Abstract
Purpose
While vitamin D is critical for optimal skeletal health, it also appears to play a significant role in vascular homeostasis. This pilot study compared arteriovenous access outcomes following cholecalciferol supplementation compared to placebo in end-stage renal disease patients preparing to undergo AV access creation.
Methods
52 adult hemodialysis patients preparing for AVF creation were randomized to receive peri-operative high dose cholecalciferol vs. placebo in this double-blind, randomized placebo-controlled pilot study. The primary outcome was mean response to high-dose oral cholecalciferol vs. placebo, and secondary outcome arteriovenous access maturation at 6 months. Logistic regression was used to assess the association between AV access maturation and baseline, post-treatment and overall change in vitamin D concentration.
Results
45% of cholecalciferol-treated and 54% of placebo-treated patients were successfully using their AVF or AVG at 6 months (p= 0.8). Baseline serum concentrations of 25(OH)D and 1,25(OH)2D did not differ between those who experienced AVF or AVG maturation and those who did not (p=0.22 and p=0.59, respectively). Similarly, there was no relationship between AVF or AVG maturation and post-treatment serum 25(OH)D and 1,25(OH)2D concentration (p=0.24 and 0.51, respectively).
Conclusions
Peri-operative high dose vitamin D3 therapy does correct 25(OH)D level but does not appear to have an association with AV access maturation rates. Future research may include extended pre-operative vitamin D3 therapy in a larger population or in certain sub-populations at high risk for AVF failure.
Keywords: AVF, Dialysis access, ESRD, Vitamin D
Introduction
Vitamin D is an essential nutrient that is often deficient in end-stage renal disease (ESRD) patients, primarily due to insufficient sunlight exposure, low dietary intake and loss of renal function. As a result, 78%-91% of ESRD patients are vitamin D deficient or insufficient at the time of dialysis initiation.1,2
While vitamin D is critical to maintaining calcium and phosphorus homeostasis for optimal skeletal health, it also appears to play a significant role in vascular homeostasis. Circulating vitamin D [(25(OH)D] is converted to 1,25(OH)2D in local tissues by the enzyme 1-alpha-hydroxylase. This further up-regulates the vitamin D receptor found in many tissues, including vascular smooth muscle cells (VSMC), and further increases the expression of 1-alpha-hydroxylase resulting in locally produced 1,25(OH)2D, which is believed to have many important immunomodulatory effects.3-11 The importance of local vitamin D production is supported by the observation that 25(OH)D deficiency is associated with many forms of vascular disease and endothelial dysfunction. 12-16 Among its many actions, vitamin D suppresses extracellular matrix remodeling factors, which play a key role in VSMC migration and development of vascular neointimal hyperplasia, modulates VSMC growth in vitro, and retards VSMC proliferation. 5,10,17-20 Furthermore, vitamin D has anti-oxidant and anti-inflammatory properties.21-24
Given vitamin D’s ability to suppress factors known to promote development of progressive venous neointimal hyperplasia and to affect expression of oxidative and inflammatory markers detected within failing AV access25-27, we hypothesized that nutritional vitamin D supplementation (25(OH)D) may improve arteriovenous fistula (AVF) outcomes by providing adequate substrate to enable local, active vitamin D production within the vasculature.
We therefore conducted a double-blind randomized controlled pilot study to provide critical estimates to determine the distribution of vitamin D levels in our ESRD patient cohort in those who receive high-dose oral vitamin D3 (cholecalciferol) compared to placebo for the planning of subsequent, larger studies examining the effect of vitamin D on AVF maturation and to show feasibility for patient recruitment in the proposed time frame.
