finding was also evident within the six individual SEER sites with data sufficient to perform this statistical analysis.
Table 1.
Annual Percentage Change (APC) in incidence of EAC by age groups in SEER 9 database years 1973 – 2008.
| Age-Group | Esophageal adenocarcinoma Annual percentage change (95% CI) | |
|---|---|---|
| Male | Female | |
| 40–44 years | 1.31 (0.98, 1.64) | 0.40 (−0.14, 0.94) |
| 45–49 years | 1.91 (1.69, 2.13) | −0.15 (−0.52, 0.21) |
| 50–54 years | 2.52 (2.34, 2.70) | −0.42 (−0.71, −0.12) |
| 55–59 years | 2.46 (2.20, 2.73) | −0.56 (−0.79, −0.33) |
| 60–64 years | 2.27 (1.88, 2.66) | −0.40 (−0.50, −0.30) |
| 65–69 years | 2.01 (1.50, 2.53) | −0.03 (−0.02, 0.07) |
| 70–74 years | 1.23 (0.65, 1.81) | 0.12 (0.0, 0.25) |
| 75–79 years | 0.33 (−0.23, 0.89) | 0.09 (−0.05, 0.24) |
| 80–84 years | −0.58 (−1.12, −0.04) | 0.09 (−0.08, 0.25) |
Figure 2.
Incidence rates (per 100,000) of esophageal adenocarcinoma listed across age groups by sex. Mean and 95% CI were generated from SEER 9 database and indicated via points with error bar spread.
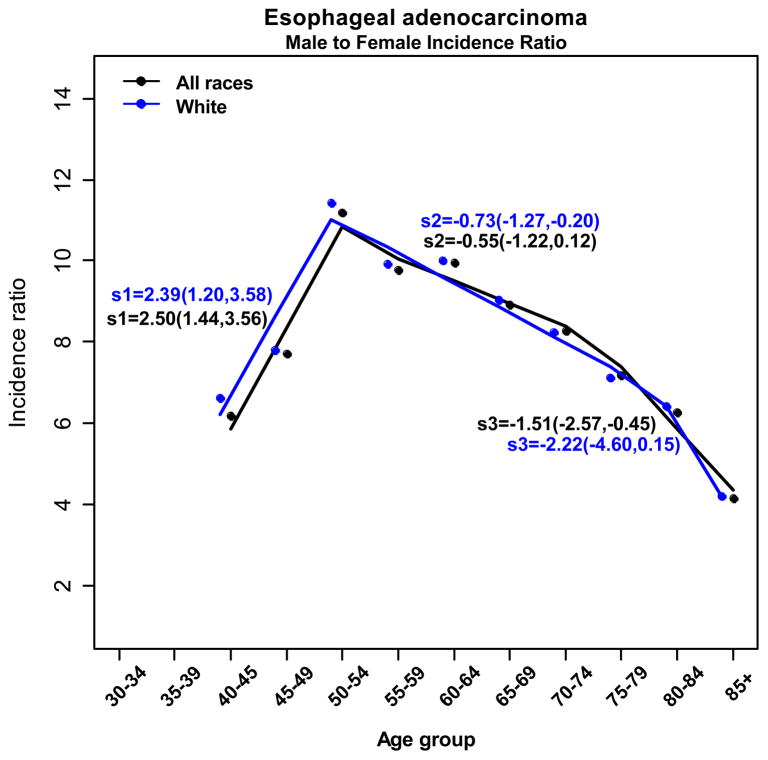
Evaluating the relationship between male and female esophageal adenocarcinoma incidence over age revealed that the overall male to female ratio of esophageal adenocarcinoma incidence is 7.66 during 1973 – 2008 (95% CI: 7.28–8.06) (Fig. 3). Even though the incidence of esophageal adenocarcinoma rises in both males and females, the esophageal adenocarcinoma male-to-female ratio is highest at 50–54 years and progressively declines thereafter. The esophageal adenocarcinoma male to female ratio decreases from a high of 11:1 at age 50–54 to a low of 4:1 in those aged 75–79. Our analysis revealed that the age-specific male to female ratio shifts abruptly (statistically significant joinpoint in 50–54 years) in the peri-menopausal period (Figure 3). We also evaluated the male to female ratio over five different time periods between 1973 and 2008 and found a decreasing sex ratio after age 50–54 in all time periods for esophageal adenocarcinoma.
