Abstract
Thyroid hormone (T3), like many other ligands of the steroid/thyroid hormone nuclear receptor superfamily is a strong inducer of liver cell proliferation in rats and mice. However, the molecular basis of its mitogenic activity, which is currently unknown, must be elucidated if its use in hepatic regenerative medicine is to be considered. F-344 rats or C57BL/6 mice were fed a diet containing T3 for 2-7 days. In rats, administration of T3 led to an increased cytoplasmic stabilization and nuclear translocation of β-catenin in pericentral hepatocytes with concomitant increase in Cyclin-D1 expression. T3 administration to wild-type (WT) mice resulted in increased hepatocyte proliferation, however no mitogenic response in hepatocytes to T3 was evident in the hepatocyte-specific β-catenin knockout mice (KO). In fact, T3 induced β-catenin-TCF4 reporter activity both in vitro and in vivo. Livers from T3-treated mice demonstrated no changes in Ctnnb1 expression, activity of Glycogen synthase kinase-3β known to phosphorylate and eventually promote β-catenin degradation, or E-cadherin-β-catenin association. However, T3 treatment increased β-catenin phosphorylation at Ser675, an event downstream of protein kinase A (PKA). Administration of PKA inhibitor during T3 treatment of mice and rats as well as in cell culture abrogated Ser675-β-catenin and simultaneously decreased Cyclin-D1 expression to block hepatocyte proliferation.
Conclusion
We have identified T3-mediated hepatocyte mitogenic response to be mediated by PKA-dependent β-catenin activation. Thus, T3 may be of therapeutic relevance to stimulate β-catenin signaling to in turn induce regeneration in selected cases of hepatic insufficiency.
Keywords: thyroid hormone, Wnt, nuclear receptor, Cyclin-D1, PKA, regeneration
Introduction
β-Catenin is an important nuclear effector of the Wnt signaling pathway that is involved in the establishment of the dorsoventral axis or the segmentation pattern in embryos (1). In addition, there is a growing evidence of the involvement of the Wnt/β-catenin pathway in liver biology (2). Despite its aberrant accumulation due to mutations in hepatocellular cancer (HCC), β-catenin is crucial for cell cycle regulation during embryonic liver development and liver regeneration after partial hepatectomy (PH) (2, 3). Suppression of β-catenin by antisense oligonucleotides in ex vivo liver cultures results in decreased cell proliferation and increased apoptosis of hepatocytes (4). Further evidence of a critical role of β-catenin in fully differentiated hepatocyte proliferation stems from recent findings showing that hepatocyte-specific β-catenin knockout mice (KO) display decreased numbers of hepatocytes in S-phase at the time of peak hepatocyte proliferation following PH, due to lack of Cyclin-D1 (5, 6).
Liver regeneration is a compensatory response to injury, in which proliferation is essential to restore hepatic mass and function. In contrast, numerous primary mitogens induce hepatocyte proliferation without causing liver injury. Unlike liver regeneration, direct hyperplasia, results in an excess of hepatic DNA and liver mass (7). Such mitogens include the peroxisome proliferators (PPs), retinoic acids (RA), thyroid hormone tri-iodothyronine (T3), and the halogenated hydrocarbon 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP), which are all ligands of nuclear receptors of the steroid/thyroid superfamily (8). As heterodimers with RXRα, these receptors function as ligand-activated transcription factors and regulate genes involved in lipid metabolism, adipogenesis, xenobiotic detoxification, and differentiation. Among these nuclear receptor ligands, T3 is particularly interesting since its mitogenic activity is associated with regression of preneoplastic lesions induced by chemical carcinogens (9, 10). T3 is known to influence a variety of physiological processes, including cell growth and metabolism in mammals, metamorphosis in amphibians, and development of the vertebrate nervous system. Most of these effects are mediated by thyroid hormone nuclear receptors, (TRs), which act as transcription factors (11, 12). Due to the ubiquitous nature of TRs, T3 has been shown to induce proliferation in several organs including liver, kidney, pancreas, heart and intestine, although its effect on liver and pancreas is predominantly via TRβ (13).
In the liver, T3-induced proliferation lacks many early events thought to be critical in liver regeneration, such as activation of latent transcription factors (AP1, NFκB) or increased expression of immediate-early transcription factors (c-fos, c-myc) and c-jun (14, 15). These differences suggest that the signaling pathways activated by T3 via TRs may be different from those activated in liver regeneration. Interestingly, T3 has been shown to induce Cyclin-D1 expression (15). Since Cyclin-D1 is regulated by the Wnt/β-catenin signaling in the liver during proliferative states, we asked if T3's effect on Cyclin-D1 expression and eventually on hepatocyte proliferation was indeed β-catenin-dependent (5, 6, 16, 17). In the current study, we use in vitro and in vivo models (rats and mice) to show that β-catenin is indeed activated by T3, which is protein kinase A (PKA) dependent. Using β-catenin KO mice, we show the requirement of β-catenin in T3-mediated hepatocytes proliferation. We eventually discuss the usability of T3 for therapeutics in select cases of hepatic insufficiency.
Materials and Methods
Animals
Eight week male F-344 rats (Charles River, Milan, Italy) were maintained on a standard laboratory diet (Ditta Mucedola, Milan, Italy) or fed a T3-supplemented diet (4 mg/kg of diet, Sigma Chemical Co., St Louis, MO) for 2 or 4 days. Eight-10 weeks male β-catenin KO mice or sex-matched littermate controls obtained from breeding homozygous floxed β-catenin mice and albumin-cre transgenic mice as described previously (6) were fed a basal or a T3-supplemented diet (4 mg/kg of diet) for 1 week. C57BL/6 mice were also fed T3 or basal diet for 4 days to harvest livers for addressing molecular changes. C57BL/6 male mice were used in the experiments with the PKA inhibitor (see below). To label hepatocytes, BrdU (5-bromodeoxyuridine) dissolved in drinking water (1 mg/ml) was given to all animals throughout the experimental period. The animals were given food and water ad libitum with a 12 h light/dark daily cycle. All studies on mice and rats were performed in strict accordance with the Institutional Animal Use and Care Committee at the University of Pittsburgh and the University of Cagliari and the National Institutes of Health guidelines.
Administration of H89, a PKA inhibitor
Three different protocols were used:
Experimental protocol 1
3-5 months old male mice (C57BL/6 strain) (n≥3) were fed a T3 diet (4mg/kg/diet) or a basal diet for 3 days. H89 (200μg/100g/bw, Merck, Billerica, MA) was injected intraperitoneally (IP) 1 hour prior to T3-feeding and 2 hours before sacrifice.