Materials and Methods
Subjects and Protocol
The protocol for this double-blind, randomized, placebo-controlled pilot study has been previously described.28 Briefly, we enrolled 52 adults with end-stage renal disease (ESRD) receiving in-center outpatient hemodialysis who were preparing to receive an AVF creation within 4 weeks. Suitability of a patient for AVF creation was based
on vein diameter >2.5 mm, and inflow artery diameter of > 2 mm.29 Enrolled subjects were randomized to 200,000 IU oral vitamin D3 (cholecalciferol) or matching placebo (Bio-Tech Pharmacal Inc, Fayetteville, AR) weekly for 3 weeks. In a recent publication, we reported that our dosing regimen of high-dose cholecalciferol versus placebo is safe and effectively increases circulating serum 25(OH)D concentrations to the normal range of > 30 ng/mL, lowers serum parathyroid hormone (PTH) concentration, and does not cause hypercalcemia.28 The study coordinator directly observed all patients taking their assigned medication, and there was 100% overall medication compliance. A blood sample was collected at study enrollment and again three weeks following study drug completion for measurement of serum 25(OH)D and 1,25(OH)2D. Monthly dialysis unit laboratory test results collected ≤ 4 weeks prior to study enrollment date and ≤ 4 weeks after study completion were abstracted to include in the analysis. The Emory University Institutional Review Board approved the study protocol, and all participants provided informed consent prior to study enrollment. The study was registered at clinicaltrials.gov (NCT00912782).
Outcome Definition
The primary study outcome was mean response to high-dose oral vitamin D3 (cholecalciferol) vs. placebo. The secondary study outcome was AVF maturation, defined as the ability to cannulate the AVF with two large bore needles at ≥ 6 consecutive dialysis sessions, and achievement of an AVF blood flow >300 ml/min, assessed at six months following AVF creation.
Analytic Methods
Serum 25(OH)D was assayed by using a chemiluminescence immunoassay (Diasorin Inc: CV over multiple runs: 6.3-12.9%) and 1,25 (OH)2D was assayed by using solid-phase extraction and radioimmunoassay by ARUP laboratories. All samples were batched and analyzed with known standards to ensure test quality. Vitamin D deficiency was defined as 20 ng/mL, and insufficiency was defined as 20 to 30 ng/mL. Levels to define vitamin D status were derived from The Endocrine Society clinical practice guidelines for vitamin D30 because the Institute of Medicine guidelines are intended for a normal healthy population, not those with chronic disease.
Statistical Analysis
Baseline characteristics and post-treatment blood chemistry measures including serum 25(OH)D concentration were compared between the placebo group and the cholecalciferol group using Wilcoxon rank sum tests for continuous variables and Fisher exact tests for categorical variables. Wilcoxon signed rank tests were used to test whether changes of blood chemistry measures from baseline to post-treatment were different from 0. AVF or AVG maturation status, was compared between the two study groups using Fisher exact tests. Logistic regression analysis was performed to test the association between AVF/AVG maturation status and baseline and post-treatment vitamin D concentration and between AVF/AVG maturation status and changes in vitamin D concentration from baseline to post-treatment, first unadjusted and then adjusted for age, diabetes, and race. The analyses were performed on an intention-to-treat basis using the free programming language and software environment for statistical computing, R. A p-value of <=0.05 was considered statistically significant.
Results
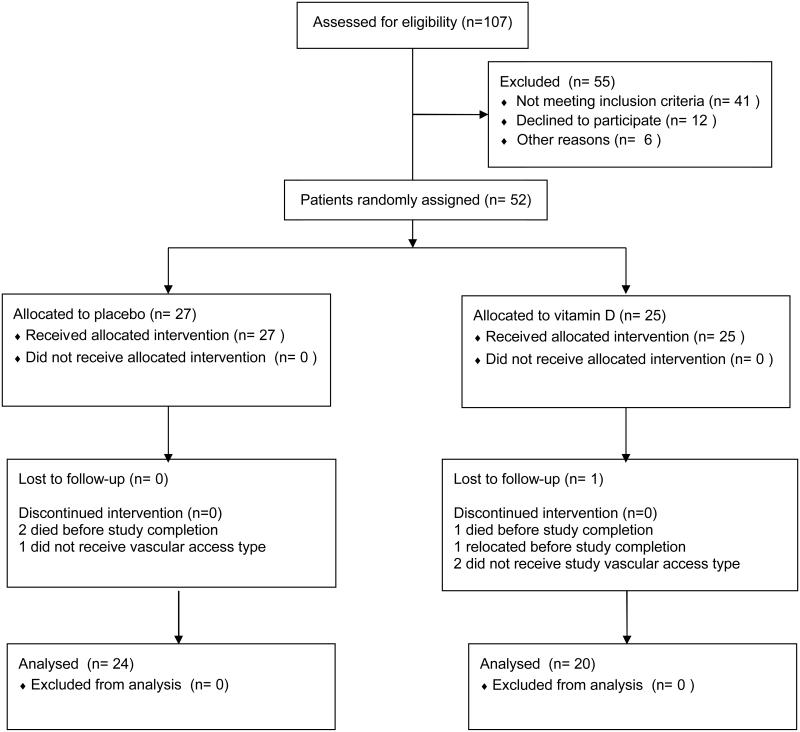
A total of 52 subjects were enrolled: 25 were randomly assigned to the cholecalciferol treatment group, and 27 to receive placebo, as previously reported.28 During follow-up, one subject was lost to follow-up, 3 died, and 4 never received a permanent vascular access. Of these 8 subjects, 5 were in the vitamin D group and 3 were in the placebo group. Characteristics of these 8 patients were similar to the remaining patient cohort, except for female gender (p=0.04). Overall, 44 subjects (24 in placebo group; 20 in cholecalciferol group) remained for analysis (Figure 1).
