Abstract
A proper response against stressors is critical for survival. In mammals, the stress response is primarily mediated by secretion of glucocorticoids via the hypothalamic-pituitaryadrenocortical (HPA) axis and release of catecholamines through adrenergic neurotransmission. Activation of these pathways results in a quick physical response to the stress and, in adaptive conditions, mediates long-term changes in the brain that lead to the formation of long-term memories of the experience. These long-term memories are an essential adaptive mechanism that allows an animal to effectively face similar demands again. Indeed, a moderate stress level has a strong positive effect on memory and cognition, as a single arousing or moderately stressful event can be remembered for up to a lifetime. Conversely, exposure to extreme, traumatic, or chronic stress can have the opposite effect and cause memory loss, cognitive impairments, and stress-related psychopathologies such as anxiety disorders, depression and post-traumatic stress disorder (PTSD). While more effort has been devoted to the understanding of the effects of the negative effects of chronic stress, much less has been done thus far on the identification of the mechanisms engaged in the brain when stress promotes long-term memory formation. Understanding these mechanisms will provide critical information for use in ameliorating memory processes in both normal and pathological conditions. Here, we will review the role of glucocorticoids and glucocorticoid receptors (GRs) in memory formation and modulation. Furthermore, we will discuss recent findings on the molecular cascade of events underlying the effect of GR activation in adaptive levels of stress that leads to strong, long-lasting memories. Our recent data indicate that the positive effects of GR activation on memory consolidation critically engage the brain-derived neurotrophic factor (BDNF) pathway. We propose and will discuss the hypothesis that stress promotes the formation of strong long-term memories because the activation of hippocampal GRs after learning is coupled to the recruitment of the growth and pro-survival BDNF/cAMP response element-binding protein (CREB) pathway, which is well-know to be a general mechanism required for long-term memory formation. We will then speculate about how these results may explain the negative effects of traumatic or chronic stress on memory and cognitive functions.
Keywords: glucocorticoid, glucocorticoid receptor, memory, BDNF, PTSD, stress, trauma, survival pathway
1. Introduction
Stress triggers physiological responses that are necessary for organisms to adapt to a changing environment and respond to immediate perturbation, threat, or danger. Animals’ survival not only depends on their immediate response to a stressor, but also relies on their ability to memorize and integrate the information learned about the stressor in order to effectively respond to similar demands in the future.
In addition to the rapid physiological responses that include increases in blood pressure, heart rate, and pulmonary ventilation and a hypervigilance state, stress produces long-lasting changes in the central nervous system (CNS) that are responsible for memorization of the event. These reactions are governed by acute adrenergic neurotransmission in the sympathetic nervous system and, following activation of the hypothalamic-pituitary-adrenal (HPA) axis, the release of glucocorticoids from the adrenal glands. As a result, adrenergic neurotransmission and glucocorticoid secretion activate specific brain regions that include the hippocampus, amygdala, and prefrontal cortex. These regions are enriched in adrenergic and glucocorticoid receptors (GRs), which, in rodents as in humans are known to play critical roles in encoding, processing, and retaining the information of emotional events (de Kloet, Joels & Holsboer, 2005; Lupien, Maheu, Tu, Fiocco & Schramek, 2007; McIntyre, McGaugh & Williams, 2012; Roozendaal, Okuda, de Quervain & McGaugh, 2006).
The various parameters that characterize emotional experiences, such as arousal or stress intensity, duration, chronicity, predictability, and controllability are known to critically affect memory and cognition (Lupien, Maheu, Tu, Fiocco & Schramek, 2007). Whereas optimal levels of stress or arousal stimulate cognitive performance and the formation of a strong long-term memory by mediating and modulating consolidation, the process whereby an experience becomes a strong and long-lasting memory, exposure to severe or chronic stress can lead to cognitive impairments and the development of psychopathologies such as anxiety disorders, depression, and post-traumatic stress disorders (PTSD) (de Kloet, Joels & Holsboer, 2005; McEwen, 2000b). In this review, we will discuss the mechanisms by which one of the major pathways activated by stress, the release of glucocorticoids and activation of GRs, promotes long-term memory formation when stress and/or arousal are adaptive. Based on our recent data, we will propose the hypothesis that the activation of GRs promotes memory consolidation and strengthening because it critically and directly engages the activation of the brain-derived neurotrophic factor (BDNF)/cAMP response element-binding protein (CREB)-dependent pro-survival/growth pathway as a response to stress. We propose that this survival response to stress has been selected by evolution in brain cells as a fundamental mechanism that mediates memory storage. We will then summarize the knowledge of how high levels of stress and/or glucocorticoids may lead to memory impairments. We will conclude with speculations about how the knowledge of the molecular mechanisms activated by GRs in adaptive conditions may also explain the negative effects of stress on cognition, which lead to cognitive impairments and psychopathologies.
2. Stress, glucocorticoids and activation of GRs
2.1 Glucocorticoids and their receptors
Glucocorticoids are steroid hormones synthesized from cholesterol in the adrenal cortex. The predominant glucocorticoid in humans is cortisol and, in rodents, corticosterone. Their release fluctuates with circadian and ultradian rhythms made up of pulses at approximately hourly intervals. Cortisol and corticosterone are also the primary hormones responsible for the stress response because their release is regulated by the HPA axis activated in response to stress. In particular, glucocorticoids play a key role in restoring homeostasis following exposure to stress, and they also modulate important physiological responses such as ion transport, glycogenolysis, immune response, and memory.
Because of their lipophilic properties, glucocorticoids can cross plasma membranes and activate two different intracellular receptors, mineralocorticoid receptors, (MRs) and GRs, also known, respectively, as Type I and Type II receptors. MRs and GRs are homologous in their structural domains, which consist of the N-terminal transactivation domain (TAD), the DNA binding domain (DBD), and the C-terminal ligand binding domain (LBD) (Lu, Wardell, Burnstein, Defranco, Fuller et al., 2006). In the absence of ligand, cytoplasmic MRs and GRs are bound to chaperone protein complexes, including heat shock protein 70 (hsp70), heat shock protein 90 (hsp90), and FK506 binding protein 5 (FKBP5) (Grad & Picard, 2007). On ligand binding, the receptors undergo conformational changes that lead to their dissociation from the chaperone complexes, their homodimerization and nuclear translocation, or their binding to other cytoplasmic proteins. In the nucleus, both MRs and GRs can bind to specific sequences of 15 nucleotides in the promoter of target genes, known as the glucocorticoid response elements (GREs) and directly activate transcription of target genes (Zalachoras, Houtman & Meijer, 2013). Nuclear MRs and GRs can also interact with other transcription factors to control gene expression (Sandi, 2004). In addition, MRs and GRs can control rapid cellular responses by mechanisms that are independent of nuclear translocation and gene expression regulation, but instead occur through genomic-independent actions (Groeneweg, Karst, de Kloet & Joels, 2011; Prager & Johnson, 2009). We will further discuss the genomic and non-genomic mechanisms of GR below.
2.2 Stress-mediated secretion of glucocorticoids and activation of GRs in the brain
A stressful experience triggers the acute release of catecholamines (adrenaline and noradrenaline) from the sympathetic nervous system, as well as activation of the HPA axis by first engaging secretion of corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus, then adrenocorticotropic hormone (ACTH) from the anterior lobe of the pituitary gland, and finally glucocorticoids from the adrenal cortex into the blood circulation. Once the stress ends, hormonal levels return to homeostasis by the negative feedback action of glucocorticoids on the HPA axis. In addition to the immediate reaction to the stressor by catecholamines and glucocorticoids, which evoke rapid physical responses (e.g., “fight or flight” response in the case of a threat), the release of glucocorticoids activate MRs and GRs in the brain. MRs and GRs are ubiquitously expressed throughout the brain in both glial cells and neurons, with highest levels in the hippocampus and amygdala, two areas that play critical roles in memories of fear, threat, and stressful experiences (de Kloet, Joels & Holsboer, 2005; Lupien, Maheu, Tu, Fiocco & Schramek, 2007). Importantly, MRs have a tenfold higher affinity for glucocorticoids than GRs and are largely occupied by the ligand in basal conditions, whereas GRs occupation highly depends on increases in glucocorticoid levels following stress response (de Kloet, Joels & Holsboer, 2005; Lupien, Maheu, Tu, Fiocco & Schramek, 2007). In this review, we will mainly discuss the mechanisms mediated by GRs.
For many years, the effects of glucocorticoids on synaptic plasticity and memory were believed to result exclusively from the classical genomic-dependent pathway of GR activation. However, it has recently been shown that many effects of GRs are also mediated by rapid, genomic-independent mechanisms (Groeneweg, Karst, de Kloet & Joels, 2011; Prager & Johnson, 2009).
As mentioned earlier, the genomic action of GRs occurs in the nucleus, where these receptors can directly activate transcription by binding to the GREs in the promoter of target genes (Karst, Karten, Reichardt, de Kloet, Schutz et al., 2000; Prager & Johnson, 2009). However, GRs can control gene expression also by interacting with other transcription factors, including activator protein 1 (AP1), nuclear factor κB (NF-κB), transcription factor IID (TFIID), signal transducer and activator of transcription 5 (STAT5), and CREB (Sandi, 2004). Studies using DNA microarray or serial analysis of gene expression (SAGE) in cultured hippocampal neurons or the hippocampus in vivo demonstrated that activation of GRs leads to the transcription of various genes, including calcium binding proteins, synaptosomal-associated proteins (SNAPs), neuronal cell-adhesion molecules (NCAMs), dynein, neurofilaments, β-actin, LIM domain kinase 1 (LIMK1) and profilin. These genes have key functions in intracellular signal transduction, metabolism, neuronal structure, synaptic plasticity, and memory, suggesting that, indeed, they may be target genes regulated by GR in long-term memory formation (Datson, Morsink, Meijer & de Kloet, 2008; Datson, van der Perk, de Kloet & Vreugdenhil, 2001; Morsink, Steenbergen, Vos, Karst, Joels et al., 2006; Sandi, 2004).