Figure 3.
Black solid line (all races) and blue solid line (white only) indicates the predicted value of male to female incidence ratio by age after fitting regression models with joint point approach on all races of esophageal adenocarcinoma patients. Fitted slopes with 95% CI were indicated next to the lines.
Discussion
We observed the previously reported epidemic increase of esophageal adenocarcinoma in white males seen over the past three decades (11). While the majority of the esophageal cancer epidemiology literature focus has been on studying this epidemic increase in males, few studies have examined the epidemiology of esophageal cancer over different time periods for different age groups by sex (12). Our study does not focus on the well-characterized trend in white male esophageal adenocarcinoma, but instead evaluates the sex disparity in esophageal adenocarcinoma incidence over time and reveals a pattern and a troubling trend for esophageal adenocarcinoma incidence within the aging post-menopausal female population. To our knowledge, there is only one age-period-cohort assessment conducted on the incidence of esophageal adenocarcinoma and that is from a SEER site, the state of Connecticut, 1935–1989 (13). The study revealed that regardless of age every male birth cohorts had rapidly increasing rates of esophageal adenocarcinoma; whereas female birth cohorts also had more slowly increasing rates for females 50 and older while females aged 40–49 experienced a qualitatively flatter trajectory. This suggests a hypothesis is needed to evaluate this age-related biologic effect with no significant generational effects.
In support of the hypothesis that a reproductive factor is protective among females, estrogen has been shown to inhibit esophageal squamous cell cancer growth both in vitro (14, 15) and in vivo (16). This anti-proliferative effect is thought to occur through the estrogen receptor ER beta, which has been shown to be expressed also in esophageal adenocarcinoma (17). Experimental studies on a colon cancer cell line suggest that estrogen may inhibit cancer progression by ER beta reducing ER alpha-induced proliferation (18). Furthermore, a recent study by Sukocheva et al suggested that esophageal adenocarcinoma and Barrett’s esophagus cells respond to treatment with selective estrogen receptor ligands, resulting in decreased cell growth and apoptosis (19). As females are exposed to higher estrogen concentrations for substantial durations (menarche to menopause) throughout their reproductive years, it is biologically plausible that the pre-menopausal endocrine milieu could inhibit esophageal carcinogenesis in females preferentially.
There is also precedence for estrogen exposure being an inhibitor of carcinogenesis in other human intestinal malignancies. A recent meta-analysis by Camargo et al supports the hypothesis that longer exposure to either ovarian or exogenous estrogen may decrease the risk of gastric cancer (20). Moreover, hormonal status not only seems to play an important role in the development and pathogenesis of colorectal adenocarcinoma, but also may be of prognostic significance (21). Furthermore, epidemiological and experimental studies suggest a protective role of estrogens against colorectal cancer (22). In the Women’s Health Initiative trial, postmenopausal hormone use was associated with a 40% decrease in colorectal cancer (23). Hormone replacement was also found to protect premenopausal women who used oral contraceptives use reducing the risk of developing colorectal cancer by 20% (24, 25). Given these similar observations of changing cancer incidence in developmentally related components of the digestive tract, it is possible that the pathophysiology seen in the colon and stomach is similar to that of the esophagus. The apparent coherence of a protective effect of endogenous and exogenous estrogen against cancers of the colon and stomach may extend to include cancers of the esophagus.
In the SEER 9 registry, esophageal adenocarcinoma in the pre-menopausal female is uncommon. Incidence of esophageal cancer is age-related increasing steadily with age irrespective of sex. We identified a novel observation that a negative annual percentage change exists for esophageal cancer between 1973 –2008 specifically for females from ages 50–54 to ages 60–64. This indicates, surprisingly and in contrast to males, that the females during reproductive years and early post-menopausal years are somehow protected and less likely to develop esophageal cancer until later in life. The explanation for this countervailing trend is unclear, but could be in part attributed to exposure to estrogen including hormonal replacement therapy, which occurred among peri- and post-menopausal women in the United States during this period of time (26, 27). Additional age-period-cohort assessment would help distinguish between true age-related (biologic) effects and birth-cohort or generational (exposure/etiologic) effects.