Experiment protocol 2
This was essentially similar to Experimental Protocol 1 except that H89 (200μg/100g/bw, LC Lab, Boston, MA) was injected IP every 24 hours for 5 days. The animals received BrdU dissolved in drinking water (1 mg/ml) during the entire experimental period. Livers were paraffin embedded, cryofixed and frozen at −80°C until use.
Experiment protocol 3
Seven weeks old male F-344 rats were given a single IP dose of T3 (20μg/100g/bw), 30 minutes after H89 (200μg/100g/bw, IP, LC lab). Rats were killed after 24 hours of treatment. The animals received BrdU in drinking water (1 mg/ml) during the entire experimental period.
Immunohistochemistry
Liver sections were analyzed by immunohistochemistry for β-catenin (Santa Cruz Biotechnology, Santa Cruz, CA), Cyclin-D1 (Thermo Scientific, Freemont, CA), glutamine synthetase (GS, Santa Cruz Biotechnology), β-galactosidase (Rockland Immunochemicals, Gilbertsville, PA), BrdU (Becton Dickinson, San Jose, CA). Briefly, formalin-fixed sections were deparaffinized. Endogenous peroxide was inactivated using 3% hydrogen peroxide (Sigma, St. Louis, MO). For β-catenin, Cyclin-D1, β-galactosidase and GS staining, slides were microwaved in citrate buffer for 20 minutes followed by blocking in the blue blocker (Shandon Lipshaw, Pittsburgh, PA). Sections were then incubated with secondary anti-mouse horseradish-peroxidase–conjugated antibody (Chemicon, Temecula, CA) for 30 minutes and the signal was detected using the ABC Elite kit (Vector Laboratories, Burlingame, CA), according to the manufacturer's instructions. BrdU was stained with mouse antibody from Becton Dickinson (San Jose, CA), as previously described (18). The labeling index (LI) was calculated as BrdU or cyclin-D1-positive hepatocyte nuclei/100 nuclei. At least 5000 hepatocyte nuclei per liver were scored.
Protein Extraction and Western Blot Analysis
Protein extraction from primary hepatocyte cultures or frozen liver tissue and Western blot analysis were performed as previously described (6, 16). The primary antibodies used were against β-catenin, GSK3β, pSer9GSK3β, Gapdh (Santa Cruz Biotechnology, Santa Cruz, CA), pSer675β-catenin (Cell Signaling Technology, Danvers, MA), Cyclin-D1 (Thermo Scientific) and β-actin (Sigma). Horseradish peroxidase–conjugated secondary antibodies were purchased from Chemicon. The proteins were detected by Super-Signal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and visualized by autoradiography.
Immunoprecipitation
Immunoprecipitation was performed with 500μg of protein extract using β-catenin-conjugated A/G agarose beads (Santa Cruz) as described previously (6, 16). Blots were probed for E-cadherin (BD Transduction Labs) and β-catenin (Santa Cruz). Equal pull down was verified by probing the immunoblot for β-catenin.
Isolation and culture of primary hepatocytes
Hepatocytes were isolated by adaptation of the calcium 2-step collagenase perfusion technique as previously described (19).
β-Catenin/Tcf Transcription Reporter Assay
Hepatocytes were plated on collagen coated six-well plates (Becton Dickinson) at 300,000 cells/well and transfected with 2.5μg of the plasmids TOPFlash (Upstate Biotechnology, Lake Placid, NY) at 80% of confluency. TOPFlash has three copies of the Tcf/Lef sites upstream of a thymidine kinase (TK) promoter and the firefly luciferase gene. All transfections were performed with Lipofectamine2000 as previously described (20). To normalize transfection efficiency, cells were co-transfected with 0.5μg of the internal control reporter Renilla reniformis luciferase driven under the TK promoter (pRL-TK; Promega, Madison, WI). Cells have been treated with or without 100nM of T3 in medium (Sigma). T3 was administered in two doses, once every 24h. Forty-eight hours after transfection the cells were harvested and lysed with Passive lysis buffer (Promega). Luciferase assay was performed using the Dual Luciferase Assay System kit according to the manufacturer's protocols (Promega). Relative luciferase activity was reported as fold induction after normalization for transfection efficiency. Experiments were repeated at least three times with 6 samples per experiment.
Real-time PCR
Total RNA was extracted by homogenizing frozen liver tissue in Trizol® reagent (Invitrogen, Carlsbad, CA). Two microgram of total RNA from each sample was reverse-transcribed after DNase treatment using Super Script III first strand kit (Invitrogen). Real-time PCR was performed on an ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) using specific Taqman Gene Expression assays (Applied Biosystems) for TRα and TRβ, and the relative expression levels were calculated after normalization to 18S. For β-catenin gene expression, Sybr green assay was used (Applied Biosystems) and the values were normalized to Cyclophilin expression.
Statistics
Data are presented as mean ± standard error (S.E.). Comparison between treated and control groups were performed by the ANOVA and Student's t test. P<0.05 (*) was considered significant throughout the study.
Results
T3-fed rats show stabilization of β-catenin and increased expression of its targets in the liver
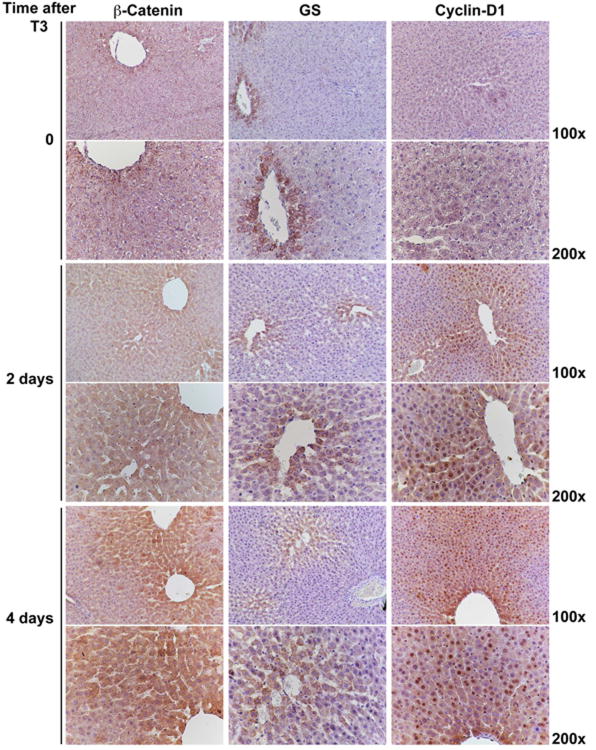
Previous studies have shown T3 to be a powerful liver mitogen in rats (9). To investigate if the Wnt/β-catenin pathway is altered after T3 treatment to then contribute towards hepatocyte proliferation, we performed immunohistochemical studies on liver sections from rats sacrificed 2 and 4 days after feeding T3-diet. Normal untreated control livers, referred henceforth as control livers, show β-catenin staining only at the hepatocyte membrane (Fig.1). T3 treatment led to increased cytoplasmic accumulation of β-catenin at day 2 followed at 4 days by enhanced cytoplasmic and nuclear localization. GS-positivity was limited to hepatocytes in the pericentral area in controls and T3-treated rats after 2 days; however, a modest increase in the number of GS-positive hepatocytes was observed in the perivenular area at 4 days after T3 treatment (Fig. 1). Control livers were negative for cyclin-D1, another target of β-catenin and a cell cycle regulator, but increased specifically after 2 to day 4 days of T3 treatment (Fig. 1). The enhanced Cyclin-D1 expression in response to T3 at 2 days was more in pericentral hepatocytes, which expanded to additional zones at 4 days. Also, this increase in Cyclin-D1 staining was disproportionately greater than that observed for GS. Thus, T3 induces β-catenin activation in the rat liver.
