Abstract
Purpose.
It has been suggested that ring-like patterns of macular pigment, as measured with dual wavelength autofluorescence, are observed less frequently in subjects with age-related maculopathy. We explored relative contributions of genetic and environmental factors in macular pigment optical density (MPOD) distributions using a classic twin study.
Methods.
As part of a previous nutritional study, 322 healthy Caucasian female twins, aged 16 to 50 (mean 40) years, underwent measurement of MPOD optical density by two-wavelength fundus autofluorescence. In the present study, the right eye MPOD profile was assessed for the presence of a ring-like pattern by two graders independently, using common criteria, with a third grader arbitrating in cases of disagreement. Concordance was calculated as 2C/(2C + D), where C is the number of twin pairs concordant, and D the number discordant, for the ring-like pattern. Also, heritability was calculated using maximum-likelihood structural equation modeling.
Results.
Images and zygosity data were available for 314 twins (88 monozygotic [MZ] and 69 dizygotic [DZ] pairs). The overall prevalence of the ring pattern was 25.8%. Respective concordances for MZ and DZ twins were 0.75 and 0.22. Additive genetic factors were estimated to contribute to 84.0% of the total variance (95% confidence intervals, 63.7%–94.6%).
Conclusions.
Concordance for MZ twins was over three times that for DZ twins, with heritability estimated at 84%, indicating that genetic factors contribute to the development of the ring structure. Studies have suggested that ring-like patterns of macular pigment can affect risk for age-related maculopathy. In a classic twin study, we found that the presence of such a pattern was highly heritable.
Keywords: macular pigment, heritability, lutein
Introduction
The concentration of macular pigment is highest at the foveal center, and declines with increasing eccentricity, although interindividual variation has been shown.1 A number of studies, using the technique of fundus autofluorescence, have evaluated the presence, in some individuals, of a ring-like pattern of macular pigment.2–5 While in most individuals, macular pigment appears to decline in density monotonically as a function of eccentricity, some individuals show a second peak in the distribution at 0.5° to 1.0° eccentricity corresponding to an annulus or ring of high pigment density at this position. It has been suggested that this ring may follow the inner plexiform layer2 although its precise anatomical correlate is unknown.
The yellow macular pigment may improve visual function (its density is associated with improved photostress recovery and chromatic contrast, and reduced glare disability6), and, by absorbing blue wavelengths that cause oxidative stress, could play a role in protection against AMD.7–15 As well as the overall amount of macular pigment, its distribution may also be pertinent. A recent study showed a lower prevalence of the ring-like pattern in individuals with age-related maculopathy,3 suggesting a potential protective effect of this configuration. However, causality cannot be established from cross-sectional studies (e.g., AMD might cause the ring-like structure to be absent), and other studies have reported such atypical profiles to be associated with age and smoking,16 both risk factors for AMD.
As much current research focuses on the effects of modulating environmental factors, such as diet on the development or progression of retinal disease, it is of considerable interest to explore the relative contributions of genetic and environmental factors to the presence or absence of the ring-like pattern. This was the purpose of the present study, in which macular pigment measurements from over 300 twins were re-analyzed and the heritability of the ring-like structure estimated. The measurements were originally made as part of a nutritional supplement study,17 and so a secondary aim of the present study was to explore whether or not supplemental lutein and zeaxanthin altered the pattern of macular pigment in the retina.
Methods
As part of the previous nutritional supplement study, which has been described in detail,17 322 healthy, female, twin volunteers (aged 16–50, mean age 39 ± 8.7 years) were recruited from the TwinsUK registry based at St. Thomas' Hospital in London. Participants were asked to take a daily macular pigment supplement for 6 months, and underwent review at baseline, 3 and 6 months. Supplements consisted of three tablets per day of “Macuvite” (Springfield, Oud-Beijerland, The Netherlands), containing 18 mg lutein and 2.4 mg zeaxanthin. The study had local research ethics committee (St. Thomas' Hospital Research Ethics Committee) approval and was conducted in accordance with the tenets of the Declaration of Helsinki.