Figure 1.
Flow diagram of patient enrollment
Clinical and laboratory characteristics
Clinical and laboratory characteristics were similar in the groups at baseline (Table 1), with the exception of baseline serum 25 (OH) D and 1,25 (OH)2 D concentrations, which were greater among the placebo group (P=0.04 and P=0.02, respectively). At baseline, 95.5% of subjects were vitamin D insufficient (serum 25(OH) D < 30 ng/mL); mean serum 25(OH)D was 16.8 ± 6.5 ng/mL, and mean serum 1,25(OH)2D was 17.4 ± 8.9 pg/mL. Following treatment, vitamin D sufficiency (25(OH)D ≥ 30 ng/mL) was achieved in 90% of cholecalciferol-treated patients (mean serum 25(OH)D= 53.4 ± 17.7 ng/mL) and only 13.6% in the placebo-treated patients (mean serum 25(OH)D= 18.4 ± 7.4 ng/mL) (p<0.001), while serum 1,25(OH)2D significantly increased in cholecalciferol-treated patients (p<0.001). There was no significant change in serum calcium, phosphorus or intact PTH.
Table 1.
Baseline characteristics for subjects with observed AV maturation outcome
| All (n=44) | Placebo (n=24) |
Cholecalciferol (n=20) |
P value | |
|---|---|---|---|---|
| Age (y) | 51.1 ±13.2 | 52.1 ± 14.9 | 49.9 ± 10.9 | 0.62 |
| Black | 40 (90.9%) | 21 (87.5%) | 19 (95%) | 0.61 |
| Female | 14 (31.8%) | 9 (37.5%) | 5 (25%) | 0.52 |
| Duration of Dialysis, days | 636 ± 1050 | 924 ±1340 | 291 ± 301 | 0.20 |
| BMI (kg/m2) | 28.2 ± 6.9 | 28.3 ± 7.9 | 28.1 ± 5.7 | 0.68 |
| Clinical blood pressure (mm Hg) |
||||
| Systolic | 152.7 ± 24.9 | 150.8 ± 23.7 | 155.1 ± 26.7 | 0.62 |
| Diastolic | 86.7 ± 17.3 | 85.3 ±16.9 | 88.3 ±18.1 | 0.93 |
| Diabetes | 21 (47.7%) | 12 (50%) | 9 (45%) | 0.77 |
| Hypertension | 38 (86.4%) | 21 (87.5%) | 17 (85%) | 1.00 |
| HIV/AIDS | 8 (18.2%) | 4 (16.7%) | 4 (20%) | 1.00 |
| Lactose intolerance | 9 (20.5%) | 3 (12.5%) | 6 (30%) | 0.261 |
| Laboratory | ||||
| Intact parathyroid hormone | 605.3 ± | |||
| (pg/mL) | 756.61 | 630.3 ± 944.8 | 575.3 ± 461.8 | 0.54 |
| Ionized calcium (mg/dL) | 8.9 ± 0.8 | 9.1 ± 0.6 | 8.8 ± 0.9 | 0.26 |
| Phosphorus (mg/dL) | 4.9 ± 1.3 | 4.7 ±1.2 | 5.0 ± 1.5 | 0.66 |
| 25(OH)D (ng/mL) | 16.8 ± 6.5 | 18.6 ± 6.6 | 14.6 ± 5.8 | 0.04 |
| 1,25(OH)2 D (pg/mL) | 17.4 ± 8.9 | 20.3 ± 9.9 | 13.9 ± 6.2 | 0.02 |
| Hemoglobin (g/dL) | 10.8 ± 1.7 | 11.2 ±1.4 | 10.4 ± 1.9 | 0.14 |
| Current use of intravenous | ||||
| vitamin D analogs | 30 (68.2%) | 16 (66.7%) | 14 (70%) | 1.00 |
Results are presented as mean ± SD or n (%)
AV access outcome
Overall, 15 radial-cephalic, 13 brachial-cephalic, 7 brachial-basilic AVFs were created and 9 AVGs surgically placed. At follow-up, 45% of cholecalciferol-treated and 54% of placebo-treated patients were successfully using their AVF or AVG at 6 months (p= 0.8). Excluding AVG patients, 41% of cholecalciferol-treated and 50% of placebo-treated patients achieved AVF maturation (p=0.7). Baseline concentrations of serum 25(OH)D and 1,25(OH)2D were similar between patients who experienced AVF or AVG maturation and those who did not (p=0.22 and p=0.59, respectively). Similarly, there was no relationship between AVF or AVG maturation and post-treatment serum 25(OH)D and 1,25(OH)2D concentration (p=0.24 and 0.51, respectively).