Although GR-mediated transcriptional activation is necessary for long-term synaptic changes in the hippocampus, studies have shown that genomic-independent actions of GRs rapidly control glutamate release and modulate synaptic transmission and plasticity (Groeneweg, Karst, de Kloet & Joels, 2011; Haller, Mikics & Makara, 2008; Prager & Johnson, 2009; Tasker, Di & Malcher-Lopes, 2006). In addition, several investigations provided evidence of genomic-independent action of GRs in modulation of the endocannabinoid system (Atsak, Roozendaal & Campolongo, 2012). While glucocorticoid-mediated release of endocannabinoids in the hypothalamus regulates activation and termination of the HPA axis (Di, Malcher-Lopes, Halmos & Tasker, 2003), endocannabinoid signaling in both the basolateral amygdala (BLA) and hippocampus appear to control cognitive processes such as emotional memory encoding (Atsak, Roozendaal & Campolongo, 2012; Hill, Patel, Campolongo, Tasker, Wotjak et al., 2010). In particular, it has been shown that genomic-independent mechanisms of GRs lead to activation of the endocannabinoid system in the BLA and hippocampus, which, in turn, enhances the consolidation of emotional memories (Bucherelli, Baldi, Mariottini, Passani & Blandina, 2006; Campolongo, Roozendaal, Trezza, Hauer, Schelling et al., 2009; de Oliveira Alvares, de Oliveira, Camboim, Diehl, Genro et al., 2005).
2.3 Non-genomic and genomic effects of GRs on glutamate transmission
Glucocorticoids are critical in modulating glutamatergic neurotransmission in several brain regions, including the hippocampus, amygdala, and medial prefrontal cortex (mPFC). Glucocorticoid-mediated regulation of the glutamatergic system engages rapid non-genomic action, as well as long-lasting genomic mechanisms controlled by GRs and directly affects synaptic transmission, plasticity, learning, and memory (Popoli, Yan, McEwen & Sanacora, 2012; Sandi, 2011).
First, glucocorticoids regulate glutamate transmission by non-genomic actions. Specifically, glucocorticoids rapidly enhance presynaptic glutamate release in the hippocampus, amygdala, and mPFC (Lowy, Gault & Yamamoto, 1993; Moghaddam, 1993; Venero & Borrell, 1999) via rapid non-genomic action of GRs (Musazzi, Milanese, Farisello, Zappettini, Tardito et al., 2010) as well as MRs (Karst, Berger, Turiault, Tronche, Schutz et al., 2005; Olijslagers, de Kloet, Elgersma, van Woerden, Joels et al., 2008). Glucocorticoids also rapidly modulate the trafficking of postsynaptic AMPA receptor subunits via genomic-independent mechanisms. Further, activation of MRs leads to lateral diffusion of GLUA1 and GLUA2 subunits to postsynaptic sites, thus increasing the frequency of hippocampal AMPA receptor-mediated current in CA1 neurons (Groc, Choquet & Chaouloff, 2008; Krugers, Hoogenraad & Groc, 2010). As a consequence, the rapid non-genomic effects of glucocorticoids on glutamate neurotransmission increase the frequency of miniature excitatory postsynaptic currents (mEPSCs) in hippocampal and amygdala neurons (Karst, Berger, Erdmann, Schutz & Joels, 2010; Olijslagers, de Kloet, Elgersma, van Woerden, Joels et al., 2008), thereby promoting long-term memory formation (Yuen, Liu, Karatsoreos, Feng, McEwen et al., 2009; Yuen, Liu, Karatsoreos, Ren, Feng et al., 2011).
Second, glucocorticoids affect glutamate neurotransmission via genomic-dependent mechanisms (Yuen, Liu, Karatsoreos, Feng, McEwen et al., 2009; Yuen, Liu, Karatsoreos, Ren, Feng et al., 2011). For example, in cultured hippocampal neurons, glucocorticoids regulate surface expression of GLUA2 subunits by a genomic-mediated process that results in increased GLUA2-containing AMPA receptors at the synapse (Martin, Henley, Holman, Zhou, Wiegert et al., 2009). Although there is no evidence that this effect is due to direct genomic regulation of GRs on AMPA receptor subunits (Martin, Henley, Holman, Zhou, Wiegert et al., 2009), indirect mechanisms have been suggested. Specifically, GRs enhance transcription of the immediate early gene serum-glucocorticoid-inducible kinase 1 (SGK1), which in turn leads to activation of the Rab4-GDI complex that facilitates AMPA receptors recycling at the synapse (Liu, Yuen & Yan, 2010; Yuen, Liu, Karatsoreos, Ren, Feng et al., 2011). Increased synaptic expression of GLUA2-containing AMPA receptors then leads to lasting enhancement of hippocampal synaptic transmission, spine formation, and long-term memory (Conboy & Sandi, 2010; Passafaro, Nakagawa, Sala & Sheng, 2003; Saglietti, Dequidt, Kamieniarz, Rousset, Valnegri et al., 2007). In line with these findings, genomic-mediated effects of GRs also regulate calcium signaling in hippocampal neurons, thus contributing to stronger firing accommodation and high frequency long-term potentiation (LTP) (Joels & Karst, 2012). GR-mediated increases in intracellular calcium levels were found to be dependent on the activation of NMDA receptors (Takahashi, Kimoto, Tanabe, Hattori, Yasumatsu et al., 2002) and L-type voltage-gated calcium channels (VGCCs) (Chameau, Qin, Spijker, Smit & Joels, 2007).
Conversely, in conditions of high or prolonged stress, activation of GRs can have a negative effect on glutamatergic transmission through the activation of NMDA receptors (Coussens, Kerr & Abraham, 1997; Shors & Servatius, 1995). In particular, high glucocorticoid concentrations in the hippocampus lead to GR-mediated activation of extrasynaptic NR2B-containing NMDA receptors (Yang, Huang & Hsu, 2005). This mechanism, which also results in endocytosis of GLUA2-containing AMPA receptors, increases hippocampal long-term depression (LTD) and impairs spatial memory retrieval (Howland & Cazakoff, 2010).
3. Glucocorticoids and memory consolidation
As mentioned earlier, a long-lasting memory is formed through a process known as consolidation, which, over time, converts a new labile memory trace into a stronger one that is resilient to disruption (Dudai, 2012; McGaugh, 2000; Squire, Stark & Clark, 2004). Memory consolidation requires an initial phase of de novo gene expression and protein synthesis, which leads to long-term synaptic plasticity and morphological changes (Alberini, 2008; 2009; Kandel, 2001; Lamprecht, Farb, Rodrigues & LeDoux, 2006). Arousal and moderate levels of stress facilitate memory consolidation and, consistent with this, emotionally arousing experiences are better remembered than neutral ones (Phelps, 2006; Roozendaal & McGaugh, 2011). Extensive evidence indicates that the release of glucocorticoids induced by arousal or stress and the consequent activation of GRs in specific brain regions critically mediates memory consolidation and modulates memory retention (McGaugh & Roozendaal, 2002).
3.1 Glucocorticoids, GRs, and their effect on memory
The contribution of glucocorticoids to the regulation of memory was first found in adrenalectomized rats, which showed impaired corticosterone expression, as well as spatial and contextual fear memory deficits (Pugh, Tremblay, Fleshner & Rudy, 1997; Roozendaal, Portillo- Marquez & McGaugh, 1996). In line with these results, systemic inhibition of glucocorticoid synthesis by administration of metyrapone impairs contextual fear conditioning, as well as spatial and inhibitory avoidance (IA) memories (Cordero, Kruyt, Merino & Sandi, 2002; Roozendaal, Bohus & McGaugh, 1996). Moreover, contextual and auditory fear conditioning, spatial and novel-object recognition, and IA memories are all enhanced by posttraining administration of corticosterone or the synthetic glucocorticoid dexamethasone (Akirav, Kozenicky, Tal, Sandi, Venero et al., 2004; Pugh, Tremblay, Fleshner & Rudy, 1997; Roozendaal & McGaugh, 1996; Roozendaal, Okuda, Van der Zee & McGaugh, 2006). In humans, oral administration of cortisol during learning or within one hour of stimulus presentation strengthens declarative memory for neutral and emotionally arousing information (Abercrombie, Kalin, Thurow, Rosenkranz & Davidson, 2003; Buchanan & Lovallo, 2001), whereas inhibition of glucocorticoid synthesis reduces long-term declarative memory (Maheu, Joober, Beaulieu & Lupien, 2004).
As described earlier, glucocorticoid-mediated effects engage both MRs and GRs, which, however, seem to have different roles in the acquisition, storage, consolidation, and retrieval of arousing information. Activation of MRs regulates the initial phase of memory encoding, including the response to novelty, whereas GRs are important in memory consolidation (de Kloet, Oitzl & Joels, 1999; ter Horst, van der Mark, Arp, Berger, de Kloet et al., 2012). Indeed, systemic inhibition of GRs with antagonists, much like targeted disruption of GR in transgenic mice, suppresses long-term spatial and contextual fear memories (Conrad, Lupien & McEwen, 1999; Cordero & Sandi, 1998; Oitzl & de Kloet, 1992; Oitzl, Reichardt, Joels & de Kloet, 2001; Pugh, Fleshner & Rudy, 1997; Revest, Di Blasi, Kitchener, Rouge-Pont, Desmedt et al., 2005). The degree of GRs activation in addition to that of MRs is critical for the effect of stress on cognitive performance, with memory facilitation occurring when high affinity MRs are fully occupied and low affinity GRs only partially activated. Whereas intermediate activation of GRs is necessary for memory consolidation, saturation of GRs has been shown to lead to memory impairments (de Kloet, Oitzl & Joels, 1999; Lupien, Maheu, Tu, Fiocco & Schramek, 2007). These studies indicate that while a relatively limited amount of glucocorticoids leads to positive effects on cognition, high concentration and/or prolonged exposure to glucocorticoids produce impairing effects.
In line with these observations, several studies have investigated the role of both MRs and GRs in glucocorticoid-mediated synaptic transmission and hippocampal LTP (Diamond, Bennett, Fleshner & Rose, 1992; Joels & Krugers, 2007). In particular, activation of MRs was found to enhance synaptic potentiation and hippocampal LTP, whereas saturation of GRs after treatment with high doses of glucocorticoids attenuated LTP and enhanced LTD (McEwen & Sapolsky, 1995; Pavlides, Ogawa, Kimura & McEwen, 1996). Other evidence indicates that enhanced activation of GRs dampens the ability of hippocampal neurons to induce LTP and elevates the threshold for synaptic strengthening, suggesting that activation of GRs may play a role in reducing the accessibility of novel information to the same neural network (Diamond, Park & Woodson, 2004; Joels, Pu, Wiegert, Oitzl & Krugers, 2006; Wiegert, Pu, Shor, Joels & Krugers, 2005).