Macular Pigment Measurements
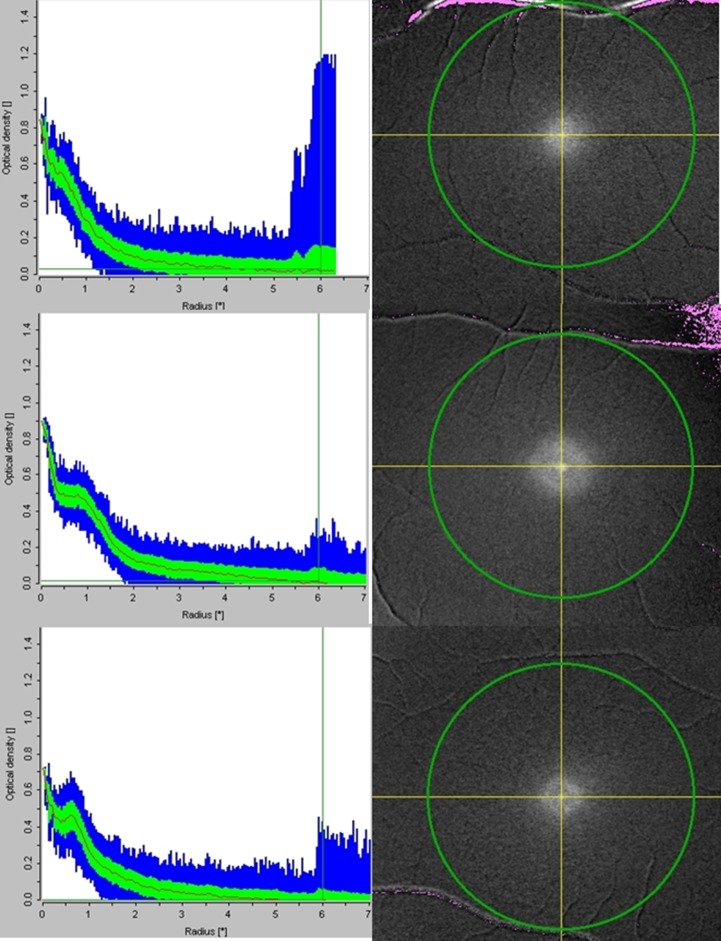
During each visit, participants underwent two-wavelength autofluorescence imaging of both eyes, following pharmacologic mydriasis, using a modified confocal scanning laser ophthalmoscope (Heidelberg Engineering, Heidelberg, Germany), which provided high resolution 20° images at 488- and 514-nm wavelengths. Members of each twin pair were imaged on the same day. The instrument's gain setting was fixed throughout the study, and the equipment was allowed to warm up for 1 hour prior to measurements. Digital subtraction of images obtained at the two wavelengths yielded a grayscale map, the intensity of which was proportional to the macular pigment optical density (MPOD) at each retinal location.18 The instrument's software was also able to generate a plot of MPOD against eccentricity, averaging MPOD measurements at each eccentricity (Fig. 1).
Figure 1.
Representative macular pigment profiles for no-ring (top), plateau (middle), and ring-like (bottom) distributions. Right panels show digital subtraction images with intensity corresponding to MPOD. Left panels show corresponding MPOD measurements plotted against eccentricity. The line plots the mean MPOD at each eccentricity; the green and blue areas show SD and extreme values, respectively.
Image Analysis
The images acquired during the previous study were re-analyzed for the present study as follows. The MPOD profile (MPOD plotted against eccentricity) was generated as above with the foveal center (zero eccentricity) manually defined as the point giving the highest MPOD measurement. If MPOD declined roughly monotonically with eccentricity, this was classified as a “no-ring” profile (N). If there were a definite secondary peak in the profile at approximately 0.5° to 1.0° eccentricity, this was classified as a “ring-like” profile (R). In some cases, the profile flattened, but did not show a secondary peak, before declining again, and these were classified initially as no-ring profiles. In a secondary analysis, these were classified separately as “plateau” profiles (P), to explore whether this had any effect on the heritability estimate. Examples are shown in Figure 1.