Following adjustment for age, diabetes and race, there was no association between AVF maturation and baseline, post-treatment or change in serum 25(OH)D concentrations (p=0.97, 0.54, 0.56, respectively), or serum 1,25(OH)2D concentrations (p=0.51, 0.61, 0.43, respectively). These findings were similar when including AVG’s in the analysis.
Discussion
We found that peri-operative correction of 25(OH)D insufficiency and deficiency was achieved with high-dose cholecalciferol, but did not appear to have an association with AV access maturation among ESRD patients who received an AVF or AVG, although serum 25(OH)D concentrations normalized in 90% of cholecalciferol-treated subjects.
While no previous study has examined serum 25(OH)D concentration as a predictor of AV access maturation, previous work supports a role for vitamin D in the regulation of vascular smooth muscle cell proliferation. Vitamin D receptors (VDR) are present in numerous tissues, including the endothelium and vascular smooth muscle cells, which also express 1-alpha-hydroxylase, the enzyme that converts 25(OH)D to 1,25(OH)2 D. 11,31
Animal experiments suggest that vitamin D concentrations are associated with intimal proliferation, and that vitamin D inhibits VSMC proliferation.4,32 Gupta et al recently demonstrated that serum 25(OH)D concentration may affect the extent of resultant neointimal thickening in an atherosclerotic swine model following arterial injury.32 Six-months following balloon angioplasty to the coronary arteries, the percent area of arterial neointimal hyperplasia was significantly greater (P< 0.05) among the group of animals fed a vitamin D-deficient high cholesterol diet (72.5 ± 4.5%) compared with those fed a vitamin D-sufficient high cholesterol diet (54.9 ± 3.7%). Proliferating cell nuclear antigen (PCNA), a marker of smooth muscle cell proliferation, was more abundant in cells within the balloon-injured arteries of vitamin-D deficient animals compared with vitamin-D sufficient animals, suggesting that vitamin D has an antiproliferative effect. Moreover, Gupta et al found that VDR expression was significantly downregulated and that TNF-alpha expression was increased in proliferating VSMC’s within neointimal lesions following vessel injury. They also showed that VDR expression in cultured VSMC’s increased with calcitriol stimulation, yet significantly decreased with TNF-alpha treatment, suggesting that the presence of inflammation following vessel injury may downregulate VSMC VDR and contribute to VSMC proliferation and restenosis.
In the mouse aortic allograft, a model of immune mediated vascular intimal hyperplasia, VDR expression is present in aortic cells. 4 Adorini et al reported that mice receiving aortic allografts that were treated with vitamin D3 analogue supplementation had significantly less intimal cell proliferation within allogeneic aortic segments 60 days after transplant, compared with vehicle mice.
While these findings suggest the potential benefit of vitamin D on the attenuation of VSMC proliferation and neointimal hyperplasia following injury from percutaneous angioplasty, they may be relevant to CKD and ESRD patients undergoing AV access creation. First, our group and others33,34 have shown that venous intimal hyperplasia pre-exists in late-stage non-dialysis CKD patients, a population with a high prevalence of vitamin D insufficiency and deficiency35. While pre-existing intimal hyperplasia has not been linked to AV access failure, it remains unclear whether these lesions progress following AV access creation. Second, it is estimated that approximately 30% of patients with newly created AVF undergo AVF PTA to promote maturation36, or develop stenosis in vessel segments typically manipulated during surgical AVF creation.37. Therefore, a cost- effective intervention that could potentially reduce the magnitude of subsequent AVF restenosis may have significant impact.