We present a detailed analysis of temporal trends in esophageal adenocarcinoma incidence, by, age and sex. We found that the age-adjusted incidence rates in the post-menopausal females above age 80 continuously increases with age unlike their above age 80 male counterparts. We also demonstrated that the age-adjusted esophageal adenocarcinoma incidence rates over age differ by sex as exemplified by the male-to-female incidence ratio of esophageal adenocarcinoma. Steady decline in the ratio from ages 50–54 years and older population suggests that the incidence of esophageal adenocarcinoma in older females is increasing disproportionately after age 50–54, leading to a decrease in the male-to female ratio in the post-menopausal period. What is surprising is that this is observed at a time when males have had an accumulated burden that is larger in magnitude compared to women. Women experience the same rates 15–25 years later than males at comparable age. (Figure 3) These data support the hypothesis that temporal exposure during the reproductive years and perhaps to estrogen is associated with a protective effect against esophageal carcinogenesis with higher rates shifted to older ages. Confirmation of this hypothesis would be supported further by data not available in the SEER database such as individual hormonal levels, and lack of support for the hypothesis may be found in examining potential confounders and known influencers of esophageal cancer occurrence (e.g. Barrett’s esophagus, gastroesophageal reflux, alcohol use, tobacco exposure, and intra-abdominal fat) in cohorts. Biologic plausibility for the hypothesis would be supported if a gastrointestinal tract developmental link or common molecular mechanisms could be confirmed.
Barrett’s esophagus is clearly associated with GERD and is part of a continuum to esophageal adenocarcinoma development. Barrett’s esophagus is the biggest risk factor for esophageal adenocarcinoma and in theory it is possible that differences between age and sex in the incidence of esophageal adenocarcinoma mirrors age and sex differences in Barrett’s esophagus. The role of age and sex disparities in Barrett’s esophagus incidence remains fairly unknown and it is unclear if the age and sex differences in esophageal adenocarcinoma are due to Barrett’s esophagus age and sex differences. According to a meta-analysis conducted by Cook and colleagues, the male to female sex ratio of Barrett’s esophagus is often based on nonsystematic estimates derived from small populations at a few selected institutes while the larger cohorts are from Veterans hospitals and these study populations may not be truly representative of the general population (28). Furthermore, Cook and colleagues’ meta-analysis stated that reported estimates of Barrett’s esophagus and erosive reflux disease are substantially lower than similar estimates for esophageal adenocarcinoma (28). They believe these differences make it important to establish why male Barrett’s esophagus and erosive reflux disease patients are at increased risk of malignancy compared to females. Like Sukocheva et al, we believe it is the estrogen-based endocrine milieu even in Barrett’s esophagus that contributes to decreasing malignancy risk for females (19). Pohl and colleagues in a case control study find that females are statistically significantly over represented in GERD while underrepresented in Barrett’s esophagus (29). This may be an indication of where in the progression to cancer, the “female” factor is most protective.
Our data imply as the aging post-menopausal female is exposed to a prolonged period of low estrogen levels their risk and incidence of esophageal adenocarcinoma increases. Females encounter a delay in age-related increase esophageal adenocarcinoma incidence rates that may be associated with the protective effects of estrogen exposure before menopause. Using age as a proxy for estrogen exposure, our findings suggest consistency with a hormonal component to the observed age-related, declining male to female esophageal adenocarcinoma incidence rate ratios. Menopause is an event that marks the end of spontaneous ovulation coupled with a significant decrease in estrogen. In the Western world, the average age of menopause is 51 years and according to the Nurse’s Health Study, prevalence menopause was documented by age: at 40 years prevalence of having undergone menopause was 0.7%, at 45 5.8%, at 50 34.9%, at 55 85.3%, at 60 98.5% and at 65 99.3% the largest accrual of menopause occurs between ages 50 and 55 and second most commonly between age 45 and 50 (30).