Figure 1. Activation of β-catenin signaling in rat livers after T3-feeding.
Immunostaining shows β-catenin localizing to the hepatocyte membrane in the control livers, while it accumulates in the hepatocyte cytoplasm in T3-fed rats at 2 days. A progressive shift of β-catenin stabilization from zone I towards zone II along with nuclear translocation of β-catenin-positive is observed at day 4 of T3 feeding. A concomitant increase in the number of GS-positive cells around central vein is observed at 4 days after T3. Progressive Cyclin-D1 nuclear staining is evident in zone I at 2 days, and in zone I and II at 4 days of T3 feeding as compared to the control livers. Four rats per group were used for this study.
β-Catenin is an absolute requirement for T3 mitogenic action in mouse liver
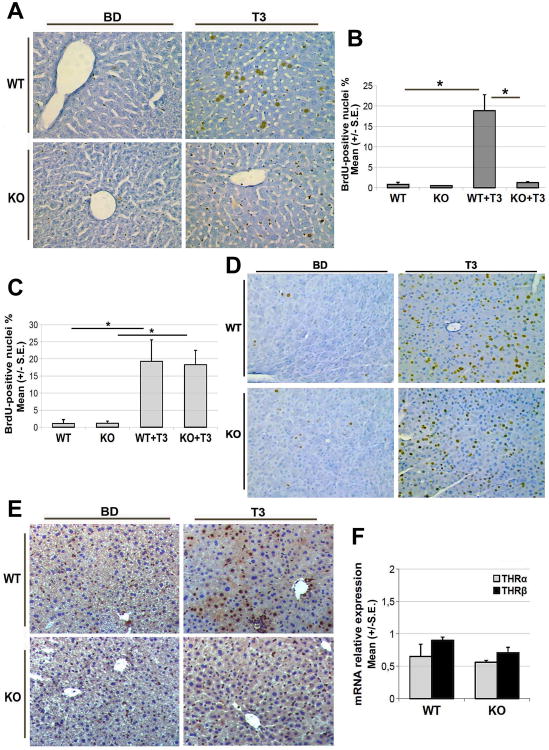
Based on the above findings, we next asked whether β-catenin plays an essential role in T3-induced mitogenesis. To this end we administered T3 to Ctnnb1lox/lox; Alb-Cre+/− or KO mice and wild-type littermate controls (WT). While WT mice fed T3 diet for 1 week exhibited several BrdU-positive hepatocytes and hence a high LI (19.8%), almost no hepatocyte proliferation was evident in KO mice (1.2%) (Fig. 2A, 2B). No significant difference in the LI was observed between untreated WT or KO mice. Non-parenchymal cells however do show BrdU incorporation in the hepatocyte-specific β-catenin KO mice in response to T3. T3 is also mitogenic for the pancreatic acinar cells (21). To eliminate the possibility that the discordant proliferative effects elicited by T3 in WT versus KO mice could be due to differences in T3 metabolism or systemic effects, we next examined the LI of BrdU in the pancreatic acinar cells, which in the same animals do express normal β-catenin. The analysis clearly indicates that the pancreatic acinar cells in the KO and WT mice respond robustly and comparably to T3 (Fig. 2C, 2D).
Figure 2. Lack of β-catenin in hepatocytes impairs hepatocyte proliferation and Cyclin-D1 expression in response to T3 diet.
A. Representative microphotographs showing immunohistochemical staining for BrdU in the WT and KO livers after T3-treatment for 4 days (200×). Several BrdU-positive hepatocyte nuclei are observed in livers of WT mice fed T3 for 4 days. KO after T3 treatment lack BrdU staining in the hepatocytes although BrdU-positive non-parenchymal cells are evident, similar to WT livers. At least three WT and KO were used throughout this study.
B. A significant (*p<0.05) decrease in BrdU LI of hepatocytes in KO versus WT mice after T3 treatment. While T3 stimulated BrdU incorporation in the WT hepatocytes, no significant increase was evident in KO hepatocytes. At least 5000 hepatocyte nuclei per liver were scored. The LI is expressed as number of BrdU-positive hepatocyte nuclei/100 nuclei. Results are expressed as mean ± standard error (S.E.) of 3 or more mice per group.
C. LI of pancreatic acinar cells in WT and KO mice shows no significant difference between the two groups following treatment with T3-supplemented diet (4 mg/kg) for 7 days. T3 stimulated BrdU incorporation in pancreatic acinar cells comparably and significantly (*p<0.05) in WT and KO. At least 2000 acinar cell nuclei per pancreas were scored. LI was expressed as number of BrdU-positive acinar cell nuclei/100 nuclei and results expressed as mean ± standard error (S.E.) of 3 or more mice per group.
D. Representative photomicrographs showing labeling of BrdU in the pancreas of WT and KO mice treated with T3 for 4 days (200×).
E. Representative microphotograph of Cyclin-D1 staining in the WT and KO livers after 4 days of T3-feeding (200×). Absence of Cyclin-D1 immunoreactivity in KO hepatocytes nuclei is clearly evident despite T3 feeding as compared to the WT.
F. qRT-PCR analysis of TRα and TRβ expression in livers of untreated WT and KO mice. 18S was used as endogenous control. Error bars represent the standard error (S.E) of TaqMan RT-PCR performed in triplicates.
As expected, a strong nuclear staining for Cyclin-D1 was observed as early as 4 days after T3 treatment in the hepatocytes in the WT mice; au contraire, almost no cyclin-D1-positive hepatocytes were seen in the KO mice (Fig. 2E). These results suggest that lack of β-catenin impairs Cyclin-D1 expression and hence affects T3-induced hepatocyte proliferation.