Successful AVF maturation is also dependent upon compensatory dilatation of the inflow artery and adequate dilation and blood flow to the outflow vein. 38-40 Data suggests that vasoactive function is influenced by vitamin D sufficiency. Tare et al 41 examined the effect of vitamin D insufficiency on endothelial and smooth muscle function in the arteries of offspring of vitamin D deficient rats. The vitamin D deficient rat offspring had only half of the endothelium-derived nitric oxide-evoked arterial dilation and virtually no endothelium-derived hyperpolarizing factor compared with control rats, reflecting a pronounced impairment in arterial relaxation. In accordance with these findings are the results showing that vitamin D therapy increases flow-mediated dilation in adult humans. 42
Other favorable effects of vitamin D on AVF maturation may include suppression of matrix metalloproteases (MMP) and a reduction in thrombotic factors. MMP’s are linked to the development of intimal hyperplasia 43,44 and identified in vein segments of failed AVF. 45 Greater local vitamin D activity may reduce intimal hyperplasia by lessening the effect of MMP remodeling within the vein, as treatment with vitamin D3 is shown to suppress MMP 2 and 3 in vitro, and circulating serum 25(OH)D is inversely associated with plasma MMP-9 concentration in ESRD patients.5,46
Reports also suggest that VDR activation has antithrombotic effects. Plasminogen activator inhibitor type 1 (PAI-1), a procoagulant, proinflammatory protein, is reduced in vitro by paricalcitol, a vitamin D analogue. 7,8 It is also noted that VDR activation in vivo decreases platelet aggregation via regulation of anti-thrombin gene expression in the liver. 47 When evaluated in humans, vitamin D appears to plays a role in coagulation. Cancer patients treated with high dose 1,25 (OH)2D had significantly fewer venous and arterial thrombotic events than did those treated with standard dose1,25(OH)2D.48
Our study has some limitations worth mentioning. Although this was a pilot study, a sample size of 50 patients (25 per group) was determined to achieve power of 0.8 when estimating that 52% of placebo-treated patients vs 88% of cholecalciferol-treated patients would experience AVF maturation. Instead, we found that 54% of placebo-treated patients vs. 45% of cholecalciferol-treated and were successfully using their AVF or AVG at 6 months. This finding suggests that any advantage that vitamin D confers is much lower than our original estimate, in which case our study was underpowered to detect it. It is possible that AV access outcome was influenced by variability in surgical technical expertise, however, we attempted to address this by limiting the vascular surgeons performing AV access procedures to two highly experienced individuals (W.M., G.S.) and there was no difference in AV access outcome. In addition, postoperative AV access cannulation and vascular access management may have varied between intervention and control subjects. We attempted to limit variation by recruiting patients from dialysis units with a uniform cannulation protocol and dialysis providers. Finally, we may have failed to see a benefit with cholecalciferol on AVF maturation because longer pre-surgical vitamin D sufficiency (> 6 months, for example) may be required.
We found that high-dose vitamin D3 therapy in patients with ESRD undergoing AVF creation successfully corrected vitamin D insufficiency and deficiency, but did not appear to improve AVF maturation rates compared to a cohort of matched controls in our pilot, double-blind randomized controlled study. Conclusive results will likely require a larger patient sample to achieve adequate effect size. Further, the role for vitamin D in AV access maturation may need to be explored in larger studies with extended pre-operative supplementation or in certain sub-populations at high risk for AVF failure.