3.2 Spatial and temporal activation of GRs in memory formation and retrieval
Memory is encoded by the concerted interplay of several brain areas and networks that interact for proper memory acquisition, consolidation, and expression (McIntyre, McGaugh & Williams, 2012; Schwabe & Wolf, 2013). Glucocorticoids are critical regulators in the activation and cooperation of these areas (Roozendaal, 2003). For example, stress-mediated secretion of glucocorticoids and/or activation of GRs directly affects hippocampal functions, thus modulating the consolidation of several types of hippocampal-dependent memories, including spatial and contextual memories in rodents and declarative memory in humans (Donley, Schulkin & Rosen, 2005; Eichenbaum, 2000; Gabrieli, 1998; Kim & Diamond, 2002; Lupien & Lepage, 2001; Roozendaal & McGaugh, 1997a; Roozendaal, Nguyen, Power & McGaugh, 1999; Squire, 2004).
Stress-induced secretion of glucocorticoids also targets the amygdala (Kim, Lee, Han & Packard, 2001; Roozendaal & McGaugh, 1997a; Roozendaal, Nguyen, Power & McGaugh, 1999). In particular, activation of GRs in the amygdala is important for fear memory encoding and hippocampal modulation. Inhibition of GRs in the BLA impairs long-term spatial and contextual fear memories, whereas infusion of GR agonist into the BLA enhances IA-mediated memory (Donley, Schulkin & Rosen, 2005; Roozendaal & McGaugh, 1997b; Roozendaal, Quirarte & McGaugh, 2002). Here we should remind that in addition to glucocorticoids, adrenergic neurotransmission in the amygdala is key element in modulating fear memories, as shown by contextual and spatial memory enhancements after amygdala infusion of norepinephrine or β-adrenergic receptors agonists (Ferry, Roozendaal & McGaugh, 1999; Hatfield & McGaugh, 1999). In contrast, inhibition of adrenergic receptors in the amygdala blocks memory enhancement induced by systemic or intrahippocampal corticosterone injections (Quirarte, Roozendaal & McGaugh, 1997; Roozendaal, Okuda, Van der Zee & McGaugh, 2006). Hence, a concerted action of adrenalin/noradrenalin and glucocorticoid is critical for memory formation and modulation. Cortical areas such as the entorhinal, parietal, perirhinal, insular, and prefrontal cortices are modulated by BLA activity, and all contribute to fear memory consolidation (McGaugh, 2002). Recent evidence indicates that glucocorticoids have a direct effect in these regions, as activation of GRs in the insular cortex and mPFC enhances long-term memory consolidation (Barsegyan, Mackenzie, Kurose, McGaugh & Roozendaal, 2010; Fornari, Wichmann, Atucha, Desprez, Eggens-Meijer et al., 2012; Roozendaal, McReynolds, Van der Zee, Lee, McGaugh et al., 2009).
Notably, the influence of stress on memory retention largely depends on the phase of memory processing the arousing or stressful event is presented with. Thus, exposure to mild stress during or immediately after learning has a positive effect on the consolidation of long-term memory, typically when the stressor is part of the learning event (Roozendaal, 2000). However, in both humans and rodents, if stress is presented shortly before retrieval, impairments in memory retention can occur (Cahill, Gorski & Le, 2003; McGaugh & Roozendaal, 2002; Roozendaal, 2002). In particular, stress is known to have an inhibitory effect on memory retrieval, as shown, for example, in rodents that exhibit retrograde amnesia when exposed to a stressor before a spatial memory retention test (de Quervain, Roozendaal & McGaugh, 1998; Diamond, Park, Heman & Rose, 1999). Activation of glucocorticoid and noradrenergic pathways in the hippocampus and BLA are important in the impairing effects of stress on memory retrieval (de Quervain, Roozendaal & McGaugh, 1998; Roozendaal, Hahn, Nathan, de Quervain & McGaugh, 2004). Also, the severity of memory impairments has been correlated with the concentration of circulating plasma corticosterone at the time of testing (de Quervain, Roozendaal & McGaugh, 1998; Diamond, Park, Heman & Rose, 1999).
In humans, the results of many studies support the importance of stressor’s timing with regard to memory retention. Increases in cortisol levels during acquisition positively correlate with the strength of memory consolidation (Abercrombie, Kalin, Thurow, Rosenkranz & Davidson, 2003; Abercrombie, Speck & Monticelli, 2006; Smeets, Sijstermans, Gijsen, Peters, Jelicic et al., 2008; Zorawski, Blanding, Kuhn & LaBar, 2006), whereas stress exposure or administration of synthetic glucocorticoids before memory testing significantly impairs retrieval of declarative memories, particularly when they are associated with emotionally arousing information (de Quervain, Roozendaal, Nitsch, McGaugh & Hock, 2000; Kuhlmann, Piel & Wolf, 2005). Hence, the effect of stress on memory performance largely depends on the timing, context, and convergence of stress hormones in the brain (Joels, Fernandez & Roozendaal, 2011; Roozendaal, 2002).
4. GR-dependent molecular mechanisms critical for long-term memory consolidation
As we discussed earlier, in adaptive conditions, the release of glucocorticoids and activation of GRs mediate and modulate memory consolidation and the storage of strong and long-lasting memories of salient events. Which are the molecular mechanisms underlying the effect of glucocorticoids and GR activation on memory consolidation? Do GRs interact with the identified transcriptional, translation and post-translational mechanisms required for memory consolidation? The understanding of the molecular mechanisms mediated by GRs in promoting memory consolidation is still partial, possibly because of the complexity of GR-mediated responses and the multiple cell types and brain regions targeted. The identification of these mechanisms will provide important information for developing new strategies for memory strengthening or weakening, hence treating memory and cognitive disorders.
Mechanisms found to be activated by GR stimulation in cell culture or by pharmacological treatments do not necessarily inform about the identity and regulation of the mechanisms occurring in vivo after an adaptive stressful/arousing experience that will be consolidated into a long-term memory. It is in fact well known that GR activation is involved in many different stress conditions and is a highly regulated mechanism that can lead to many different types of responses depending on the stress levels, duration and type. Hence, it is important to identify which mechanisms occur physiologically in vivo after learning as a consequence of GR activation, and also characterize their temporal and spatial progression as well as regulation.
A few studies, including a recent one from our laboratory (Chen, Bambah-Mukku, Pollonini & Alberini, 2012) have demonstrated that, after learning, GRs regulate several intracellular signaling pathways known to be required for memory consolidation. These include the pathways activated by CREB, mitogen-activated protein kinase (MAPK), calcium/calmodulin-dependent protein kinase II (CamK II), and BDNF. In addition, GRs control epigenetic modifications that influence long-term memory. Specifically, activation of GRs by different types of psychological stress such as forced swimming or predator exposure increase the phosphorylation and acetylation of histone H3 in dentate gyrus granule neurons (Bilang- Bleuel, Ulbricht, Chandramohan, De Carli, Droste et al., 2005; Chandramohan, Droste, Arthur & Reul, 2008). The concomitant activation of GRs, NMDA receptors, and MAPK signaling pathway is required for phospho-acetylation of histone H3 in the dentate gyrus (Bilang-Bleuel, Ulbricht, Chandramohan, De Carli, Droste et al., 2005; Chandramohan, Droste, Arthur & Reul, 2008). Phospho-acetylation of histone H3 in turn modulates stress-induced behavioral responses such as immobility after forced swimming (Bilang-Bleuel, Ulbricht, Chandramohan, De Carli, Droste et al., 2005) and object recognition memory (Stefanko, Barrett, Ly, Reolon & Wood, 2009). In the insular cortex, activation of GRs enhances the interaction between phospho-CREB and CREB-binding protein (CBP), leading to the histone deacetylase (HDAC)-mediated chromatin modification necessary for memory consolidation for novel object recognition and object location (Roozendaal, Hernandez, Cabrera, Hagewoud, Malvaez et al., 2010).
Using fear conditioning in mice, two studies demonstrated the critical role of GRs in the induction of MAPK phosphorylation, expression of early growth response protein 1 (Egr-1), and phosphorylation of Synapsin-Ia/Ib during fear memory consolidation (Revest, Di Blasi, Kitchener, Rouge-Pont, Desmedt et al., 2005; Revest, Kaouane, Mondin, Le Roux, Rouge-Pont et al., 2010). More specifically, activation of hippocampal GRs recruits the MAPK signaling pathway, which in turn leads to induction of the downstream immediate early gene Egr-1 (also known as Zif268), which is a key transcription factor in memory consolidation (Jones, Errington, French, Fine, Bliss et al., 2001; Kelleher, Govindarajan, Jung, Kang & Tonegawa, 2004). In agreement, exogenous administration of glucocorticoids or constitutive activation of GR in transgenic mice results in activation of MAPK and induction of Egr-1 expression in the hippocampus. In line with these findings, inhibition of the MAPK pathway in the hippocampus abolishes the increase in contextual fear conditioning induced by glucocorticoids (Revest, Di Blasi, Kitchener, Rouge-Pont, Desmedt et al., 2005). GR-mediated activation of MAPK and Egr-1 then leads to the increased expression and phosphorylation of Synapsin-Ia/Ib necessary for contextual memory consolidation (Revest, Kaouane, Mondin, Le Roux, Rouge-Pont et al., 2010). Phosphorylation of Synapsin Ia/Ib has been shown to facilitate activity-dependent release of glutamate from presynaptic vesicles of pyramidal neurons (Chi, Greengard & Ryan, 2003; Jovanovic, Czernik, Fienberg, Greengard & Sihra, 2000). Collectively, these studies have identified the MAPK-activated pathway, with subsequent regulation of Egr-1 expression and phosphorylation of Synapsin-Ia/Ib, as a sequence of events targeted by activation of GRs that leads to fear memory consolidation.