Finally, to establish whether the lack of response of KO mouse hepatocytes to T3 could be due to down-regulation of liver thyroid hormone receptors, we performed qRT-PCR. The results show no significant difference in the mRNA levels of either TRα or TRβ between the WT and KO mice (Fig. 2F). This was further validated by examining the previously published microarray analysis of untreated WT and KO livers for thyroid receptor targets (6). No notable differences in the expression of several relevant target genes of thyroid receptor signaling (22) were obvious as shown in Table 1 representing three pooled livers for each group. Thus it is unlikely that that the impaired response of KO to T3 is due to decreased transcriptional activation of thyroid hormone receptors in the absence of β-catenin in the hepatocytes.
T3 treatment induces β-catenin activity both in vitro and in vivo in the liver cells
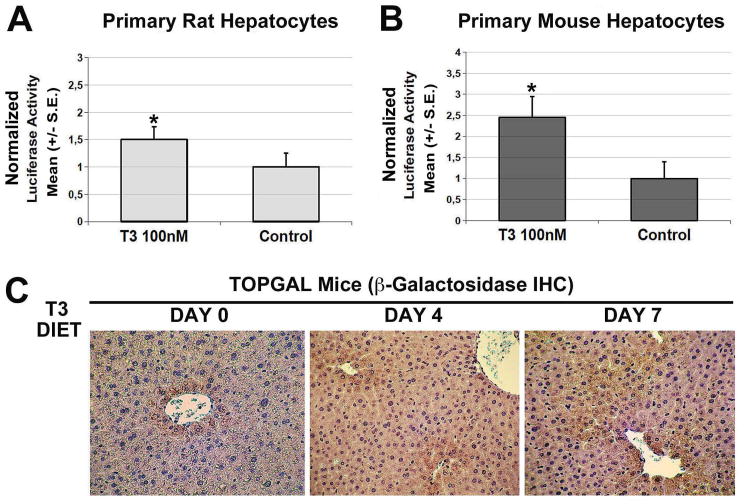
To establish whether T3 could directly induce β-catenin activation, we measured luciferase activity in TOPFlash plasmid-transfected mouse and rat primary hepatocytes as described in the methods. T3 treatment promoted binding of β-catenin to the TCF-binding elements leading to an increase in the luciferase activity in primary hepatocytes in both species suggesting that T3 can indeed activate β-catenin signaling (Fig. 3A, 3B). A control FOPFlash reporter that contains mutated TCF-binding elements showed no luciferase activity in absence or presence of T3 (data not shown).
Figure 3. T3 induces β-catenin activation in vitro and in vivo.
A. TOPflash reporter assay shows an increase in luciferase activity 48 hours after T3 treatment of primary rat hepatocytes. A vector containing renilla luciferase was used as an internal control for transfection efficiency, and results are expressed as relative firefly/renilla luciferase activity. The results presented are the mean ± standard error (S.E.) for three experiments; *p < 0.05.
B. TOPflash reporter assay shows an increase in luciferase activity 48 hours after T3 treatment of primary murine hepatocytes. A vector containing renilla luciferase was used as an internal control for transfection efficiency, and results are expressed as relative firefly/renilla luciferase activity. The results presented are the mean ± standard error (S.E.) for three experiments; *p < 0.05.
C. TOPGAL mice fed T3 diet for 4 and 7 days shows increased β-galactosidase expression by indirect immunohistochemistry. At baseline β-galactosidase expression was detected in pericentral hepatocytes only, whereas T3 feeding led to widening of the expression to several hepatocytes layers around the central vein demonstrating an increase in the activity of β-catenin-TCF complex. A total of three TOPGAL mice were used for this study.
Next, TOPGAL reporter mice that harbor a transgene comprising β-galactosidase gene (LacZ) downstream of TCF binding elements were fed T3-diet for 4 or 7 days. Livers sections from these mice were stained for β-galactosidase by indirect immunostaining. While TOPGAL mice on basal diet showed β-galactosidase expression only in pericentral hepatocyte rim as reported elsewhere (23), T3-feeding led to expansion of β-galactosidase-positive hepatocytes to additional few layers around the central vein, which is suggestive of enhanced in vivo β-catenin activation in response to T3 (Fig. 3C).
Mechanism of β-catenin activation brought about by T3-treatment in vivo
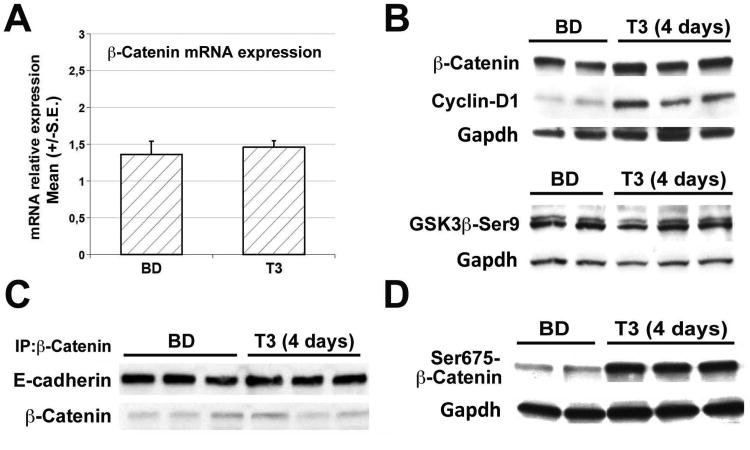
To address the mechanism by which T3 induces β-catenin activation, livers from T3-fed mice for 4 days were assessed. qRT-PCR analysis of β-catenin mRNA levels did not show any difference between T3-treated and untreated mice (Fig. 4A). While Cyclin-D1 protein was consistently increased after 4 days of T3 treatment, no notable changes total β-catenin or GSK3β-Ser9 (inactive form) were observed suggesting that β-catenin activation downstream of T3 may not be via canonical Wnt pathway (Fig. 4B). Yet another pool of β-catenin is at the membrane where it associates to E-cadherin and is part of the adherens junction (24). T3-treatment did not alter E-cadherin-β-catenin association as shown in a representative immunoprecipitation analysis (Fig. 4C). Interestingly, a striking increase in the levels of Ser675-β-catenin was found in the T3-treated mouse livers (Fig. 4D).
Figure 4. T3 induced β-catenin activation via Ser675-phosphorylation.
A. qRT-PCR analysis of β-catenin expression (normalized to cyclophilin A) in C57BL6 mice treated with T3 for 4 days shows no change. Error bars represent the standard error (S.E.) of TaqMan RT-PCR performed in triplicates.
B. Representative western blots show T3 feeding for 4 days does not lead to a notable increase in total β-catenin when compared to basal diet fed mice. However Cyclin-D1 levels are consistently increased. GSK3β-Ser9 levels remain unaffected at 4 days of T3 treatment. Gapdh verifies equal loading. Each lane represents an individual sample.