For heritability and concordance calculations the baseline visit was used (this had the greatest number of participants as not all attended for all three visits), and the MPOD profile of the right eye was assessed independently by two graders (AT and OAM) according to the above criteria. In cases where the two graders differed, a third grader (CJH) assessed the image, acting as arbitrator to provide the final grade. Kappa statistics were calculated to assess agreement between the two initial graders. Additionally, one grader (AT) also assessed all of the other profiles (both eyes, all available visits), so that further comparisons could be made, both between eyes and between visits (to explore whether supplementation had any discernible effect on the prevalence of the ring-like pattern).
When analyzing profiles, graders were blinded to the zygosity and also to the grade for the other member of the twin pair. This was achieved by analyzing profiles with the dataset ordered alphabetically by first name (rather than by last name or by study identification number, both of which were similar for twin pairs); thus, profiles of members of a twin pair were not analyzed consecutively.
Calculating Concordance
Case-wise concordance for presence of the ring-like structure was calculated as
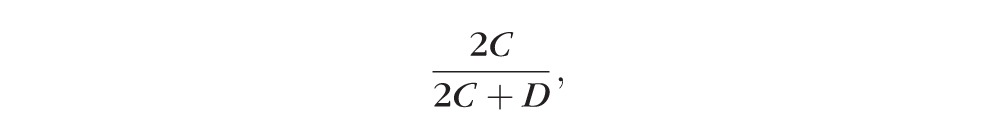 |
where C is the number of twin pairs concordant, and D the number discordant, for the ring-like pattern. The calculation was done for monozygotic (MZ) and for dizygotic (DZ) twin pairs.
Calculating Heritability
Heritability calculations were performed with maximum likelihood structural equation twin modeling, using the OpenMx package (in the public domain, http://openmx.psyc.virginia.edu) in R (in the public domain, http://www.r-project.org). In this technique the variance of a trait is estimated by the contributions of three factors: the additive genetic component (A), the shared environment (C) or the nonadditive genetic component (D), and the unique environment (E). Threshold liability ACE/ADE models were executed with standardized path coefficients and expected variance and covariance matrices. The goodness of fit of the full and reduced ACE/ADE models were compared with the observed data. Heritability was calculated as the proportion of total variance of the trait (V) due to the additive genetic effect (A) in the best fitting model. In the first instance, the binary grading of ring or no-ring was used. Subsequently, the plateau profiles were separated from the no-ring group as a third grade, to explore any effect on the estimate of contributions of additive genetic factors.
Results
Zygosity status was not known (one twin pair) or data were not available for one or both members of the twin pair (three pairs), therefore 8 of 322 twin participants were excluded from analysis. Of the 314 remaining twins (157 pairs), 88 pairs were MZ and 69 pairs DZ. Results of grading for right eyes were as follows: 81 individuals (25.8%) had ring-like structures, 47 individuals (15.0%) had plateau-like pigment profiles, and 186 individuals (59.2%) had neither.
Concordances and Heritability
Casewise concordances for presence of the ring-like structure were 0.75 for MZ twins and 0.22 for DZ twins. Model-fitting analyses identified the best-fit model was the AE model for both grading methods of the data. Estimated contribution of additive genetic factors (heritability) was 84.0% (95% confidence intervals [CIs], 63.7%–94.6%) when the binary grades of ring and no-ring were used. Taking into account plateau profiles as a separate grade altered the estimate only slightly to 83.7% (95% CI, 69.4%–92.0%).
Intergrader Agreement
Kappa measures of agreement between the two initial graders were 0.705 (95% CI, 0.617–0.793) for the binary classification of ring versus no-ring, and 0.685 (weighted kappa) when plateau profiles were classified as a separate intermediate grade. Both indicate a good level of agreement.