Our laboratory has recently identified several critical molecular mechanisms underlying glucocorticoid-mediated long-term memory consolidation in the rat hippocampus using an IA learning paradigm. We found that recruitment of hippocampal GRs after training controls the activation of several intracellular pathways that are critical for memory consolidation (Chen, Bambah-Mukku, Pollonini & Alberini, 2012). Specifically, GRs control the rapid learning-dependent hippocampal increase of CREB phosphorylation and expression of the immediate early gene-activity-regulated cytoskeleton-associated protein (Arc), as well as the increase in synaptic phospho-CAMKIIα, phospho-Synapsin-1, and GluA1 expression. All of these rapid changes, except Arc induction, result from non-genomic actions of GRs (Chen, Bambah-Mukku, Pollonini & Alberini, 2012). These results extend previous findings that expression of Arc increases in hippocampal synapses following memory-enhancing administration of corticosterone (McReynolds, Donowho, Abdi, McGaugh, Roozendaal et al., 2010); that stress-dependent Arc expression is impaired in the hippocampus of GR-deficient (GR+/−) mice (Molteni, Calabrese, Chourbaji, Brandwein, Racagni et al., 2010); and that corticosterone increases AMPA receptor trafficking in pyramidal neuronal cultures from prefrontal cortex (Liu, Yuen & Yan, 2010). We also found that inhibition of GRs in rat hippocampus significantly reduces phosphorylation of the tropomysosin receptor kinase B (TrkB), extracellular-signal regulated kinase 1/2 (ERK1/2), Akt, and phospholipase C γ (PLCγ) (Chen, Bambah-Mukku, Pollonini & Alberini, 2012). Because these pathways are all canonical activation pathways downstream of BDNF, these findings suggest that the BDNF-dependent pathway is a key downstream effector of GR activation during memory consolidation.
In addition, we established that the long-lasting molecular modifications induced by training that are required for memory consolidation, including the persistent phosphorylation of CREB, CamKIIα, and Synapsyn1a, are all dependent on GR activation, most likely because they require the early and rapid molecular activation described just above. In fact, memory consolidation appears to be mediated by a BDNF-dependent autoregulatory loop that activates the CREB/CCAAT enhancer-binding protein (C/EBP)-dependent gene cascade, which in turn regulates BDNF expression. This BDNF-dependent autoregulatory loop is required to complete the consolidation necessary for long-term memory persistence (unpublished observations). Notably, we then found that intrahippocampal injections of BDNF, but not of other neurotrophins, such as nerve growth factor (NGF) and neurotrophin 3 (NT3), rescue both the molecular impairments and the amnesia caused by GR inhibition (Chen, Bambah-Mukku, Pollonini & Alberini, 2012). The effect is selective for GRs because BDNF does not rescue behavioral deficits caused by the inhibition of β-adrenergic receptors (Chen, Bambah-Mukku, Pollonini & Alberini, 2012), underscoring the critical and specific role of the BDNF-mediated signaling pathway in the GR-dependent molecular activations required for memory consolidation. Our findings extended to memory formation previous observations byJeanneteau et al. (2008) who reported that administration of dexamethasone in the hippocampus of rats or in hippocampal or cortical cell cultures induces TrkB phosphorylation (Jeanneteau, Garabedian & Chao, 2008). However, in these studies, TrkB transactivation was found to require a genomic effect mediated by GR, contrasting with the non-genomic effect of GRs on TrkB phosphorylation observed in our study (Chen, Bambah-Mukku, Pollonini & Alberini, 2012. See also discussion of this manuscript).
If hippocampal GR activation upon learning takes place upstream of the BDNF-dependent activation required for long-term memory, how does that occur? Although a direct interaction between GRs and TrkB has been reported in cortical neurons in vitro (Numakawa, Kumamaru, Adachi, Yagasaki, Izumi et al., 2009), our attempts do not yet indicate any direct interaction between GRs and TrkB in rat dorsal hippocampi in vivo. Hence, we speculate that the non-genomic effect of GRs on TrkB phosphorylation may target the regulation of BDNF release and/or TrkB membrane trafficking. GRs may also regulate BDNF-mediated signaling pathways by mechanisms that depend on cAMP-mediated trafficking and activation of TrkB (Ji, Pang, Feng & Lu, 2005). Moreover, it appears that the persistence of the long lasting molecular changes necessary for memory consolidation recruit additional genomic-dependent mechanisms such as BDNF transcription. Although there is no classical GRE sequence in the BDNF promoter, in situ hybridization demonstrated that BDNF mRNA expression is enhanced after intrahippocampal injection of corticosterone, presumably by the indirect transcriptional effects of GRs (Chao & McEwen, 1994; Hansson, Sommer, Rimondini, Andbjer, Stromberg et al., 2003). In contrast, expression of BDNF is altered in the hippocampus of GR-deficient mice (Alboni, Tascedda, Corsini, Benatti, Caggia et al., 2011; Ridder, Chourbaji, Hellweg, Urani, Zacher et al., 2005).
In view of all these data, we propose a model that explains, at least in part, the nature of the biological mechanisms involved in the formation of long-term memories elicited by stressful or arousing experiences: we suggest that evolution has selected mechanisms of growth and pro-survival response to stress as the fundamental molecular pathways activated by learning and recruited in brain cells to form long-term memories. In other words, as in any other cell, in the brain, adaptive levels of stress (via glucocorticoids) trigger cellular events that are responsible to restore homeostasis, hence adaptation to the new environment. To do so, these cellular events must produce persistent changes in the state of the cell, which lead to a new homoestatic state. Thus, the new homeostatic state, by means of its underlying long-lasting cellular and molecular changes, is the result of long-term changes, in other words result in the formation and persistence of a cellular long-term memory of the salient stimulus. Indeed, glucocorticoids, like hypoxia, metabolic or thermal stresses are cellular stressors and apoptotic promoters. Furthermore, whereas, as described earlier, in animal and humans, adaptive responses to environmental stressors depend on the activation of the HPA axis, in less differentiated organisms and cultured cells it induce the "stress response" (Pagliacci, Migliorati, Smacchia, Grignani, Riccardi et al., 1993). This cellular stress response generally comprises a pro-survival response that promotes growth and survival and protects cells from death. In neurons, the pro-survival response to stress consists of activation of growth pathways, which actually leads, in addition to the pro-survival response, to synaptic growth. Synaptic growth is known to underlie the formation and persistence of memory. Hence, it is plausible that evolution has indeed chosen the cellular response to stress as the fundamental mechanisms that in brain cells promote long-term memory formation.
Based on our data, we suggest that the pro-survival response to stress, which mediates long-term memory formation, occurs in the brain through sequential events: exposure to a salient event leads to the release of glucocorticoids that activate GRs in brain regions such as the hippocampus, which are critical for memory consolidation. GR activation recruits the BDNF/CREB-dependent pathways, which through their downstream as well as additional parallel events lead to cellular growth and promote survival. This activation of survival and growth pathways results in synaptic changes that underlie long-term maintenance of the information (Fig. 1). Our findings experimentally support and are in line with numerous evidence obtained in studies of chronic stress and glucocorticoid treatments indicating that glucocorticoids and BDNF critically influence each other (Bath, Schilit & Lee, 2013; Gray, Milner & McEwen, 2013; Jeanneteau & Chao, 2013; Numakawa, Adachi, Richards, Chiba & Kunugi, 2013; Rothman & Mattson, 2013; Suri & Vaidya, 2013).
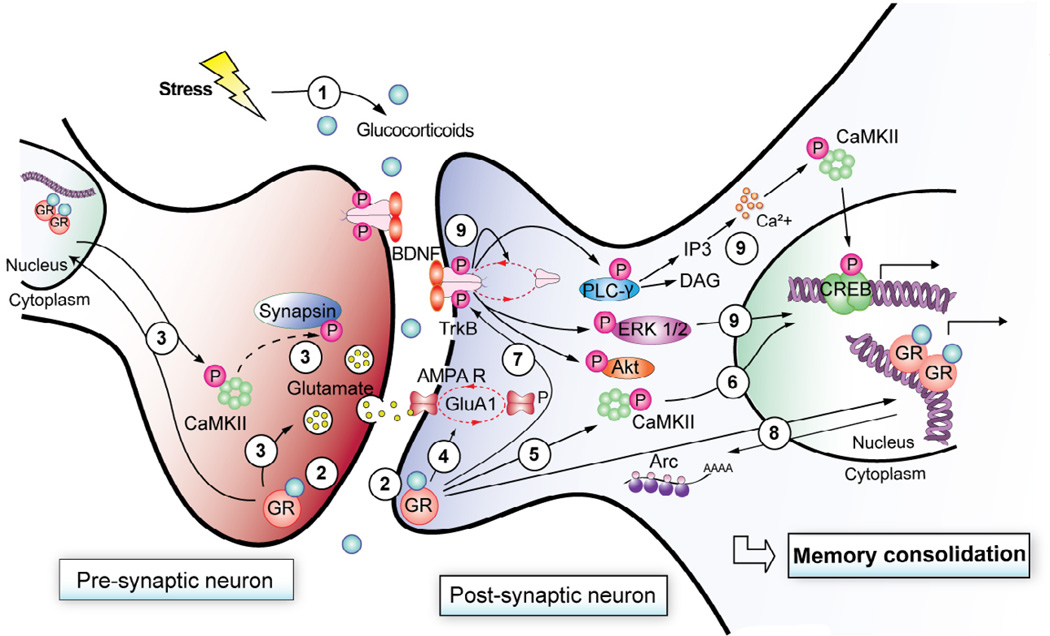
Fig. 1. The GR/TrkB model of memory consolidation.
Exposure to a stress triggers release of glucocorticoids through activation of the HPA axis (1). Glucocorticoids released in the circulation cross the blood-brain barrier and activate glucocorticoid receptors (GRs) at the synapse of hippocampal neurons (2). In presynaptic neurons, GRs regulate release of glutamate by genomic-dependent and -independent mechanisms (3). Postsynaptically, GRs stimulate rapid non-genomic increases in synaptic GluA1 expression (4) and phosphorylation of CamKII (5), CREB (6), and TrkB (7), as well as genomic-dependent increases in Arc expression (8). TrkB-mediated signaling pathways activated by BDNF converge on CREB phosphorylation (9). Activation of presynaptic and postsynaptic GRs in hippocampal neurons, together with recruitment of the BDNF-mediated signaling pathways, is necessary for stress-mediated memory consolidation. These data are adapted from Chen, Bambah-Mukku, Pollonini, and Alberini (2012).