C. Immunoprecipitation studies from three representative livers show no change in association of β-catenin and E-cadherin in the livers of 4 days T3- versus basal diet-fed C57BL/6 mice.
D. Representative western blot shows a noteworthy increase in pSer675-β-catenin levels in 4 days T3-fed as compared to control diet-fed C57BL/6 mice. Gapdh verified equal loading.
To verify if T3 also induced Ser675-β-catenin phosphorylation in vitro, primary cultures of mouse hepatocytes were treated with T3 for 30 minutes. Analysis of whole cell lysates from these cells also showed a clear increase in Ser675-β-catenin levels (Fig. 5A). Phosphorylation at Ser675 has been identified as a mechanism of β-catenin activation downstream of cyclic AMP-dependent protein kinase A (PKA) (25, 26). Since CREB is a known substrate of PKA (27), we next asked if T3 also induced CREB-phosphorylation. Indeed increased pSer133-CREB was observed by western blot analysis after 30 minutes of T3 treatment (Fig. 5A). Eventually, to directly address if T3-induced serine phosphorylation of β-catenin and CREB were PKA-dependent, primary hepatocytes were treated with T3 in the presence of H89, a small molecule inhibitor of PKA as indicated in the methods (28). H89 prevented any increase in Ser675-β-catenin and pSer133-CREB in primary hepatocytes in response to T3 treatment (Fig. 5A).
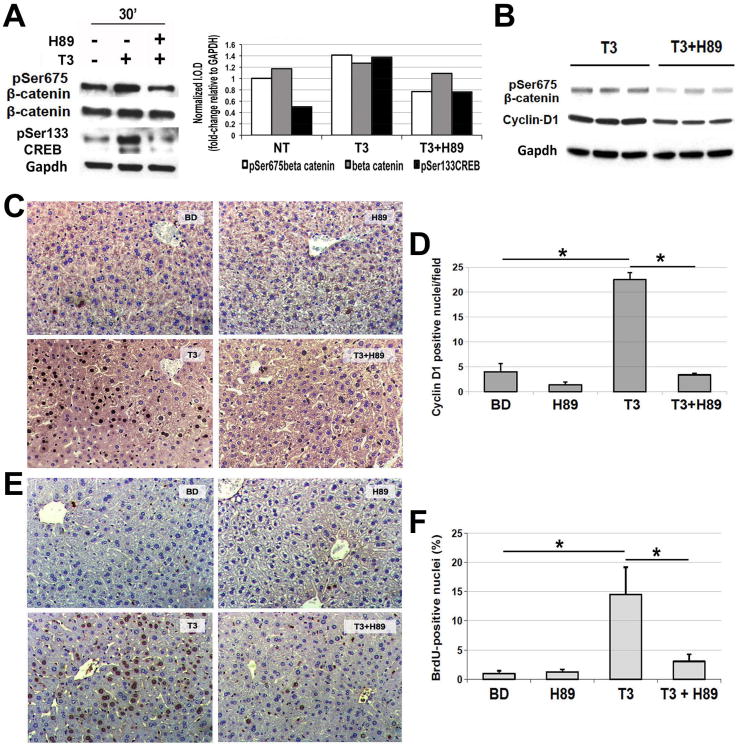
Figure 5. Blockade of Protein Kinase A impairs T3's effect on β-catenin, Cyclin-D1 and hepatocyte proliferation in mice.
A. A representative western blot using pooled samples from three wells per condition (left) shows increased levels of pSer675-β-catenin and pSer133-CREB in primary mouse hepatocytes after 30 minutes of T3 treatment. Inclusion of PKA inhibitor H89 (1 μM) in the media 30 minutes prior to the addition of T3 (100 nM) showed a notable decrease in pSer675-β-catenin and pSer133-CREB levels. Densitometry on the representative WB (right) shows an increase in pSer675-β-catenin and pSer133-CREB after T3 treatment, which was blocked by H89 treatment. (I.O.D. – integrated optical density).
B. A representative western blot shows a decrease in the hepatic levels of pSer675-β-catenin and Cyclin-D1 when H89 was injected twice IP in 3-day T3 fed mice as compared to 3 day T3 only group. Gapdh verifies equal loading. Each lane represents an individual sample.
C. A representative micrograph (200×) illustrates a decrease in the number of Cyclin-D1-positive hepatocytes when H89 was injected twice to the 3-day T3-fed mice as compared to T3 only group. Three or more mice per group were used for this study.
D. Quantification of the Cyclin-D1-positive hepatocytes shows a significant decrease in positive cells in H89+T3 as compared to T3 only group (*p<0.05).
E. A representative micrograph (200×) illustrates a noteworthy increase in BrdU uptake by the hepatocytes in mice after 5 days of T3 feeding, which was dramatically decreased in animals simultaneously administered H89 IP every 24 hours. Three or more mice per group were used for this study.
F. Quantification of BrdU positive hepatocytes shows a significant (*p<0.05) decrease in the LI in T3+H89 group as compared to T3 only.
H89 blocks T3-induced β-catenin activation, Cyclin-D1 expression and hepatocyte proliferation in mice and rats
To determine relevance of PKA in T3-mediated β-catenin activation and hepatocyte proliferation in vivo, mice were fed T3 diet along with H89 administration as discussed in methods (see Experimental protocol 1). Total liver lysates from such treatment show that H89 successfully abrogated T3-mediated increase in Ser675-β-catenin in mice, which was also accompanied by decreased levels of Cyclin-D1 (Fig. 5B). Immunohistochemistry confirmed a significant decrease in the number of cyclin-D1-positive hepatocytes in mice that were administered H89 in addition to T3 (Fig. 5C, 5D). Intriguingly, BrdU incorporation in hepatocytes in mice undergoing H89 and T3 treatment showed a decrease as compared to T3 only fed mice, although it missed statistical significance due to variation in the T3-fed animals (not shown). Since an appreciable mitogenic effect of T3 in mouse liver is achieved between 4-5 days (13), we modified the protocol by administering both T3 and H89 for 5 days (see experimental protocol 2). This modification led to a more uniform BrdU incorporation in control mice and an almost complete abrogation of T3-induced hepatocyte proliferation in T3+H89 group of mice (LI was 4% in animals receiving H89+T3 vs. 16% in mice treated only with T3) (Fig. 5E, 5F).
