Abstract
Background and Objective
Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase and have anti-inflammatory effects independent of cholesterol lowering. Recent clinical studies have indicated that statin intake has beneficial effect on periodontal disease. However, the underlying mechanisms have not been well understood. In the current study, we employed a rat model with lipopolysaccharide (LPS)-induced periodontal disease and determined the effect of simvastatin, a commonly prescribed statin, on osteoclastogenesis, gingival inflammation and alveolar bone loss.
Material and Methods
Sprague-Dawley rats were injected with A. actinomycetemcomitans LPS in periodontal tissue 3 times per week for 8 weeks and part of the rats with LPS injection were also given simvastatin via gavage. After the treatments, the rat maxillae were scanned by microcomputed tomography and the images were analyzed to determine alveolar bone loss. To explore the underlying mechanisms, the effect of simvastatin on osteoclastogenesis and gingival expression of pro-inflammatory cytokines were also determined by tartate-resistant acid phosphatase staining and real-time polymerase chain reaction assays, respectively.
Results
Results showed that LPS treatment markedly increased bone loss, but administration of simvastatin significantly alleviated the bone loss. Results also showed that LPS treatment stimulated osteoclastogenesis and the expression of inflammatory cytokines, but simvastatin significantly modulate the stimulatory effect of LPS on osteoclastogenesis and cytokine expression.
Conclusion
This study demonstrated that simvastatin treatment inhibits LPS-induced osteoclastogenesis and gingival inflammation and reduces alveolar bone loss, indicating that the intake of simvastatin may hinder the progression of periodontal disease.
Keywords: Simvastatin, periodontal disease, lipopolysaccharide, inflammation, alveolar bone
INTRODUCTION
Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, are drugs for lowering serum cholesterol (1). Since the first statin drug was approved for the treatment of hypercholesterolemia in 1987, a large number of studies have demonstrated that statins have anti-inflammatory effects independent of cholesterol lowering (2, 3). Clinical trials have shown that statins are effective for the treatment of not only hypercholesterolemia, atherosclerosis and cardiovascular disease, but also other inflammation-associated diseases such as rheumatoid arthritis (4). Considering that periodontal disease is also an inflammation-associated disease in which lipopolysaccharide (LPS) from Gram-negative bacteria plays an important role in triggering periodontal inflammation and tissue destruction (5, 6), it is important to determine if statin intake attenuates the progression of periodontal disease. The potential efficacy of statins in controlling periodontal disease is supported by in vitro findings from our and other laboratories that statins inhibited LPS-induced expression of pro-inflammatory cytokines and matrix metalloproteinases (MMPs) in mononuclear cells (7–12). Based on our in vitro studies, we hypothesized that statin is capable of reducing periodontal inflammation and alveolar bone loss in rats with LPS-induced periodontal disease.
Several clinical studies have appraised the effect of statins on periodontal disease. For example, a retrospective study reported that patients with periodontitis who took statins had 37% lower number of pathological periodontal pockets than those without statin medication (13). A recent study reported that subgingivally delivered simvastatin, a commonly prescribed statin with the trade name Zocor, was effective in treatment of patients with chronic periodontitis (14). To elucidate the underlying mechanism, animal studies have demonstrated the effect of simvastatin treatment on periodontal bone loss and gingival inflammation (15, 16). Recently, Dalcico et al. used a rat model with ligature-induced periodontitis and found that simvastatin reduced gingival inflammatory cytokine expression, oxidative stress, and bone loss (15). However, the effect of simvastatin on osteoclastogenesis remains uninvestigated and studies using different animal models and approaches are necessary to further document the beneficial effect of statins on periodontal disease.
In the present study, we employed a rat model with LPS-induced periodontal disease and treated the rats with simvastatin for 8 weeks concurrently with LPS injection. After the treatment, we examined alveolar bone loss using micro computed tomography (microCT), determined osteoclastogenesis using tartate-resistant acid phosphatase (TRAP) staining, and analyzed gingival expression of proinflammatory molecules using real-time PCR and PCR array. We found that simvastatin treatment significantly reduced LPS-induced alveolar bone loss and inhibited LPS-induced osteoclastogenesis and expression of pro-inflammatory molecules in periodontal tissue.