Using age as a proxy for female reproductive age exposures has limitations as menopause can be induced surgically (i.e. bilateral oopherectomy) or medically (e.g. chemotherapy or pelvic irradiation) at any age. We also are aware that estrogen production during natural menopause does not stop abruptly as five to seven years before the onset of the last menstrual period, ovarian function begins to diminish. In addition, premature menopause can occur at or before age 40 and in the United States its prevalence is estimated 1 case per 1000 women by age 30, 1 case per 250 women by age 35 and 1 case per 100 women by age 40. Taking these data together, it is not entirely surprising that the annual percentage change starts to decrease at the age group before the majority of women have entered menopause. Our inability to quantify hormone levels of individual patients from the SEER database made age the best available surrogate.
Our data confirm sex differences in incidence previously observed in esophageal adenocarcinoma and suggest that the endocrine milieu in the pre and peri-menopausal females serves as a protective factor against the development of esophageal adenocarcinoma. Estrogen appears to protect females during their reproductive years. The hypothesized protective pre-menopausal effect of estrogen in females disappears with time as seen in the 60–64 age group and above post-menopausal female, in which the rate of esophageal adenocarcinoma incidence in those groups increases. This lost of protection from esophageal carcinoma incidence may also be due to an increasing period of time without endogenous or exogenous estrogen protection. Furthermore, a latency period may exist for esophageal adenocarcinoma development after the removal of endogenous and exogenous estrogen in the post menopausal female.
The rate of female esophageal adenocarcinoma incidence continuously increases into later age relative to males and post-menopausal women comprise an increasingly large portion of the population. As the putative protective effects of estrogen exposure dissipate in post-menopausal females, the continuous increase in esophageal adenocarcinoma into the oldest age groups is a concern and the implications are significant. Given the frailty of this population, judicious early detection and optimization of endoscopic therapy for this deadly cancer for those at risk will be essential. Likewise, close surveillance of trends by sex in population cohorts will be necessary to corroborate the factors causal of gastrointestinal cancer, including esophageal adenocarcinoma. Our initial epidemiological observation of sex-age differences warrants translation into a molecular epidemiology study with the use of relevant biomarkers to further explore the possible protective role of hormones against esophageal adenocarcinoma. Identification of protective factors and populations at highest risk may shift the balance of risk and benefits surrounding the now controversial use of estrogen replacement therapy in post-menopausal women.
Acknowledgments
Funding Source
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Division of Oncology. National Institute of Health/National Institute on Minority Health Disparities Loan Repayment Program.
Footnotes
Meeting Presentation
2012 ASCO General Meeting (Poster presentation) 2013 AACR Annual Conference (Poster presentation)
Disclaimer
Charles Rudin, MD: Consultant for Lilly.
List of References
- 1.el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am. 2002;31:421–40. doi: 10.1016/s0889-8553(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 2.Vizcaino AP, Moreno V, Lambert R, et al. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973–1995. Int J Cancer. 2002;99:860–868. doi: 10.1002/ijc.10427. [DOI] [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235–56. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 4.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 5.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1–9. doi: 10.1016/s0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 6.Lagergren J, Nyren O. Do sex hormones play a role in the etiology of esophageal adenocarcinoma? A new hypothesis tested in a population-based cohort of prostate cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7:913–5. [PubMed] [Google Scholar]
- 7.Wang QM, Qi YJ, Jiang Q, et al. Relevance of serum estradiol and estrogen receptor beta expression from a high-incidence area for esophageal squamous cell carcinoma in China. Med Oncol. 2011;28:188–193. doi: 10.1007/s12032-010-9457-8. [DOI] [PubMed] [Google Scholar]