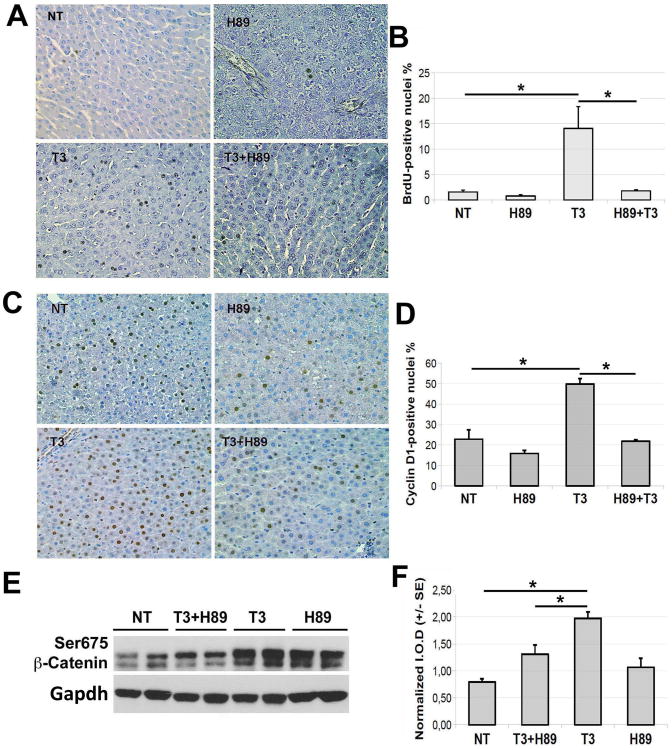
Since T3 also induced β-catenin activation, Cyclin-D1 expression and hepatocyte proliferation in ratlivers as shown in Fig. 1, we investigated if this effect was via PKA. It is known that, in rats, a single dose of T3 induces a peak of hepatocyte proliferation 24 hours after treatment (15). Therefore, in this study we administered the PKA inhibitor 30 minutes prior to T3 treatment and sacrificed the animals 24 hours thereafter (Experimental Protocol 3). PKA inhibition almost completely prevented BrdU incorporation (LI of H89+T3 was 1.8% vs. 14.0% of the T3 group) (Fig. 6A, 6B). In concordance, Cyclin-D1 nuclear expression was also significantly reduced in this group of animals (Fig. 6C, 6D). To address the efficacy of PKA inhibition in decreasing β-catenin activity brought about by T3, Ser675-β-catenin levels were assessed. Indeed, PKA blockade before T3 administration led to a significant decrease in Ser675-β-catenin levels as assessed at 90 minutes after H89 or 60 minutes after T3 treatment (Fig. 6E, 6F).
Figure 6. Blockade of Protein Kinase A impairs T3's effect on β-catenin, Cyclin-D1 and hepatocyte proliferation in rats.
A. Representative microphotographs (200×) illustrate the effect of H89 on T3-induced rat hepatocyte proliferation by BrdU immunohistochemistry. H89 was given 1 hour prior to a single dose of T3 (20 μg/100 g) and the rats were sacrificed 24 hours later. Minimum four rats per group were used for this entire study.
B. Quantification of BrdU positive hepatocytes in A shows a significant (*p<0.05) increase in the LI after T3 treatment, which was significantly abrogated in the presence of H89 (*p<0.05).
C. Representative microphotographs (200×) show increased nuclear Cyclin-D1 expression in hepatocyte following a single injection of T3, which was decreased in the group that simultaneously received H89 as well.
D. Quantification of Cyclin-D1-positive hepatocytes to calculate LI shows a significant increase after T3 treatment (*p<0.05), which was reduced significantly in the H89 pretreatment group (*p<0.05).
E. A representative western blot shows the effect of H89 on T3-induced Ser675-β-catenin levels in rat liver. Gapdh depicts protein loading.
F. Densitometric analysis of E (Ser675-β-catenin normalized to Gapdh) using the ImageJ software shows a significant (*p<0.05) decrease in Ser675-β-catenin levels in T3+H89 as compared to T3 only group. (I.O.D. – integrated optical density).
Discussion
The unique capability of the liver to regenerate is known since 1930s (29). Vast body of literature has led to an improved understanding of the cellular and molecular basis of liver regeneration and it has become clear now that tremendous redundancy exists in these processes that enable successful initiation and execution of the liver regeneration process (30). This is highly relevant since liver is located strategically to perform key functions indispensable to survival but at the same time is the portal of entry to toxins and other harmful molecules through the portal circulation. Due to its critical function in synthesis, metabolism and detoxification, and its rather vulnerable location, liver is bestowed with a capacity to regenerate to maintain hepatic health and homeostasis on a day-to-day basis. An overwhelming insult or any mechanism that may impair the liver regeneration process due to acute or chronic injury, may lead to the end stage liver disease (ESLD). The majority of these patients with ESLD may require a liver transplant, which despite being a major advance still faces issues of donor organ scarcity and associated morbidity. Indeed major efforts are underway to discover not only improved transplantation technologies, but also research is underway to assess alternate strategies such as cell therapy, stem cell differentiation and transplantation, tissue engineering and regenerative therapies. Thus it is of significance to discern the signaling pathways naturally activated to enhance liver regeneration, which may be further stimulated through use of naturally occurring or synthetic agents as basis for regenerative therapies (31).
Role of the Wnt/β-catenin signaling in liver regeneration has now been identified in multiple species ranging from zebrafish to patients and in various models ranging from PH to toxicant-induced liver injury (5, 6, 32-35). β-Catenin's stabilization and nuclear translocation is an early event in rat and mouse liver regeneration (17, 34, 36). Acetaminophen-induced liver regeneration also leads to β-catenin stabilization and absence of β-catenin impaired regenerative response to an equitoxic injury in the KO mice (32). The major basis of β-catenin's role in liver regeneration is its ability to induce expression of cyclin-D1, a major G1 to S phase regulator (6, 17). Indeed loss of β-catenin in hepatocytes led to delay in liver regeneration due to decreased Cyclin-D1 levels (5, 6). Conversely, transgenic expression of N-truncated β-catenin results in increased proliferation and hepatomegaly, while mice expressing point mutant of β-catenin display accelerated regeneration after PH (16, 37). Also Wnt-1 gene therapy promoted liver regeneration after PH due to enhanced Cyclin-D1 expression (16). Thus it appears highly relevant to find a suitable modality to stimulate the Wnt signaling pathway as a means of regenerative therapy for the liver.
In the current study, we followed up on the observation where T3-induced hepatocyte proliferation also depended solely on the induction of Cyclin-D1 expression (15). T3's ability to stimulate hepatocyte proliferation was in fact reported in two models of impaired liver regeneration (9). T3 stimulated Cyclin-D1 expression and liver regeneration in old rats subjected to PH. It also improved BrdU LI via increased Cyclin-D1 expression in rats subjected to 90% hepatectomy. However, how T3 stimulated Cyclin-D1 expression has remained an enigma and the current study demonstrates an imperative role of β-catenin in this process.