MATERIALS AND METHODS
Animal Treatments
To assess the effect of simvastatin on periodontal disease, we employed an established rat model of periodontal disease induced by A. actinomycetemcomitans LPS (17–19). Female Sprague-Dawley rats (10-week old and 250 g weight), purchased from Charles River Laboratory (Wilmington, MA), were fed regular rat chow and tap water ad libitum. All animal-related work was performed in accordance with the National Institute of Health Guidelines. The Institutional Animal Care and Use Committee at the Medical University of South Carolina approved all experimental protocols. LPS from Aggregatibacter actinomycetemcomitans (strain Y4, serotype B) was extracted by the hot phenol-water method as described (18, 19) and diluted in phosphate-buffered saline (PBS). Each rat was injected with 20 μg/rat of the LPS through the palatal gingiva between the maxillary 1st and 2nd molars 3 times per week for 8 weeks (n=8). Rats injected with PBS were used as control animals (n=7). To determine the effect of simvastatin on LPS-induced periodontal disease, rats were treated with both LPS via periodontal injection and simvastatin (20 mg/kg/day) daily via oral gavage for 8 weeks (n=13). Considering oral gavage-associated trauma or death (20), more rats were included in this group. The selection of the dose of simvastatin was based on two studies: 1. Nassar et al. reported that oral administration of simvastatin at 20 mg/kg/day led to a reversal of the cyclosporine A-induced bone loss in rats (16). 2. Aoki et al. reported that oral administration of simvastatin at 25 mg/kg/day suppresses the development of cerebral aneurysms by inhibiting inflammatory reactions in rats (21).
MicroCT and Quantification of Alveolar Bone Loss
Nondemineralized rat maxillae were scanned in 70% ethanol by a cone beam microCT system (Scanco Medical). The voltage of the X-ray was 70 kV and the beam current was 114 μA. The scanning was performed without frame average and filter. Each scan was done at 20 μm resolution. The GEHC Microview software (version 2.1.2) was used for rotation of the images and quantitation. Three liner measurements of the distance from the cement-enamel junction (CEJ) to the alveolar bone crest (ABC) were taken between the first and second molars as described previously (22).
RNA Isolation from Gingival Tissue
RNA was extracted from gingival tissue surrounding the injection sites using the RNeasy Mini Kit (Qiagen, Santa Clarita, CA). Briefly, the gingival tissue was homogenized in 350 μl of RNeasy Lysis Buffer with 1% β-mercapthoethanol and RNA was isolated by following the instruction provided by the manufacturer. The isolated RNA was stored at −80 °C.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
First-strand complementary DNA (cDNA) was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) using 20 μl of reaction mixture containing 0.25 μg of total RNA, 4 μl of 5× iScript reaction mixture, and 1 μl of iScript reverse transcriptase. The complete reaction was cycled for 5 minutes at 25°C, 30 minutes at 42°C and 5 minutes at 85°C using a PTC-200 DNA Engine (MJ Research, Waltham, MA). The reverse transcription (RT) reaction mixture was then diluted 1:10 with nuclease-free water and used for PCR amplification of cDNA in the presence of primers. The primers for real time PCR were purchased from Qiagen [interleukin 6 (IL-6), catalog #: PPR06483B-200, MMP-9, catalog #: PPR44728C-200; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), catalog #: PPR06557B-200]. The real-time PCR was performed in duplicate using 25 μl of reaction mixture containing 1.0 μl of RT mixture, 0.2 μM of both primers, and 12.5 μl of iQ™ SYBR Green Supermix (Bio-Rad Laboratories). Real-time PCR was run in the iCycler™ real-time detection system (Bio-Rad Laboratories) with a two-step method. The hot-start enzyme was activated (95°C for 3 min) and cDNA was then amplified for 40 cycles consisting of denaturation at 95°C for 10 sec and annealing/extension at 60°C for 60 sec. A melt-curve assay was then performed (55°C for 1 min and then temperature was increased by 0.5°C every 10 sec) to detect the formation of primer-derived trimers and dimmers. Data were analyzed with the iCycler iQ™ software (Bio-Rad Laboratories). The average starting quantity (SQ) of fluorescence units was used for analysis. Quantification was calculated using the SQ of targeted cDNA relative to that of GAPDH cDNA in the same sample.
PCR Array
Rat toll-like receptor (TLR) signal pathway PCR array (Catalog # PARN-018Z, Qiagen) was used to profile gene expression according to the instructions from the manufacturer. RNA from two randomly selected rats in each group was converted to cDNA and then amplified by PCR and the means were used for data analysis. The PCR array was performed in duplicate. The delta threshold cycle (Ct) was calculated by subtracting Ct for GAPDH from Ct for genes of interest.