Although the GR/BDNF pathway recruitment is critical for proper memory consolidation in conditions at which the stress levels are controllable, evidence indicates that chronic stress and elevated glucocorticoids levels in pathological situations negatively regulate the BDNF pathway (Allen & Dawbarn, 2006). In agreement, chronic exposures to corticosterone or dexamethasone suppress BDNF-mediated release of glutamate in cultured cortical neurons, providing a possible mechanism for the negative impact of chronic stress on cognitive functions (Numakawa, Kumamaru, Adachi, Yagasaki, Izumi et al., 2009). In depression, the negative impact of chronic stress on spine density, synaptic plasticity, and neuronal survival in the hippocampus and prefrontal cortex is mediated, at least in part, by glucocorticoid-dependent downregulation of BDNF expression (Duman, Heninger & Nestler, 1997; Duman & Monteggia, 2006). Our results and working model, together with the literature on chronic stress, suggest that the convergence between GR and BDNF pathways may be an important node of dysfunction in stress-related cognitive impairments and affective disorders.
5. Glucocorticoids, cognitive impairments, and psychopathologies
5.1. Stress and memory: an inverted U-shaped relationship
Chronic stress or even single severely traumatic experiences can have a negative impact on cognitive functions and lead to the development of several psychopathologies (de Kloet, Joels & Holsboer, 2005; de Quervain, Aerni, Schelling & Roozendaal, 2009). The effect of stress on cognitive functions is largely dependent on the characteristics of the stressor. Stress intensity, duration, chronicity, controllability, and predictability are major characteristics that affect cognition and memory (Lupien, Maheu, Tu, Fiocco & Schramek, 2007).
Many studies have established that the intensity of a stressor is a critical factor that modulates cognitive performance. Specifically, in both humans and rodents, stress intensity and memory are known to follow an inverted U-shaped relationship, with maximal memory strength at an intermediate level of stress. In the early 20th century, this relationship was originally described by Yerkes and Dodson in a paradigm that measured the cognitive performance of mice in a discrimination task after exposure to electrical shocks of different intensities (Calabrese, 2008). The so-called Yerkes-Dodson law postulates that an optimal level of stress or arousal leads to maximal performance of a specific cognitive task. Importantly, this law also emphasizes that the inverted U effect shifts to a linear relationship as the task becomes simple. An important component of this theory therefore relies on the complexity of the task and brain structures involved in memory processing (Diamond, Campbell, Park, Halonen & Zoladz, 2007; Sandi & Pinelo-Nava, 2007).
Following the seminal observation of Yerkes and Dodson, various studies have characterized the nonlinear relationship between stress intensity and cognitive performance in rodent models and humans. For example, variation of the intensity of a stress intrinsic to the learning paradigm, such as water temperature in a radial arm maze, demonstrated the inverted U effect of stress on learning and memory performance in rats (Salehi, Cordero & Sandi, 2010). Similarly, systemic administration of corticosterone shortly after training modulates long-term object-recognition memory with an inverted U effect (Okuda, Roozendaal & McGaugh, 2004), while exposure to electrical footshocks of different intensities accompanied by intrahippocampal administration of corticosterone leads to a similar effect on contextual fear memories (Kaouane, Porte, Vallee, Brayda-Bruno, Mons et al., 2012). Notably, memory-impairing effects of high stress on spatial tasks are largely mediated by the action of glucocorticoids and GRs in the hippocampus and BLA (Roozendaal, Griffith, Buranday, De Quervain & McGaugh, 2003; Roozendaal, Hahn, Nathan, de Quervain & McGaugh, 2004), and become more pronounced as cognitive task gains complexity (Celerier, Pierard, Rachbauer, Sarrieau & Beracochea, 2004; Diamond, Park, Heman & Rose, 1999). In contrast, increasing stressor intensity leads to enhanced fear memory strength for simpler cognitive tasks, as shown in studies using classical Pavlovian contextual and cued fear conditioning (Cordero, Kruyt, Merino & Sandi, 2002; Rau, DeCola & Fanselow, 2005).
In humans, studies also support the conclusion of an inverted U-shaped relationship between stress intensity and performance of complex cognitive tasks (Diamond, Campbell, Park, Halonen & Zoladz, 2007; Lupien, Maheu, Tu, Fiocco & Schramek, 2007). Consolidation of declarative memories follows a bell-shaped curve associated with levels of cortisol secreted after stress exposure, with peak memory performance occurring at an intermediate cortisol level (Andreano & Cahill, 2006). Similarly, injections of increasing doses of cortisol rapidly modulate declarative memory retrieval with a dose-dependent effect that follows an inverted U profile (Abercrombie, Kalin, Thurow, Rosenkranz & Davidson, 2003; Domes, Rothfischer, Reichwald & Hautzinger, 2005; Schilling, Kolsch, Larra, Zech, Blumenthal et al., 2013). In addition, psychological stressors or administration of high doses of cortisol lead to impairment in spatial cognitive tasks and declarative memory, particularly when associated with emotionally laden material (Kirschbaum, Wolf, May, Wippich & Hellhammer, 1996; Kuhlmann, Piel & Wolf, 2005; Newcomer, Selke, Melson, Hershey, Craft et al., 1999). In contrast to the impairing effect of severe stress on complex memories and cognitive functions, it is known that exposure to traumatic events can lead to the development of pathological memories referred as “hypermnesia” or “flashbulb memories” during which subjects experience a particularly strong and vivid autobiographical memory for a specific highly arousing experience (Berntsen & Thomsen, 2005; Tekcan & Peynircioglu, 2002).
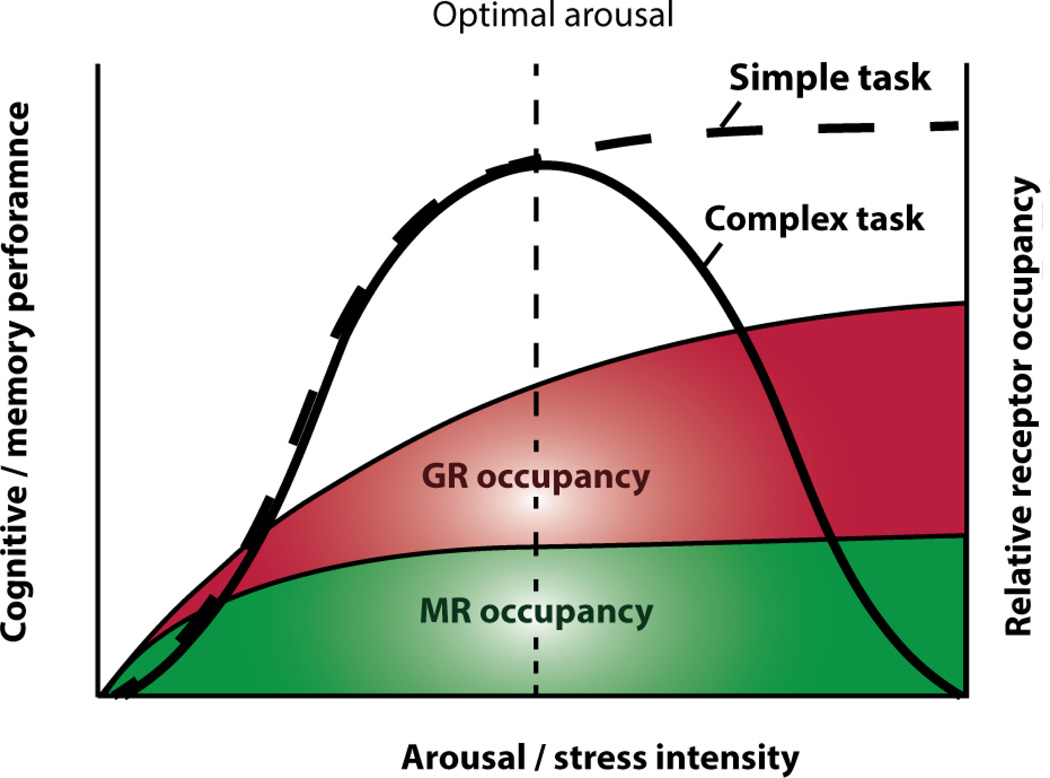
Together, studies on animal models and humans have shown that, in line with Yerkes and Dodson’s original observation, memory performance associated with complex cognitive tasks is sensitive to stress in an inverted U fashion, whereas simple forms of fear memory induced by traumatic experiences can be strong and persistent (Fig 2).
Fig. 2. Effect of arousal or stress intensity on cognitive performances and steroid receptor occupancy.
The intensity of a stressor is a critical parameter that modulates cognitive and memory performance. Exposure to an intermediate level of stress that leads to optimal cognitive performance triggers secretion of glucocorticoids in a range that fully activates the high affinity MRs and partially activates the low affinity GRs. An inverted U-shaped relationship between stress level and cognitive performance is observed in rodents and humans for complex tasks (e.g., decision making process, declarative and spatial memories), whereas high stress leads to an asymptotic effect on memory performance for simpler cognitive tasks (e.g., flashbulb memories, fear memories).
5.2. Glucocorticoid-mediated mechanisms of memory impairments
Exposure to an acute strong stress triggers the secretion of high levels of glucocorticoids and leads to memory impairment (de Kloet, Joels & Holsboer, 2005; Kim & Diamond, 2002; Sandi, 2004). For example, impairments of spatial memory have been observed in rats exposed to acute stress, social stress, following administration of corticosterone and in transgenic mice with endogenous elevated corticosterone levels (de Quervain, Roozendaal & McGaugh, 1998; Diamond, Park, Heman & Rose, 1999; Heinrichs, Stenzel-Poore, Gold, Battenberg, Bloom et al., 1996; Luine, Spencer & McEwen, 1993).