In the current study, we report that the absence of β-catenin in hepatocytes impairs the ability of T3 to induce Cyclin-D1 expression and hence T3 was unable to stimulate hepatocyte proliferation in the KO mice. Further studies demonstrate that T3 was able to cause direct activation of the β-catenin signaling. However, this effect was not through the canonical Wnt signaling pathway or through disruption of adherens junctions, which is yet another independent pool of β-catenin in a cell (24). Since T3 is known to have both genomic and non-genomic effects (38), we interrogated if T3 could impact gene expression of CTNNB1. The genomic effects of T3 are mediated by recruitment of thyroid hormone receptor-associated protein (TRAP)/Mediator (Med) complex, histone acetyl transferases such as p300 and p160/steroid receptor coactivator to the promoters of various genes (39). Notably, recent studies have shown that intestinal epithelial cell proliferation is controlled by thyroid hormones, and that TRα directly controls transcription of the β-catenin gene in these cells, suggesting a direct correlation between T3, β-catenin and a positive regulation of proliferation-controlling genes, such as type D cyclins (40). However, no difference in the mRNA expression of β-catenin in the liver was observed secondary to T3-treatment, in vivo or in vitro. It should be mentioned that hepatocytes are known to express higher levels of TRβ and selective agonists for this receptor subtype are in clinical trials for their ability to decrease blood lipids through reverse cholesterol transport in hepatocytes (13, 41). Intriguingly, β-catenin phosphorylation at Ser675 site was greatly induced by T3. This site has been shown to cause β-catenin activation through second messenger cAMP-mediated PKA activation (25, 26). Indeed one of the major non-genomic effects of thyroid hormone is via cAMP-dependent protein kinase activation (42). Thus it appears that T3 activates PKA to in turn induce β-catenin activation. These findings were strengthened by inhibition of PKA through application of a small molecule H89, which abrogated T3-induced PKA activity leading to an impairment of CREB and β-catenin's phosphorylation both in vitro and in vivo(27, 43). Simultaneously, H89 administration to T3 fed mice or rats blocked Cyclin-D1 expression and resulted in dramatically lowering hepatocyte proliferation. It was interesting to note that T3 treatment led to a discordant β-catenin activation, since its two downstream targets were not comparably induced. While Cyclin-D1 was notably enhanced, GS was only modestly increased. The molecular basis of this observation remains obscure and may be due to the co-factor function of β-catenin, which may be binding to a set of specific transcription factors and other regulatory proteins upon specific signaling. Indeed, other studies have demonstrated such partial and discordant activation, for example in the context of Lgr5-positive stem cell activation in the liver (44).
Thus T3 is a plausible and practical modality to induce β-catenin signaling as a potential regenerative therapy. Additionally, selective TRβ agonists may possess a similar property without any untoward side effects associated with TRα activation predominantly in the heart (10, 13, 41). This is of even greater relevance since TRβ-agonists induced regression of preneoplastic lesions in rodent livers (10). Regenerative therapies depend on stimulation of surviving hepatocytes in a liver after acute or chronic insult and can be envisioned to be of benefit in live donor setting, small for size syndrome, and even toxicant induced liver injury such as acetaminophen overdose (31). Indeed, in another study from our lab, β-catenin-positive hepatocytes that existed in KO mice due to leaky albumin-cre, showed growth and survival advantage in the face of chronic liver injury brought about by administration of 0.1% 3,5-diethoxycarbonyl-1,4-dihydro-collidin diet (45). These β-catenin-positive hepatocytes eventually repopulated the adult KO liver to maintain hepatic function and survival. Thus, stimulation of β-catenin signaling through T3 or other analogues may be of translational relevance in the setting of liver transplantation, cell therapy and in artificial liver devices and hepatic tissue engineering (2).
Acknowledgments
Funding support: This study was funded by NIH grants 1R01DK62277 and Endowed Chair for Experimental Pathology to SPSM; by Associazione Italiana Ricerca sul Cancro (IG-11821), Ministero Università e Ricerca Scientifica (PRIN 2010LC747T), and Regione Autonoma Sardegna (RAS 2012) to AC.
Footnotes
Conflict of interest: SPSM is a consultant for PhaseRx and Merck Pharmaceuticals.
References
- 1.Sylvie J, Ellen C, Kris V. The role of Wnt in cell signaling and cell adhesion during early vertebrate development. Front Biosci. 2011;16:2352–2366. doi: 10.2741/3858. [DOI] [PubMed] [Google Scholar]
- 2.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lade AG, Monga SP. Beta-catenin signaling in hepatic development and progenitors: which way does the WNT blow? Dev Dyn. 2011;240:486–500. doi: 10.1002/dvdy.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monga SP, Monga HK, Tan X, Mule K, Pediaditakis P, Michalopoulos GK. Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology. 2003;124:202–216. doi: 10.1053/gast.2003.50000. [DOI] [PubMed] [Google Scholar]
- 5.Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- 6.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Columbano A, Shinozuka H. Liver regeneration versus direct hyperplasia. FASEB J. 1996;10:1118–1128. doi: 10.1096/fasebj.10.10.8751714. [DOI] [PubMed] [Google Scholar]
- 8.Columbano A, Ledda-Columbano GM. Mitogenesis by ligands of nuclear receptors: an attractive model for the study of the molecular mechanisms implicated in liver growth. Cell Death Differ. 2003;10(1):S19–21. doi: 10.1038/sj.cdd.4401113. [DOI] [PubMed] [Google Scholar]
- 9.Columbano A, Simbula M, Pibiri M, Perra A, Deidda M, Locker J, Pisanu A, et al. Triiodothyronine stimulates hepatocyte proliferation in two models of impaired liver regeneration. Cell Prolif. 2008;41:521–531. doi: 10.1111/j.1365-2184.2008.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perra A, Kowalik MA, Pibiri M, Ledda-Columbano GM, Columbano A. Thyroid hormone receptor ligands induce regression of rat preneoplastic liver lesions causing their reversion to a differentiated phenotype. Hepatology. 2009;49:1287–1296. doi: 10.1002/hep.22750. [DOI] [PubMed] [Google Scholar]
- 11.Boelen A, Kwakkel J, Fliers E. Thyroid hormone receptors in health and disease. Minerva Endocrinol. 2012;37:291–304. [PubMed] [Google Scholar]