Acknowledgments
Support: This study was supported by a University Research Committee Grant, Emory University (H.W.) an NIH K23 DK65634 (H.W.), and a PHS Grant (UL1 RR02008, KL2 RR025009 or TL1 RR025010, and UL1TR000454) from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resource
Footnotes
Transparency Declarations:
None to declare. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron. Clinical practice. 2007;105(3):c132–138. doi: 10.1159/000098645. [DOI] [PubMed] [Google Scholar]
- 2.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney international. 2007 Oct;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 3.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. The Journal of clinical endocrinology and metabolism. 2001 Feb;86(2):888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 4.Adorini L, Amuchastegui S, Daniel KC. Prevention of chronic allograft rejection by Vitamin D receptor agonists. Immunology letters. 2005 Aug 15;100(1):34–41. doi: 10.1016/j.imlet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi H, Asano K, Kanai K, Suzaki H. Suppressive activity of vitamin D3 on matrix metalloproteinase production from cholesteatoma keratinocytes in vitro. Mediators of inflammation. 2005 Aug 31;2005(4):210–215. doi: 10.1155/MI.2005.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? Qjm. 2002 Dec;95(12):787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 7.Wu-Wong JR, Nakane M, Ma J. Effects of vitamin D analogs on the expression of plasminogen activator inhibitor-1 in human vascular cells. Thrombosis research. 2006;118(6):709–714. doi: 10.1016/j.thromres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Ruth Wu-Wong J, Nakane M, Ma J, Cook AL. Vitamin D analogs down-regulate plasminogen activator inhibitor-1 in human coronary artery smooth muscle cells. J Thromb Haemost. 2005 Jul;3(7):1545–1546. doi: 10.1111/j.1538-7836.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- 9.Gouni-Berthold I, Krone W, Berthold HK. Vitamin D and cardiovascular disease. Current vascular pharmacology. 2009 Jul;7(3):414–422. doi: 10.2174/157016109788340686. [DOI] [PubMed] [Google Scholar]
- 10.Mitsuhashi T, Morris RC, Jr., Ives HE. 1,25-dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. The Journal of clinical investigation. 1991 Jun;87(6):1889–1895. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somjen D, Weisman Y, Kohen F, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005 Apr 5;111(13):1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 12.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008 Mar;25(3):320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 13.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007 May;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez JA, Law J, Coakley KE, et al. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. The American journal of clinical nutrition. 2012 Sep;96(3):672–679. doi: 10.3945/ajcn.112.040642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melamed ML, Muntner P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008 Jun;28(6):1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? Journal of the American College of Cardiology. 2003 Jan 1;41(1):105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 17.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989 Jun;13(6 Pt 2):954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 18.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006 May;186(1):20–28. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 19.Dean DD, Boyan BD, Schwart Z, et al. Effect of 1alpha,25-dihydroxyvitamin D3 and 24R,25-dihydroxyvitamin D3 on metalloproteinase activity and cell maturation in growth plate cartilage in vivo. Endocrine. 2001 Apr;14(3):311–323. doi: 10.1385/endo:14:3:311. [DOI] [PubMed] [Google Scholar]
- 20.Chen S LC, Gardner DG. Vitamin D-dependent suppression of endothelin-induced vascular smooth muscle cell proliferation through inhibition of CDK2 activity. The Journal of steroid biochemistry and molecular biology. 2009 Dec 2; doi: 10.1016/j.jsbmb.2009.11.002. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu-Wong JR, Nakane M, Ma J. Vitamin D analogs modulate the expression of plasminogen activator inhibitor-1, thrombospondin-1 and thrombomodulin in human aortic smooth muscle cells. Journal of vascular research. 2007;44(1):11–18. doi: 10.1159/000097812. [DOI] [PubMed] [Google Scholar]
- 22.Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004 Jan;26(1):21–28. doi: 10.1002/bies.10368. [DOI] [PubMed] [Google Scholar]
- 23.Lin AM, Chen KB, Chao PL. Antioxidative effect of vitamin D3 on zinc-induced oxidative stress in CNS. Annals of the New York Academy of Sciences. 2005 Aug;1053:319–329. doi: 10.1196/annals.1344.028. [DOI] [PubMed] [Google Scholar]
- 24.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. The American journal of clinical nutrition. 2006 Apr;83(4):754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 25.Weiss MF, Scivittaro V, Anderson JM. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis. 2001 May;37(5):970–980. doi: 10.1016/s0272-6386(05)80013-7. [DOI] [PubMed] [Google Scholar]