Intereye Agreement
In total, 316 individuals had grades available for both eyes (by one grader) at the first visit. Of these, 87% showed agreement between the two eyes: both eyes had ring-like structures in 57 participants (18%), and both eyes were graded as no-ring in 218 participants (69%); the remaining 41 (13%) were graded as ring-like in one eye, and no-ring in the other.
Changes in Proportion With Ring-Like Structure Over the Course of Supplementation
Macular pigment optical density images were available for all visits for 126 twin pairs (252 individuals), and were graded by one grader. The proportion with a ring-like structure was 25.8% at baseline and 31.0% at 6 months for right eyes, and 21.8% and 27.4% for left eyes (P = 0.20 for right eyes and 0.15 for left eyes). Macular pigment optical density profiles for twins who appeared to change grades between visits were re-analyzed by both graders. In all cases, the changes appeared to be marginal: on one visit, the twin may have had a plateau profile, and on another visit a ring (Fig. 2), but if the designated position of the foveal center was altered slightly the grading result would be altered. There were no cases found where an unequivocal monotonic decline in MPOD with eccentricity changed into a ring-like profile.
Figure 2.
Macular pigment optical density images and profiles for a subject who apparently went from a plateau distribution to a ring-like distribution on a subsequent visit. It was found that by altering the position of the foveal center on the digital subtraction images (right) the profiles could alternate between ring-like and plateau distributions for the same visit, indicating a level of variability or subjectivity in the measurements.
Discussion
The present study investigated heritability of ring-like patterns of macular pigment in a classic twin study. Concordance in MZ twins was more than three times that found in DZ twins, and additive genetic factors were estimated to account for 84% of the overall variance in pigment distributions, suggesting that the phenotype is highly heritable.
Some potential limitations in our study deserve mention. Measurement noise could affect grading of distributions as could the subjectivity of individual graders. Also, it has been shown that other factors, such as cataract, can affect MPOD measurements,19 although whether these factors would affect the observed distribution of pigment is not clear. We strove to minimize errors related to subjectivity by using two independent graders (who were blinded to twin zygosity and who did not grade members of a twin pair consecutively). We found good agreement between graders. Also, given the relatively young age of our cohort, we would expect the prevalence of significant lens opacity to be low. Such measurement or grading errors (unless they systematically increased correlation in MZ more than DZ twins) would be expected to lower the estimate of heritability. On the other hand, the classic twin study can also yield a higher estimate of heritabity than other family study designs, so it could be argued that our estimate may represent an upper limit.
The proportion of individuals with the ring-like structure in our all-female population was 25.8% (95% CI, 21.0%–30.6%), which is very similar to the proportion of 25.1% reported for females in the Muenster Aging and Retinal Study (MARS).4 That study found that females had a significantly higher prevalence than males: overall, the proportion was 19.8%. We also found a moderate to good level of agreement between the two eyes as found in previous studies. Other studies have reported higher proportions of around 50%2 or even 68% (in a white, non-Hispanic population) and 86% (in African subjects).20 It is possible that differences in measurement techniques and classification criteria may underlie these widely different estimates; for example, it may be that profiles that would have been classified as plateau in the present study were graded as ring-like in other studies. For consistency, we attempted to adhere to the classification described in the MARS study4 (with our plateau profile corresponding broadly to their “intermediate” classification), particularly as this was the study showing a lower prevalence of ring-like structure in AMD. This may partly explain the close similarity in prevalence estimates. Our classification method appeared to be reproducible as evidenced by good measures of agreement between the two initial graders. Also, it is likely, as has been shown previously, that different ethnic populations will yield different findings.20 The cohort in the present study were all twins of European origin, relatively young (mean age 40 years), and all female, which might limit the generalizability of the findings to other populations, although we believe twins to be broadly representative of the general population.21
In a recent study, Hogg et al.22 also explored heritability of spatial pigment distributions by analyzing images from 43 twin pairs. Interestingly, their measure of spatial distribution did not appear to show significant heritability. However, there were some important differences between that study and ours that may account for the apparently conflicting finding. They quantified spatial distribution by fitting a best fit curve to the horizontal macular pigment density profile, and taking the width at half peak as a measure of “relative spatial distribution of macular pigment,” rather than classifying profiles as ring-like or not. It could be that the presence or absence of a ring-like profile would not affect this measure in a consistent way, although findings from one study suggest that the presence of a ring does increase the so-called “half width” (eccentricity where half the peak MPOD is reached).4 Also, their sample size was less than one-third of the present study and comprised an older cohort. Their study also only took into account distribution over the horizontal meridian, while the present study looked at the overall profile with eccentricity in all directions from the foveal center. In addition, pigment density was quantified from single wavelength autofluorescence images, while the present study used two-wavelength autofluorescence, which has been shown to be more precise and accurate.23 Greater measurement error is likely to reduce the size of a heritability estimate, acting effectively as an additional environmental factor that will contribute to the measured variance.