- 12.Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122:3035–3043. doi: 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowalik MA, Perra A, Pibiri M, Cocco MT, Samarut J, Plateroti M, Ledda-Columbano GM, et al. TRbeta is the critical thyroid hormone receptor isoform in T3-induced proliferation of hepatocytes and pancreatic acinar cells. J Hepatol. 2010;53:686–692. doi: 10.1016/j.jhep.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Leoni VP, Ledda-Columbano GM, Pibiri M, Saliba C, Perra A, Kowalik MA, Grober OM, et al. Expression of c-jun is not mandatory for mouse hepatocyte proliferation induced by two nuclear receptor ligands: TCPOBOP and T3. J Hepatol. 2011;55:1069–1078. doi: 10.1016/j.jhep.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Pibiri M, Ledda-Columbano GM, Cossu C, Simbula G, Menegazzi M, Shinozuka H, Columbano A. Cyclin D1 is an early target in hepatocyte proliferation induced by thyroid hormone (T3) FASEB J. 2001;15:1006–1013. doi: 10.1096/fj.00-0416com. [DOI] [PubMed] [Google Scholar]
- 16.Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Jr, Dar MJ, Khillan J, Dai C, et al. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–1613. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torre C, Benhamouche S, Mitchell C, Godard C, Veber P, Letourneur F, Cagnard N, et al. The transforming growth factor-alpha and cyclin D1 genes are direct targets of beta-catenin signaling in hepatocyte proliferation. J Hepatol. 2011;55:86–95. doi: 10.1016/j.jhep.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Ledda-Columbano GM, Curto M, Piga R, Zedda AI, Menegazzi M, Sartori C, Shinozuka H, et al. In vivo hepatocyte proliferation is inducible through a TNF and IL-6-independent pathway. Oncogene. 1998;17:1039–1044. doi: 10.1038/sj.onc.1202018. [DOI] [PubMed] [Google Scholar]
- 19.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardmo C, Kotokorpi P, Helander H, Mode A. Transfection of adult primary rat hepatocytes in culture. Biochem Pharmacol. 2005;69:1805–1813. doi: 10.1016/j.bcp.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Ledda-Columbano GM, Perra A, Pibiri M, Molotzu F, Columbano A. Induction of pancreatic acinar cell proliferation by thyroid hormone. J Endocrinol. 2005;185:393–399. doi: 10.1677/joe.1.06110. [DOI] [PubMed] [Google Scholar]
- 22.Dong H, Yauk CL, Williams A, Lee A, Douglas GR, Wade MG. Hepatic gene expression changes in hypothyroid juvenile mice: characterization of a novel negative thyroid-responsive element. Endocrinology. 2007;148:3932–3940. doi: 10.1210/en.2007-0452. [DOI] [PubMed] [Google Scholar]
- 23.Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25:9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taurin S, Sandbo N, Yau DM, Sethakorn N, Dulin NO. Phosphorylation of beta-catenin by PKA promotes ATP-induced proliferation of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2008;294:C1169–1174. doi: 10.1152/ajpcell.00096.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Servillo G, Della Fazia MA, Sassone-Corsi P. Coupling cAMP signaling to transcription in the liver: pivotal role of CREB and CREM. Exp Cell Res. 2002;275:143–154. doi: 10.1006/excr.2002.5491. [DOI] [PubMed] [Google Scholar]
- 28.Geilen CC, Wieprecht M, Wieder T, Reutter W. A selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-bromocinnamyl(amino)ethyl]-5-isoquinolinesulfonamide (H-89), inhibits phosphatidylcholine biosynthesis in HeLa cells. FEBS Lett. 1992;309:381–384. doi: 10.1016/0014-5793(92)80811-t. [DOI] [PubMed] [Google Scholar]
- 29.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 30.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karp SJ. Clinical implications of advances in the basic science of liver repair and regeneration. Am J Transplant. 2009;9:1973–1980. doi: 10.1111/j.1600-6143.2009.02731.x. [DOI] [PubMed] [Google Scholar]
- 32.Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175:1056–1065. doi: 10.2353/ajpath.2009.080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- 34.Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodhi D, Micsenyi A, Bowen WC, Monga DK, Talavera JC, Monga SP. Morpholino oligonucleotide-triggered beta-catenin knockdown compromises normal liver regeneration. J Hepatol. 2005;43:132–141. doi: 10.1016/j.jhep.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura I, Fernandez-Barrena MG, Ortiz-Ruiz MC, Almada LL, Hu C, Elsawa SF, Mills LD, et al. Activation of the Transcription Factor GLI1 by WNT Signaling Underlies the Role of SULFATASE 2 as a Regulator of Tissue Regeneration. J Biol Chem. 2013;288:21389–21398. doi: 10.1074/jbc.M112.443440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cadoret A, Ovejero C, Saadi-Kheddouci S, Souil E, Fabre M, Romagnolo B, Kahn A, et al. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–3249. [PubMed] [Google Scholar]
- 38.Davis PJ, Davis FB. Nongenomic actions of thyroid hormone. Thyroid. 1996;6:497–504. doi: 10.1089/thy.1996.6.497. [DOI] [PubMed] [Google Scholar]
- 39.Sharma D, Fondell JD. Ordered recruitment of histone acetyltransferases and the TRAP/Mediator complex to thyroid hormone-responsive promoters in vivo. Proc Natl Acad Sci U S A. 2002;99:7934–7939. doi: 10.1073/pnas.122004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plateroti M, Kress E, Mori JI, Samarut J. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol Cell Biol. 2006;26:3204–3214. doi: 10.1128/MCB.26.8.3204-3214.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perra A, Simbula G, Simbula M, Pibiri M, Kowalik MA, Sulas P, Cocco MT, et al. Thyroid hormone (T3) and TRbeta agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 2008;22:2981–2989. doi: 10.1096/fj.08-108464. [DOI] [PubMed] [Google Scholar]
- 42.D'Arezzo S, Incerpi S, Davis FB, Acconcia F, Marino M, Farias RN, Davis PJ. Rapid nongenomic effects of 3,5,3′-triiodo-L-thyronine on the intracellular pH of L-6 myoblasts are mediated by intracellular calcium mobilization and kinase pathways. Endocrinology. 2004;145:5694–5703. doi: 10.1210/en.2004-0890. [DOI] [PubMed] [Google Scholar]
- 43.Wieprecht M, Wieder T, Geilen CC. N-[2-bromocinnamyl(amino)ethyl]-5-isoquinolinesulphonamide (H-89) inhibits incorporation of choline into phosphatidylcholine via inhibition of choline kinase and has no effect on the phosphorylation of CTP:phosphocholine cytidylyltransferase. Biochem J. 1994;297(Pt 1):241–247. doi: 10.1042/bj2970241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson MD, Wickline ED, Bowen WB, Lu A, Singh S, Misse A, Monga SP. Spontaneous repopulation of beta-catenin null livers with beta-catenin-positive hepatocytes after chronic murine liver injury. Hepatology. 2011;54:1333–1343. doi: 10.1002/hep.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]