Chronic stress also leads to impairment in hippocampal-dependent learning and memory tasks. Chronic immobilization, unpredictable randomized stressors, repetitive social stressors, or chronic injections of corticosterone have all been found to provoke deficits in hippocampal-dependent forms of memory (Liu, Betzenhauser, Reiken, Meli, Xie et al., 2012; Luine, Villegas, Martinez & McEwen, 1994; Luine, Spencer & McEwen, 1993; Yuen, Wei, Liu, Zhong, Li et al., 2012). Interestingly, chronically stressed rats did not exhibit any deficits in certain memory paradigms such as cued and contextual fear conditioning, presumably because of the predominant role of the amygdala in these tasks (Conrad, LeDoux, Magarinos & McEwen, 1999; Sandi, Merino, Cordero, Touyarot & Venero, 2001). Together, these studies indicate that chronic stress mostly impairs hippocampal-dependent mechanisms and functions.
Exposure to severe acute or chronic stress can have dramatic consequences on neuronal morphology in the hippocampus. Chronic stress or corticosterone administration leads to dendritic atrophy in the CA1, CA3, and dentate gyrus (Magarinos & McEwen, 1995; McEwen, 2000a; Vyas, Mitra, Shankaranarayana Rao & Chattarji, 2002), as well as loss of excitatory synapses in the CA3 area (Sousa, Lukoyanov, Madeira, Almeida & Paula-Barbosa, 2000). Acute stress and social defeat stress also lead to neurodegeneration and inhibition of neurogenesis in the adult dentate gyrus (Gould & Tanapat, 1999; Lehmann, Brachman, Martinowich, Schloesser & Herkenham, 2013; Sapolsky, 2000). In particular, binding of glucocorticoids to GRs has been shown to mediate the negative effect of severe or chronic stress on hippocampal morphology and function, therefore likely contributing to the negative effect of stress on hippocampal-dependent memories (Conrad, Lupien & McEwen, 1999; Kirschbaum, Wolf, May, Wippich & Hellhammer, 1996; McEwen, 2000a).
In contrast to what is observed in the hippocampus, severe and chronic stresses enhance neuronal activity, synaptic transmission, spine formation, and dendritic growth in the amygdala (Roozendaal, McEwen & Chattarji, 2009). Stress-mediated neuronal activity and morphological changes in the amygdala in turn lead to increased anxiety-like behavior (Roozendaal, McEwen & Chattarji, 2009). The release of glucocorticoids on exposure to severe stress therefore has contrasting physiological and morphological effects in different brain regions that in turn control specific behavioral responses.
5.3. Glucocorticoids, GRs, and traumatic memories in PTSD: potential clinical applications
Improper regulation of the stress response and subsequent abnormal secretion of cortisol are often associated with stress-related psychopathologies such as anxiety disorders, depression, and PTSD (de Kloet, Joels & Holsboer, 2005; de Quervain, Aerni, Schelling & Roozendaal, 2009). Chronic hypercortisolemia in depression, advanced aging, or Cushing’s disease have been associated with declarative memory impairments (Lupien, de Leon, de Santi, Convit, Tarshish et al., 1998; Parker, Schatzberg & Lyons, 2003; Starkman, Gebarski, Berent & Schteingart, 1992). In PTSD, most studies have found reduced levels of circulating cortisol levels (Anisman, Griffiths, Matheson, Ravindran & Merali, 2001; Delahanty, Raimonde, Spoonster & Cullado, 2003; Neylan, Brunet, Pole, Best, Metzler et al., 2005; Yehuda, 2004; Yehuda, McFarlane & Shalev, 1998), although others did not find such correlation (Lindauer, Olff, van Meijel, Carlier & Gersons, 2006; Meewisse, Reitsma, de Vries, Gersons & Olff, 2007; Muhtz, Wester, Yassouridis, Wiedemann & Kellner, 2008; Pfeffer, Altemus, Heo & Jiang, 2007). In particular, individuals with PTSD exhibit enhanced suppression of cortisol release after administration of low doses of dexamethasone, indicating that chronic hypocortisolemia is caused by excessive negative feedback in the HPA axis (Grossman, Yehuda, New, Schmeidler, Silverman et al., 2003; Newport, Heim, Bonsall, Miller & Nemeroff, 2004; Yehuda, Halligan, Golier, Grossman & Bierer, 2004).
The action of GRs in PTSD has also been shown by human genetic studies (DeRijk & de Kloet, 2005). The BclI polymorphism of the GR gene that leads to glucocorticoid hypersensitivity has notably been associated with the development of PTSD symptoms after cardiac surgery (Hauer, Weis, Papassotiropoulos, Schmoeckel, Beiras-Fernandez et al., 2011; van Rossum, Koper, van den Beld, Uitterlinden, Arp et al., 2003) and at the onset of major depression (van Rossum, Binder, Majer, Koper, Ising et al., 2006). In healthy subjects, the BclI polymorphism is associated with increased memory performance for emotional pictures, suggesting its contribution to interindividual differences in emotional memory formation in nonpathological situations (Ackermann, Heck, Rasch, Papassotiropoulos & de Quervain, 2013). In line with these findings, a polymorphism in the gene encoding the GR chaperone FKBP5 has also been correlated with increased sensitivity of GR to cortisol and risk of PTSD (Mehta, Gonik, Klengel, Rex-Haffner, Menke et al., 2011). Identification of polymorphisms in the GR gene and associations with genetic variability of other regulators of the stress response is particularly important to better characterize the genetic bases underlying trauma-related psychopathologies such as PTSD.
Because glucocorticoids and GRs have key roles in the formation of traumatic memories, potential clinical application of steroid-based therapies for the treatment of stress-related psychopathologies has been investigated. Promising findings have shown that exogenous administration of low doses of cortisol dampens the strength of traumatic memories developed in PTSD, presumably via the inhibitory effect of glucocorticoids on memory retrieval or expression (Aerni, Traber, Hock, Roozendaal, Schelling et al., 2004; Bentz, Michael, de Quervain & Wilhelm, 2010; de Quervain & Margraf, 2008). Other evidence suggests the potential use of cortisol to treat phobic disorders. Cortisol administration in patients with different types of phobias reduces their fear response during the anticipation, exposure, and recovery phases after phobic cue presentation (Soravia, Heinrichs, Aerni, Maroni, Schelling et al., 2006). In addition to inhibiting the fear response, low doses of cortisol may facilitate extinction of a traumatic memory by enhancing the consolidation of a novel corrective experience dissociated from the original memory trace (de Quervain & Margraf, 2008). In mice, administration of glucocorticoids after memory reactivation impairs retrieval of an established fear memory (Cai, Blundell, Han, Greene & Powell, 2006), whereas inhibition of glucocorticoid synthesis during memory reactivation enhances retrieval and inhibits extinction of fear memory in mice (Blundell, Blaiss, Lagace, Eisch & Powell, 2011).
Memory reconsolidation, the process whereby a retrieved memory returns to a fragile state and becomes reconsolidated (Alberini, 2011), can be targeted to weaken traumatic memories. Studies from our laboratory have shown that in rats postretrieval inhibition of amygdalar GRs with the antagonist RU38486 (mifepristone) persistently weakens IA memory (Tronel & Alberini, 2007). Similarly, postretrieval inhibition of GRs by systemic treatment with RU38486 disrupts reconsolidation of an IA traumatic memory in rats, suggesting the importance of GR inhibitors in combination with trauma reactivation as potential novel therapeutic approach (Taubenfeld, Riceberg, New & Alberini, 2009). In agreement with this idea, a recent pilot study reported significant benefit with mifepristone in combat-related PTSD (Golier, Caramanica, Demaria & Yehuda, 2012). Hence, the combination of glucocorticoid-targeting treatments with behavioral or psychological therapy aimed at reactivating the old memory trace and disrupting its reconsolidation or evoking a new memory in a safe context may represent promising leads for novel therapeutic strategies against stress-related psychopathologies. A better understanding of the cascade of molecular events occurring in the brain after activation of GRs in high stress or traumatic conditions will be key to the design of novel selective interventions against these psychopathologies.
6. Conclusion
Stress modulates memory consolidation by orchestrated activation of neuroendocrine pathways in a specific spatial and temporal manner. Consolidating a strong memory after a salient or stressful event is an adaptive response that is necessary for appropriate reactions to similar demands in the future. In part, the molecular changes elicited by glucocorticoids, which are released in response to stress and play a critical role in mediating and modulating long-term memory formation and retention have been elucidated. These changes include rapid nongenomic synaptic modifications as well as long-term genomic changes triggered by GRs. Activation of GRs in adaptive conditions engages multiple intracellular signaling pathways, possibly because GRs target multiple brain areas, cell populations and memory phases. The GR-dependent mechanisms interact with fundamental process of neural transmission and plasticity such as glutamate neurotransmission and neurotrophic factor-mediated long-term responses.
Importantly, in adaptive response to stress, activation of GRs recruits the BDNF/CREB pathways, which in turn mediate and control memory consolidation. We propose that evolution has selected growth and pro-survival mechanisms in response to stress, and particularly those mediated by the BDNF/CREB pathways, as general mechanisms underlying memory consolidation. In contrast, although it is known that glucocorticoids and GRs also play a critical role in memory impairments following chronic stress or traumatic experience, the underlying molecular mechanisms have not yet been identified. Given the negative correlations between BDNF expression and chronic stress or cognitive impairments, our studies agree with the hypothesis proposed by previous authors (Duman, Heninger & Nestler, 1997; Nestler, Barrot, DiLeone, Eisch, Gold et al., 2002) indicating that dysregulation of the GR-mediated pathway may lead to depletion or disruption of BDNF expression and signaling. Such depletion would explain the associated memory and trauma-or stress-induced cognitive impairments. Characterization of the molecular pathways engaged by glucocorticoids in conditions of chronic or maladaptive stress that lead to cognitive impairments will be of particular importance in pursuit of the development for novel specific therapeutic strategies against stress-related psychopathologies.