- 8.Derakhshan MH, Liptrot S, Paul J, et al. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- 9.Marmo R, Rotondano G, Piscopo R, et al. Combination of age and sex improves the ability to predict upper gastrointestinal malignancy in patients with uncomplicated dyspepsia. a prospective multicentre database study. Am J Gastroenterol. 2005;100:784–91. doi: 10.1111/j.1572-0241.2005.40085.x. [DOI] [PubMed] [Google Scholar]
- 10.Chandanos E, Lagergren J. The mystery of male dominance in oesophageal cancer and the potential protective role of oestrogen. Eur J Cancer. 2009;45:3149–55. doi: 10.1016/j.ejca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 12.Nordenstedt H, El-Serag H. The influence of age, sex, and race on the incidence of esophageal cancer in the United States (1992–2006) Scand J Gastroenterol. 2011;46:597–602. doi: 10.3109/00365521.2011.551890. [DOI] [PubMed] [Google Scholar]
- 13.Zheng T, Mayne ST, Holford TR, Boyle P, Wenliang L, Chen Y, Mador M, Flannery J. Time trend and age-period-cohort effects on incidence of esophageal cancer in Connecticut, 1935–89. Cancer Causes and Control. 1992;3:481– 492. doi: 10.1007/BF00051361. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka H, Sugimachi K, Ueo H, et al. Sex hormone response of a newly established squamous cell line derived from clinical esophageal carcinoma. Cancer Res. 1987;47:4134–4140. [PubMed] [Google Scholar]
- 15.Ueo H, Matsuoka H, Sugimachi K, et al. Inhibitory effects of estrogen on the growth of a human esophageal carcinoma cell line. Cancer Res. 1990;50:7212–7215. [PubMed] [Google Scholar]
- 16.Utsumi Y, Nakamura T, Nagasue N, et al. Role of estrogen receptors in the growth of human esophageal carcinoma. Cancer. 1989;64:88–93. doi: 10.1002/1097-0142(19890701)64:1<88::aid-cncr2820640116>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Hennessy BA, Harvey BJ, Healy V. 17beta-Estradiol rapidly stimulates c-fos expression via the MAPK pathway in T84 cells. Mol Cell Endocrinol. 2005;229:39–47. doi: 10.1016/j.mce.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Hartman J, Edvardsson K, Lindberg K, et al. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 2009;69:6100–6106. doi: 10.1158/0008-5472.CAN-09-0506. [DOI] [PubMed] [Google Scholar]
- 19.Sukocheva OA, Wee C, Ansar A, Hussey DJ, Watson DI. Effect of estrogen on growth and apoptosis in esophageal adenocarcinoma cells. Disease of the Esophagus. 2012 doi: 10.1111/dote.12000. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Camargo MC, Goto Y, Zabaleta J, et al. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:20–38. doi: 10.1158/1055-9965.EPI-11-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohanes P, Yang D, Chhibar RS, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol. 2012;30:2265–72. doi: 10.1200/JCO.2011.38.8751. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendifar A, Yang D, Lenz F, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15:6391–7. doi: 10.1158/1078-0432.CCR-09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–92. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannaford PC, Selvaraj S, Elliott AM, et al. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner’s oral contraception study. BMJ. 2007;335:651. doi: 10.1136/bmj.39289.649410.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez E, La Vecchia C, Braga C, et al. Hormone replacement therapy and risk of colon and rectal cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:329–33. [PubMed] [Google Scholar]
- 26.Beral V, Banks E, Reeves G, et al. Use of HRT and the subsequent risk of cancer. J Epidemiol Biostat. 1999;4:191–210. discussion 210–5. [PubMed] [Google Scholar]
- 27.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Cook MB, Wild CP, Forman D. A Systematic review and meta-analysis of the sex ratio for Barrett’s esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol. 2005;162(11):1050–61. doi: 10.1093/aje/kwi325. [DOI] [PubMed] [Google Scholar]
- 29.Pohl H, Wrobel K, Bojarski C, Voderholzer W, Sonnernberg A, Rosch T, Baumgart DC. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol. 2013;108(2):200–7. doi: 10.1038/ajg.2012.387. [DOI] [PubMed] [Google Scholar]
- 30.Rosner B, Colditz G. Age at menopause: imputing age at menopause for women with a hysterectomy with application to risk of postmenopausal breast cancer. Ann Epidemiol. 2011:450–460. doi: 10.1016/j.annepidem.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]