TRAP Staining and Quantification of Osteoclasts
Formalin-fixed specimens were decalcified in a 10% EDTA solution for 2 weeks at 4 C. The EDTA solution was changed three times per week. The maxillas were paraffin-embedded, and 7 μm sagittal sections were prepared. TRAP staining was performed in tissue sections using a leukocyte acid phosphatase kit (Sigma Aldrich, St. Louis, MO). The tissue sections were counterstained with hematoxylin after TRAP staining. Active osteoclasts were defined as multinucleated TRAP-positive cells in contact with bone surface. The region for counting TRAP positive cells is the entire region under the crown of the first molar and the second molar for each section. Three sections per sample were counted.
Immunohistochemical Analysis of MMP-9 Expression
Gingival tissues were fixed in 10% formalin for 10 min and frozen sections were made using a cryostate. Sections were rinsed in 0.01 M phosphate buffer saline (PBS), pH 7.4, and then incubated in 0.3% H2O2/methanol for 30 minutes to quench endogenous peroxidase. After being rinsed with 0.01 M PBS, sections were incubated with 3% normal serum in 0.01 M PBS for 1 hour to block nonspecific binding. Sections were then blocked with avidin-biotin solution from the ABC Elite kit (Vector Laboratories, Burlingame, CA) for 30 minutes and incubated with goat anti-MMP-9 antibody (1 μg/ml) (R&D Systems, Minneapolis, MN) overnight at 4°C. Sections were incubated with secondary biotinylated-antibody (5 μg/ml) from the ABC Elite kit (Vector Laboratories) for 1 hour and then the ABC reagent (Vector Laboratories) for 30 minutes. Slides were rinsed in 0.01 M PBS and covered with diaminobenzidine peroxidase substrate solution from the Impack DAB kit (Vector Laboratories) for 2 minutes and then rinsed in water. Counterstaining was performed with hematoxylin. Slides were then dehydrated using increasing concentrations of ethanol and xylenes and mounted. Slides were further dehydrated in 99.5% xylene (Sigma Aldrich) and mounted in xylene based Cytoseal-XYL mounting media (Fisher Scientific). Photomicrographs of tissue sections were taken using an Olympus BX53 digital microscope with Cellsens digital image software (Olympus American Inc., Center Valley, PA).
Image Analysis
Images were analyzed with the Photoshop software (version 10; Adobe Systems, San Jose, CA). The method to use “Similar” feature to select a particular color staining on a digitized immunohistochemical image has previously been described in detail (23). Briefly, a standard was created by selecting an area of 0.5 cm × 0.5 cm from a tissue section that had desired brown color from immunostaining. The Magic Wand tool was used first to highlight the standard and the “Similar” command was then used to highlight all the areas with the brown color that is similar to the standard. The quantification was done using the Histogram, which showed the pixels of the highlighted area. The extent of the positive immunostaining of MMP-9 protein was presented as the average pixels per rat (pixels/rat) from three sections of gingival tissue of each rat.
Complete Blood Count
Peripheral blood was obtained from tails of euthanized rats and a complete blood count was done for each peripheral blood sample using a HemaVet® 950FS Cell Counter (Drew Scientific, Dallas, TX) by following sample processing instruction from the manufacturer.
Statistic Analysis
GraphPad Instat statistical software (Version 3.1a) (GraphPad Software, Inc. La Jolla, CA) was used for statistical analysis. One-way ANOVA was performed to determine the statistical significance of differences of alveolar bone loss, osteoclast number and gene expression level among three experimental groups, and data were presented as mean ± SD. The Student t test was performed to compare two groups. Nonparametric ANOVA using Kruskal-Wallis procedure was used determine the differences in MMP-9 levels among three groups. A value of P< 0.05 was considered significant.