Acknowledgement
This work was supported by National Institute of Mental Health (NIMH) R01 MH065635 and R01 MH074736 to C.M.A and Swiss National Science Foundation fellowship PBLAP3_140173 to C.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience. 2003;117:505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31:187–196. doi: 10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Ackermann S, Heck A, Rasch B, Papassotiropoulos A, de Quervain DJ. The BclI polymorphism of the glucocorticoid receptor gene is associated with emotional memory performance in healthy individuals. Psychoneuroendocrinology. 2013;38:1203–1207. doi: 10.1016/j.psyneuen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, et al. Lowdose cortisol for symptoms of posttraumatic stress disorder. American Journal of Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learning and Memory. 2004;11:188–195. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. The role of protein synthesis during the labile phases of memory: revisiting the skepticism. Neurobiology of Learning and Memory. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiological Reviews. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Frontiers in Behavioral Neuroscience. 2011;5:12. doi: 10.3389/fnbeh.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboni S, Tascedda F, Corsini D, Benatti C, Caggia F, Capone G, et al. Stress induces altered CRE/CREB pathway activity and BDNF expression in the hippocampus of glucocorticoid receptor-impaired mice. Neuropharmacology. 2011;60:1337–1346. doi: 10.1016/j.neuropharm.2011.01.050. [DOI] [PubMed] [Google Scholar]
- Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clinical Science (London, England: 1979) 2006;110:175–191. doi: 10.1042/CS20050161. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychological Science. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Anisman H, Griffiths J, Matheson K, Ravindran AV, Merali Z. Posttraumatic stress symptoms and salivary cortisol levels. American Journal of Psychiatry. 2001;158:1509–1511. doi: 10.1176/appi.ajp.158.9.1509. [DOI] [PubMed] [Google Scholar]
- Atsak P, Roozendaal B, Campolongo P. Role of the endocannabinoid system in regulating glucocorticoid effects on memory for emotional experiences. Neuroscience. 2012;204:104–116. doi: 10.1016/j.neuroscience.2011.08.047. [DOI] [PubMed] [Google Scholar]
- Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Schilit A, Lee FS. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–156. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- Bentz D, Michael T, de Quervain DJ, Wilhelm FH. Enhancing exposure therapy for anxiety disorders with glucocorticoids: from basic mechanisms of emotional learning to clinical applications. Journal of Anxiety Disorders. 2010;24:223–230. doi: 10.1016/j.janxdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Berntsen D, Thomsen DK. Personal memories for remote historical events: accuracy and clarity of flashbulb memories related to World War II. Journal of Experimental Psychology: General. 2005;134:242–257. doi: 10.1037/0096-3445.134.2.242. [DOI] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JM. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptor-dependent behavioural response. European Journal of Neuroscience. 2005;22:1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Lagace DC, Eisch AJ, Powell CM. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiology of Learning and Memory. 2011;95:453–460. doi: 10.1016/j.nlm.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Bucherelli C, Baldi E, Mariottini C, Passani MB, Blandina P. Aversive memory reactivation engages in the amygdala only some neurotransmitters involved in consolidation. Learning and Memory. 2006;13:426–430. doi: 10.1101/lm.326906. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learning and Memory. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WH, Blundell J, Han J, Greene RW, Powell CM. Postreactivation glucocorticoids impair recall of established fear memory. Journal of Neuroscience. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Stress biology and hormesis: the Yerkes-Dodson law in psychology--a special case of the hormesis dose response. Critical Reviews in Toxicology. 2008;38:453–462. doi: 10.1080/10408440802004007. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier A, Pierard C, Rachbauer D, Sarrieau A, Beracochea D. Contextual and serial discriminations: a new learning paradigm to assess simultaneously the effects of acute stress on retrieval of flexible or stable information in mice. Learning and Memory. 2004;11:196–204. doi: 10.1101/lm.65604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, Qin Y, Spijker S, Smit AB, Joels M. Glucocorticoids specifically enhance Ltype calcium current amplitude and affect calcium channel subunit expression in the mouse hippocampus. Journal of Neurophysiology. 2007;97:5–14. doi: 10.1152/jn.00821.2006. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Arthur JS, Reul JM. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. European Journal of Neuroscience. 2008;27:2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- Chao HM, McEwen BS. Glucocorticoids and the expression of mRNAs for neurotrophins, their receptors and GAP-43 in the rat hippocampus. Brain Research: Molecular Brain Research. 1994;26:271–276. doi: 10.1016/0169-328x(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIalpha-BDNF-CREB pathways to mediate memory consolidation. Nature Neuroscience. 2012;15:1707–1714. doi: 10.1038/nn.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron. 2003;38:69–78. doi: 10.1016/s0896-6273(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35:674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for type II adrenal steroid receptors in spatial memory. Neurobiology of Learning and Memory. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Kruyt ND, Merino JJ, Sandi C. Glucocorticoid involvement in memory formation in a rat model for traumatic memory. Stress. 2002;5:73–79. doi: 10.1080/1025389029000124404. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Research. 1998;786:11–17. doi: 10.1016/s0006-8993(97)01420-0. [DOI] [PubMed] [Google Scholar]
- Coussens CM, Kerr DS, Abraham WC. Glucocorticoid receptor activation lowers the threshold for NMDA-receptor-dependent homosynaptic long-term depression in the hippocampus through activation of voltage-dependent calcium channels. Journal of Neurophysiology. 1997;78:1–9. doi: 10.1152/jn.1997.78.1.1. [DOI] [PubMed] [Google Scholar]
- Datson NA, Morsink MC, Meijer OC, de Kloet ER. Central corticosteroid actions: Search for gene targets. European Journal of Pharmacology. 2008;583:272–289. doi: 10.1016/j.ejphar.2007.11.070. [DOI] [PubMed] [Google Scholar]
- Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. European Journal of Neuroscience. 2001;14:675–689. doi: 10.1046/j.0953-816x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews: Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Oliveira Alvares L, de Oliveira LF, Camboim C, Diehl F, Genro BP, Lanziotti VB, et al. Amnestic effect of intrahippocampal AM251, a CB1-selective blocker, in the inhibitory avoidance, but not in the open field habituation task, in rats. Neurobiology of Learning and Memory. 2005;83:119–124. doi: 10.1016/j.nlm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: a novel therapeutic approach. European Journal of Pharmacology. 2008;583:365–371. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E, Cullado M. Injury severity, prior trauma history, urinary cortisol levels, and acute PTSD in motor vehicle accident victims. Journal of Anxiety Disorders. 2003;17:149–164. doi: 10.1016/s0887-6185(02)00185-8. [DOI] [PubMed] [Google Scholar]
- DeRijk R, de Kloet ER. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine. 2005;28:263–270. doi: 10.1385/ENDO:28:3:263. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. Journal of Neuroscience. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Woodson JC. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus. 2004;14:281–291. doi: 10.1002/hipo.10186. [DOI] [PubMed] [Google Scholar]
- Domes G, Rothfischer J, Reichwald U, Hautzinger M. Inverted-U function between salivary cortisol and retrieval of verbal memory after hydrocortisone treatment. Behavioral Neuroscience. 2005;119:512–517. doi: 10.1037/0735-7044.119.2.512. [DOI] [PubMed] [Google Scholar]
- Donley MP, Schulkin J, Rosen JB. Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behavioural Brain Research. 2005;164:197–205. doi: 10.1016/j.bbr.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The restless engram: consolidations never end. Annual Review of Neuroscience. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Reviews: Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. Journal of Neuroscience. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornari RV, Wichmann R, Atucha E, Desprez T, Eggens-Meijer E, Roozendaal B. Involvement of the insular cortex in regulating glucocorticoid effects on memory consolidation of inhibitory avoidance training. Frontiers in Behavioral Neuroscience. 2012;6:10. doi: 10.3389/fnbeh.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annual Review of Psychology. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Golier JA, Caramanica K, Demaria R, Yehuda R. A Pilot Study of Mifepristone in Combat- Related PTSD. Depress Res Treat. 2012;2012:393251. doi: 10.1155/2012/393251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biological Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Grad I, Picard D. The glucocorticoid responses are shaped by molecular chaperones. Molecular and Cellular Endocrinology. 2007;275:2–12. doi: 10.1016/j.mce.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. doi: 10.1016/j.neuroscience.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nature Neuroscience. 2008;11:868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. Journal of Endocrinology. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Grossman R, Yehuda R, New A, Schmeidler J, Silverman J, Mitropoulou V, et al. Dexamethasone suppression test findings in subjects with personality disorders: associations with posttraumatic stress disorder and major depression. American Journal of Psychiatry. 2003;160:1291–1298. doi: 10.1176/appi.ajp.160.7.1291. [DOI] [PubMed] [Google Scholar]
- Haller J, Mikics E, Makara GB. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Frontiers in Neuroendocrinology. 2008;29:273–291. doi: 10.1016/j.yfrne.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Sommer W, Rimondini R, Andbjer B, Stromberg I, Fuxe K. c-fos reduces corticosterone-mediated effects on neurotrophic factor expression in the rat hippocampal CA1 region. Journal of Neuroscience. 2003;23:6013–6022. doi: 10.1523/JNEUROSCI.23-14-06013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiology of Learning and Memory. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Hauer D, Weis F, Papassotiropoulos A, Schmoeckel M, Beiras-Fernandez A, Lieke J, et al. Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Critical Care Medicine. 2011;39:643–650. doi: 10.1097/CCM.0b013e318206bae6. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Stenzel-Poore MP, Gold LH, Battenberg E, Bloom FE, Koob GF, et al. Learning impairment in transgenic mice with central overexpression of corticotropin-releasing factor. Neuroscience. 1996;74:303–311. doi: 10.1016/0306-4522(96)00140-6. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. Journal of Neuroscience. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, Cazakoff BN. Effects of acute stress and GluN2B-containing NMDA receptor antagonism on object and object-place recognition memory. Neurobiology of Learning and Memory. 2010;93:261–267. doi: 10.1016/j.nlm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience. 2013;239:173–195. doi: 10.1016/j.neuroscience.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F, Garabedian MJ, Chao MV. Activation of Trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4862–4867. doi: 10.1073/pnas.0709102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nature Neuroscience. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- Joels M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn Sci. 2011;15:280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H. Corticosteroid effects on calcium signaling in limbic neurons. Cell Calcium. 2012;51:277–283. doi: 10.1016/j.ceca.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Joels M, Krugers HJ. LTP after stress: up or down? Neural Plasticity. 2007;2007:93202. doi: 10.1155/2007/93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nature Neuroscience. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nature Neuroscience. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kaouane N, Porte Y, Vallee M, Brayda-Bruno L, Mons N, Calandreau L, et al. Glucocorticoids can induce PTSD-like memory impairments in mice. Science. 2012;335:1510–1513. doi: 10.1126/science.1207615. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Karten YJ, Reichardt HM, de Kloet ER, Schutz G, Joels M. Corticosteroid actions in hippocampus require DNA binding of glucocorticoid receptor homodimers. Nature Neuroscience. 2000;3:977–978. doi: 10.1038/79910. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews: Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. Journal of Neuroscience. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sciences. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nature Reviews: Neuroscience. 2010;11:675–681. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Farb CR, Rodrigues SM, LeDoux JE. Fear conditioning drives profilin into amygdala dendritic spines. Nature Neuroscience. 2006;9:481–483. doi: 10.1038/nn1672. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M. Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. Journal of Neuroscience. 2013;33:2961–2972. doi: 10.1523/JNEUROSCI.3878-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer RJ, Olff M, van Meijel EP, Carlier IV, Gersons BP. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biological Psychiatry. 2006;59:171–177. doi: 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Liu W, Yuen EY, Yan Z. The stress hormone corticosterone increases synaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors via serum- and glucocorticoid-inducible kinase (SGK) regulation of the GDI-Rab4 complex. Journal of Biological Chemistry. 2010;285:6101–6108. doi: 10.1074/jbc.M109.050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Betzenhauser MJ, Reiken S, Meli AC, Xie W, Chen BX, et al. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell. 2012;150:1055–1067. doi: 10.1016/j.cell.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extra-cellular glutamate concentrations in the hippocampus. Journal of Neurochemistry. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacological Reviews. 2006;58:782–797. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Research. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine VN, Spencer RL, McEwen BS. Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotonergic function. Brain Research. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can't live with it, can't live without it. Behavioural Brain Research. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Joober R, Beaulieu S, Lupien SJ. Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behavioral Neuroscience. 2004;118:420–428. doi: 10.1037/0735-7044.118.2.420. [DOI] [PubMed] [Google Scholar]
- Martin S, Henley JM, Holman D, Zhou M, Wiegert O, van Spronsen M, et al. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PloS One. 2009;4:e4714. doi: 10.1371/journal.pone.0004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biological Psychiatry. 2000a;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Research. 2000b;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Current Opinion in Neurobiology. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends in Neurosciences. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neuroscience and Biobehavioral Reviews. 2012;36:1750–1762. doi: 10.1016/j.neubiorev.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre CK. Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiology of Learning and Memory. 2010;93:312–321. doi: 10.1016/j.nlm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and posttraumatic stress disorder in adults: systematic review and meta-analysis. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J, et al. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Archives of General Psychiatry. 2011;68:901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. Journal of Neurochemistry. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Chourbaji S, Brandwein C, Racagni G, Gass P, et al. Depression-prone mice with reduced glucocorticoid receptor expression display an altered stress-dependent regulation of brain-derived neurotrophic factor and activity-regulated cytoskeleton-associated protein. J Psychopharmacol. 2010;24:595–603. doi: 10.1177/0269881108099815. [DOI] [PubMed] [Google Scholar]
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joels M, De Kloet ER, et al. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. Journal of Neuroendocrinology. 2006;18:239–252. doi: 10.1111/j.1365-2826.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- Muhtz C, Wester M, Yassouridis A, Wiedemann K, Kellner M. A combined dexamethasone/corticotropin-releasing hormone test in patients with chronic PTSD--first preliminary results. Journal of Psychiatric Research. 2008;42:689–693. doi: 10.1016/j.jpsychires.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PloS One. 2010;5:e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Archives of General Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biological Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, et al. PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology. 2005;30:373–381. doi: 10.1016/j.psyneuen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H. Brain-derived neurotrophic factor and glucocorticoids: reciprocal influence on the central nervous system. Neuroscience. 2013;239:157–172. doi: 10.1016/j.neuroscience.2012.09.073. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Kumamaru E, Adachi N, Yagasaki Y, Izumi A, Kunugi H. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate release via a glutamate transporter. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:647–652. doi: 10.1073/pnas.0800888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neuroscience. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Reichardt HM, Joels M, de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olijslagers JE, de Kloet ER, Elgersma Y, van Woerden GM, Joels M, Karst H. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. European Journal of Neuroscience. 2008;27:2542–2550. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- Pagliacci MC, Migliorati G, Smacchia M, Grignani F, Riccardi C, Nicoletti I. Cellular stress and glucocorticoid hormones protect L929 mouse fibroblasts from tumor necrosis factor alpha cytotoxicity. Journal of Endocrinological Investigation. 1993;16:591–599. doi: 10.1007/BF03347677. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Hormones and Behavior. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424:677–681. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Ogawa S, Kimura A, McEwen BS. Role of adrenal steroid mineralocorticoid and glucocorticoid receptors in long-term potentiation in the CA1 field of hippocampal slices. Brain Research. 1996;738:229–235. doi: 10.1016/s0006-8993(96)00776-7. [DOI] [PubMed] [Google Scholar]
- Pfeffer CR, Altemus M, Heo M, Jiang H. Salivary cortisol and psychopathology in children bereaved by the september 11, 2001 terror attacks. Biological Psychiatry. 2007;61:957–965. doi: 10.1016/j.biopsych.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews: Neuroscience. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Johnson LR. Stress at the synapse: signal transduction mechanisms of adrenal steroids at neuronal membranes. Sci Signal. 2009;2:re5. doi: 10.1126/scisignal.286re5. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Fleshner M, Rudy JW. Type II glucocorticoid receptor antagonists impair contextual but not auditory-cue fear conditioning in juvenile rats. Neurobiology of Learning and Memory. 1997;67:75–79. doi: 10.1006/nlme.1996.3741. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behavioral Neuroscience. 1997;111:503–511. [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Revest JM, Di Blasi F, Kitchener P, Rouge-Pont F, Desmedt A, Turiault M, et al. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nature Neuroscience. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- Revest JM, Kaouane N, Mondin M, Le Roux A, Rouge-Pont F, Vallee M, et al. The enhancement of stress-related memory by glucocorticoids depends on synapsin-Ia/Ib. Molecular Psychiatry. 2010;15(1125):1140–1151. doi: 10.1038/mp.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. Journal of Neuroscience. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:1213–1223. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, De Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. Journal of Neuroscience. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, et al. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. Journal of Neuroscience. 2010;30:5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews: Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiology of Learning and Memory. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. European Journal of Neuroscience. 1997a;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiology of Learning and Memory. 1997b;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Memory modulation. Behavioral Neuroscience. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. Journal of Neuroscience. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Portillo-Marquez G, McGaugh JL. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behavioral Neuroscience. 1996;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor--cAMP/cAMP/PKA system in influencing memory consolidation. European Journal of Neuroscience. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Mattson MP. Activity-dependent, stress-responsive BDNF signaling and the quest for optimal brain health and resilience throughout the lifespan. Neuroscience. 2013;239:228- 240. doi: 10.1016/j.neuroscience.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learning and Memory. 2010;17:522–530. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nature Reviews: Neuroscience. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Sandi C. Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends in Neurosciences. 2011;34:165–176. doi: 10.1016/j.tins.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plasticity. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Schilling TM, Kolsch M, Larra MF, Zech CM, Blumenthal TD, Frings C, et al. For whom the bell (curve) tolls: Cortisol rapidly affects memory retrieval by an inverted U-shaped doseresponse relationship. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress and multiple memory systems: from 'thinking' to 'doing'. Trends Cogn Sci. 2013;17:60–68. doi: 10.1016/j.tics.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. Stress-induced sensitization and facilitated learning require NMDA receptor activation. Neuroreport. 1995;6:677–680. doi: 10.1097/00001756-199503000-00023. [DOI] [PubMed] [Google Scholar]
- Smeets T, Sijstermans K, Gijsen C, Peters M, Jelicic M, Merckelbach H. Acute consolidation stress enhances reality monitoring in healthy young adults. Stress. 2008;11:235–245. doi: 10.1080/10253890701754076. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, et al. Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiology of Learning and Memory. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biological Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kimoto T, Tanabe N, Hattori TA, Yasumatsu N, Kawato S. Corticosterone acutely prolonged N-methyl-d-aspartate receptor-mediated Ca2+ elevation in cultured rat hippocampal neurons. Journal of Neurochemistry. 2002;83:1441–1451. doi: 10.1046/j.1471-4159.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Riceberg JS, New AS, Alberini CM. Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors. Biological Psychiatry. 2009;65:249–257. doi: 10.1016/j.biopsych.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekcan AI, Peynircioglu ZF. Effects of age on flashbulb memories. Psychology and Aging. 2002;17:416–422. doi: 10.1037/0882-7974.17.3.416. [DOI] [PubMed] [Google Scholar]
- ter Horst JP, van der Mark MH, Arp M, Berger S, de Kloet ER, Oitzl MS. Stress or no stress: mineralocorticoid receptors in the forebrain regulate behavioral adaptation. Neurobiology of Learning and Memory. 2012;98:33–40. doi: 10.1016/j.nlm.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Tronel S, Alberini CM. Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biological Psychiatry. 2007;62:33–39. doi: 10.1016/j.biopsych.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, et al. Polymorphisms of the glucocorticoid receptor gene and major depression. Biological Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Koper JW, van den Beld AW, Uitterlinden AG, Arp P, Ester W, et al. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clinical Endocrinology. 2003;59:585–592. doi: 10.1046/j.1365-2265.2003.01888.x. [DOI] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. European Journal of Neuroscience. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert O, Pu Z, Shor S, Joels M, Krugers H. Glucocorticoid receptor activation selectively hampers N-methyl-D-aspartate receptor dependent hippocampal synaptic plasticity in vitro. Neuroscience. 2005;135:403–411. doi: 10.1016/j.neuroscience.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. Journal of Neuroscience. 2005;25:4288–4293. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Risk and resilience in posttraumatic stress disorder. Journal of Clinical Psychiatry. 2004;65(Suppl 1):29–36. [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biological Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Molecular Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalachoras I, Houtman R, Meijer OC. Understanding stress-effects in the brain via transcriptional signal transduction pathways. Neuroscience. 2013;242:97–109. doi: 10.1016/j.neuroscience.2013.03.038. [DOI] [PubMed] [Google Scholar]
- Zorawski M, Blanding NQ, Kuhn CM, LaBar KS. Effects of stress and sex on acquisition and consolidation of human fear conditioning. Learning and Memory. 2006;13:441–450. doi: 10.1101/lm.189106. [DOI] [PMC free article] [PubMed] [Google Scholar]