Whether supplementation might affect macular pigment profile patterns, and risk of AMD, is an important question. Although there appeared to be a numeric increase in the proportion of ring-like structures over the course of supplementation, this was not statistically significant; careful post hoc analysis of the actual images suggested some potential subjectivity in grading, particularly in those twins appearing to change grade over the course of supplementation. A sample size approximately twice as big would be required for sufficient power to detect whether the change in proportion from 26% to 31% was real. Also a longer study would help determine whether the possible trend identified reflects a real effect; in the present study, participants only took supplements for 6 months, and the rise in MPOD was small (approximately 5%).17 Our present findings also concur with those of Zeimer et al.5 In an analysis of images from 97 participants who had taken supplements for 6 months, they found no examples of ring-like structures appearing de novo or disappearing following supplementation.
The main novel conclusion of the present study is the high heritability of the ring-like distribution, indicating an important genetic contribution. Future studies could explore what these genetic associations might be and whether any of these affect risk of AMD. Such investigations would improve our understanding of AMD pathogenesis, and potentially our ability to prevent its development or progression.
Acknowledgments
The authors thank Joel Kosmin for valuable help with archiving the autofluorescence images.
Supported by grants from Wellcome Trust (CJH), Fight for Sight UK (OAM), Medical Research Center (KMW), and Research to Prevent Blindness (University of Minnesota). TwinsUK also receives support from the National Institute for Health Research (NIHR) BioResource Clinical Research Facility and Biomedical Research Centre based at Guy's and St. Thomas' National Heath Service Foundation Trust and King's College London.
Disclosure: A. Tariq, None; O.A. Mahroo, None; K.M. Williams, None; S.H.M. Liew, Novartis (E); S. Beatty, Nutrasight Consultancy Ltd. (C), Howard Foundation (C), Macuhealth (C), Macuvision Europe Ltd. (C), P; C.E. Gilbert, None; F.J. Van Kuijk, None; C.J. Hammond, None
References
- 1. Hammond BR Jr, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A Opt Image Sci Vis. 1997; 14: 1187–1196 [DOI] [PubMed] [Google Scholar]
- 2. Berendschot TT, van Norren D. Macular pigment shows ring-like structures. Invest Ophthalmol Vis Sci. 2006; 47: 709–714 [DOI] [PubMed] [Google Scholar]
- 3. Delori FC, Goger DG, Keilhauer C, Salvetti P, Staurenghi G. Bimodal spatial distribution of macular pigment: evidence of a gender relationship. J Opt Soc Am A Opt Image Sci Vis. 2006; 23: 521–538 [DOI] [PubMed] [Google Scholar]
- 4. Dietzel M, Zeimer M, Heimes B, Pauleikhoff D, Hense HW. The ring-like structure of macular pigment in age-related maculopathy: results from the Muenster Aging and Retina Study (MARS). Invest Ophthalmol Vis Sci. 2011; 52: 8016–8024 [DOI] [PubMed] [Google Scholar]
- 5. Zeimer M, Dietzel M, Hense HW, Heimes B, Austermann U, Pauleikhoff D. Profiles of macular pigment optical density and their changes following supplemental lutein and zeaxanthin: new results from the LUNA study. Invest Ophthalmol Vis Sci. 2012; 53: 4852–4859 [DOI] [PubMed] [Google Scholar]