- 26.Misra S, Fu AA, Rajan DK, et al. Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. J Vasc Interv Radiol. 2008 Feb;19(2 Pt 1):252–259. doi: 10.1016/j.jvir.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Misra S, Lee N, Fu AA, et al. Increased expression of a disintegrin and metalloproteinase thrombospondin 1 in thrombosed hemodialysis grafts. J Vasc Interv Radiol. 2008 Jan;19(1):111–119. doi: 10.1016/j.jvir.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Wasse H, Huang R, Long Q, Singapuri S, Raggi P, Tangpricha V. Efficacy and safety of a short course of very-high-dose cholecalciferol in hemodialysis. The American journal of clinical nutrition. 2012 Feb;95(2):522–528. doi: 10.3945/ajcn.111.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical Practice Guidelines for Vascular Access. American Journal of Kidney Disease. 2006;48(S):176–273. doi: 10.1053/j.ajkd.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011 Jul;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 31.Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002 Mar;13(3):621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 32.Gupta GK, Agrawal T, Del Core MG, Hunter WJ, 3rd, Agrawal DK. Decreased expression of vitamin D receptors in neointimal lesions following coronary artery angioplasty in atherosclerotic swine. PLoS One. 2012;7(8):e42789. doi: 10.1371/journal.pone.0042789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasse H, Huang R, Naqvi N, Smith E, Wang D, Husain A. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. The journal of vascular access. 2012 Apr-Jun;13(2):168–174. doi: 10.5301/jva.5000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee T, Chauhan V, Krishnamoorthy M, et al. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant. 2011 Jul;26(7):2264–2270. doi: 10.1093/ndt/gfq733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez J, Wasse H, Tangpricha V. Vitamin D supplementation in pre-dialysis chronic kidney disease: A systematic review. Dermato-endocrinology. 2012 Apr 1;4(2):118–127. doi: 10.4161/derm.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee T, Tindni A, Roy-Chaudhury P. Improved Cumulative Survival in Fistulas Requiring Surgical Interventions to Promote Fistula Maturation Compared with Endovascular Interventions. Seminars in dialysis. 2012 Mar 9; doi: 10.1111/j.1525-139X.2012.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badero OJ, Salifu MO, Wasse H, Work J. Frequency of swing-segment stenosis in referred dialysis patients with angiographically documented lesions. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008 Jan;51(1):93–98. doi: 10.1053/j.ajkd.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney international. 2002 Oct;62(4):1109–1124. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 39.Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg. 1996 Aug;12(2):207–213. doi: 10.1016/s1078-5884(96)80108-0. [DOI] [PubMed] [Google Scholar]
- 40.Korten E, Toonder IM, Schrama YC, Hop WC, van der Ham AC, Wittens CH. Dialysis fistulae patency and preoperative diameter ultrasound measurements. Eur J Vasc Endovasc Surg. 2007 Apr;33(4):467–471. doi: 10.1016/j.ejvs.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 41.Tare M, Emmett SJ, Coleman HA, et al. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol. 2011 Oct 1;589(Pt 19):4777–4786. doi: 10.1113/jphysiol.2011.214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris RA, Pedersen-White J, Guo DH, et al. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011 May;24(5):557–562. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circulation research. 2002 Feb 22;90(3):251–262. [PubMed] [Google Scholar]
- 44.Magid R, Murphy TJ, Galis ZS. Expression of matrix metalloproteinase-9 in endothelial cells is differentially regulated by shear stress. Role of c-Myc. The Journal of biological chemistry. 2003 Aug 29;278(35):32994–32999. doi: 10.1074/jbc.M304799200. [DOI] [PubMed] [Google Scholar]
- 45.Chang CJ, Ko YS, Ko PJ, et al. Thrombosed arteriovenous fistula for hemodialysis access is characterized by a marked inflammatory activity. Kidney international. 2005 Sep;68(3):1312–1319. doi: 10.1111/j.1523-1755.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 46.Wasse H, Cardarelli F, De Staercke C, Hooper C, Veledar E, Guessous I. 25-hydroxyvitamin D concentration is inversely associated with serum MMP-9 in a cross-sectional study of African American ESRD patients. BMC nephrology. 2011;12:24. doi: 10.1186/1471-2369-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aihara K, Azuma H, Akaike M, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. The Journal of biological chemistry. 2004 Aug 20;279(34):35798–35802. doi: 10.1074/jbc.M404865200. [DOI] [PubMed] [Google Scholar]
- 48.Beer TM, Venner PM, Ryan CW, et al. High dose calcitriol may reduce thrombosis in cancer patients. British journal of haematology. 2006 Nov;135(3):392–394. doi: 10.1111/j.1365-2141.2006.06322.x. [DOI] [PubMed] [Google Scholar]