RESULTS
Simvastatin Treatment Reduced LPS-Induced Alveolar Bone Loss
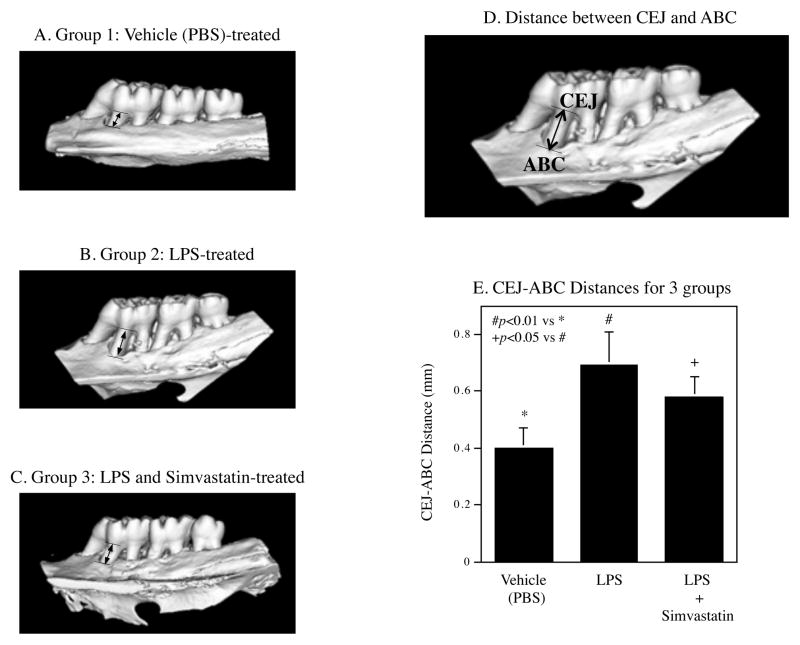
After 8-week treatments, the rat maxillae were scanned by microCT to reveal the alveolar bone (Fig. 1A–C). The CEJ-ABC distances for all rats were measured as indicated in Fig. 1D to determine the bone loss. Results showed that LPS treatment markedly increased the CEJ-ABC distance, but simvastatin treatment reduced the increase by 31% (Fig. 1E).
Figure 1.
Inhibition of LPS-induced alveolar bone loss by simvastatin treatment. Rats were treated with phosphate-buffered saline (PBS) (Group 1, n=7), lipopolysaccharide (LPS) (Group 2, n=8), or LPS plus simvastatin (Group 3, n=13) for 8 weeks and the maxillae were scanned by micro computed tomography (microCT) as described in Methods. Representative microCT images from 3 groups (A–C) were shown. The liner distances between the cement-enamel junction (CEJ) to the alveolar bone crest (ABC) were measured as indicated (D). The distances between CEJ and ABC were compared among 3 groups (E) (n=7, 8 and 13 in Group 1, 2, and 3, respectively). The data in E are mean ± standard deviation (SD). The data were from one of two studies with similar results.
Simvastatin Treatment Significantly Inhibited LPS-Stimulated Osteoclastogenesis
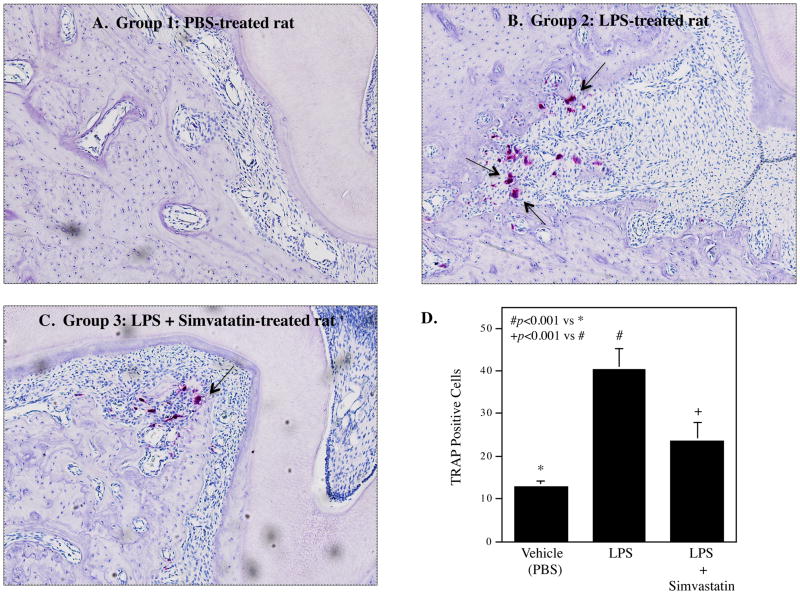
It has been reported that periodontal LPS injection in rats stimulates osteoclastogenesis in the alveolar bone (18). In this study, we determined if simvastatin treatment modulates LPS-induced osteoclastogenesis. Results showed that LPS treatment, when compared with the vehicle (PBS) treatment, led to a remarkable increase in TRAP-stained osteoclasts, but simvastatin treatment significantly reduced LPS-increased osteoclasts (Fig. 2), indicating that simvastatin treatment attenuates LPS-induced osteoclastogenesis.
Figure 2.