- 6. Hammond BR Jr, Fletcher LM, Elliott JG. Glare disability, photostress recovery, and chromatic contrast: relation to macular pigment and serum lutein and zeaxanthin. Invest Ophthalmol Vis Sci. 2013; 54: 476–481 [DOI] [PubMed] [Google Scholar]
- 7. Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995; 62 (6 suppl): 1448S–1461S [DOI] [PubMed] [Google Scholar]
- 8. Beatty S, Koh H, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000; 45: 115–134 [DOI] [PubMed] [Google Scholar]
- 9. Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001; 385: 28–40 [DOI] [PubMed] [Google Scholar]
- 10. Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003; 23: 171–201 [DOI] [PubMed] [Google Scholar]
- 11. Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006; 84: 4–15 [DOI] [PubMed] [Google Scholar]
- 12. Whitehead AJ, Mares JA, Danis RP. Macular pigment: a review of current knowledge. Arch Ophthalmol. 2006; 124: 1038–1045 [DOI] [PubMed] [Google Scholar]
- 13. Nolan JM, Stack J, O' Donovan O, Loane E, Beatty S. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp Eye Res. 2007; 84: 61–74 [DOI] [PubMed] [Google Scholar]
- 14. Loane E, Kelliher C, Beatty S, Nolan JM. The rationale and evidence base for a protective role of macular pigment in age-related maculopathy. Br J Ophthalmol. 2008; 92: 1163–1168 [DOI] [PubMed] [Google Scholar]
- 15. Kernt M, Walch A, Neubauer AS, et al. Filtering blue light reduces light-induced oxidative stress, senescence and accumulation of extracellular matrix proteins in human retinal pigment epithelium cells. Clin Experiment Ophthalmol. 2012; 40: e87–e97 [DOI] [PubMed] [Google Scholar]
- 16. Kirby ML, Beatty S, Loane E, et al. A central dip in the macular pigment spatial profile is associated with age and smoking. Invest Ophthalmol Vis Sci. 2010; 51: 6722–6728 [DOI] [PubMed] [Google Scholar]
- 17. Hammond CJ, Liew SH, Van Kuijk FJ, et al. The heritability of macular response to supplemental lutein and zeaxanthin: a classic twin study. Invest Ophthalmol Vis Sci. 2012; 53: 4963–4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wustemeyer H, Moessner A, Jahn C, Wolf S. Macular pigment density in healthy subjects quantified with a modified confocal scanning laser ophthalmoscope. Graefes Arch Clin Exp Ophthalmol. 2003; 241: 647–651 [DOI] [PubMed] [Google Scholar]
- 19. Sasamoto Y, Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Nishida K. Effect of cataract in evaluation of macular pigment optical density by autofluorescence spectrometry. Invest Ophthalmol Vis Sci. 2011; 52: 927–932 [DOI] [PubMed] [Google Scholar]
- 20. Wolf-Schnurrbusch UE, Röösli N, Weyermann E, Heldner MR, Höhne K, Wolf S. Ethnic differences in macular pigment density and distribution. Invest Ophthalmol Vis Sci. 2007; 48: 3783–3787 [DOI] [PubMed] [Google Scholar]
- 21. Sanfilippo PG, Medland SE, Hewitt AW, et al. Ophthalmic phenotypes and the representativeness of twin data for the general population. Invest Ophthalmol Vis Sci. 2011; 52: 5565–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hogg RE, Ong EL, Chamberlain M, et al. Heritability of the spatial distribution and peak density of macular pigment: a classical twin study. Eye (Lond). 2012; 26: 1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trieschmann M, Heimes B, Hense HW, Pauleikhoff D. Macular pigment optical density measurement in autofluorescence imaging: comparison of one- and two-wavelength methods. Graefes Arch Clin Exp Ophthalmol. 2006; 244: 1565–1574 [DOI] [PubMed] [Google Scholar]