Inhibition of lipopolysaccharide (LPS)-induced osteoclastogenesis by simvastatin treatment. The tartate-resistant acid phosphatase (TRAP) staining was performed on bone tissue sections of the maxillae from PBS-treated (A), LPS-treated (B) or LPS plus simvastatin-treated rats (C). Representative tissue sections from 3 groups were shown. The arrows indicate multinucleated osteoclasts. The numbers of multinucleated TRAP-positive osteoclasts were compared between 3 groups (n=5 for each group) (D).
Simvastatin Inhibited LPS-Induced Expression of Inflammatory Cytokines and MMPs
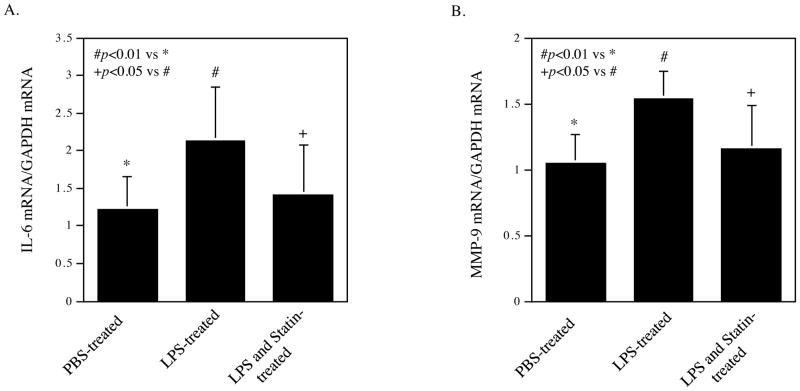
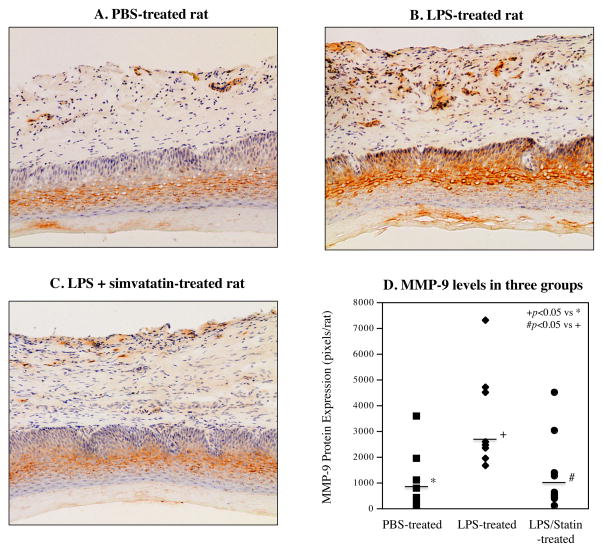
In addition to the above study on osteoclastogenesis, we also determined the effect of LPS injection and simvastatin treatment on inflammatory gene expression in gingival tissue. To elucidate the effect of simvastatin treatment on periodontal inflammation, we profiled periodontal gene expression of pro-inflammatory molecules using a TLR pathway-focused PCR array. As shown in Table 1, while LPS upregulated pro-inflammatory cytokines such as IL-6, tumor necrosis factor (TNF)α, IL-1α, IL-1β, colony-stimulating factor (CSF)2, CSF3 and monocyte chemotactic protein (MCP)-1, and several TLR family members, simvastatin treatment reduced the stimulatory effect of LPS on these cytokines significantly. For example, LPS stimulated IL-6 expression by 3.3-fold, but simvastatin reduced the stimulated expression by 42%. To confirm the above results from PCR array, we performed quantitative real-time PCR on IL-6 expression in gingival tissue of rats. The results of the real-time PCR were consistent with those from PCR array showing that simvastatin significantly suppressed the stimulatory effect of LPS on IL-6 expression (Fig. 3A). Since it is known that MMP-9 plays an important role in periodontal tissue destruction in periodontal disease (24, 25), we further determined if simvastatin reduced MMP-9 mRNA level in gingival tissue of rats. Results showed that while LPS increased MMP-9 mRNA level, simvastatin significantly inhibited the stimulation of MMP-9 mRNA level by LPS (Fig. 3B). Furthermore, immunohistochemistry was performed to detect MMP-9 protein in gingival tissue. Results showed that LPS increased MMP-9 protein level but simvastatin significantly reduced it (Fig. 4).
Table 1.
Inhibition of LPS-stimulated Gene Expression in Rat Gingival Tissue by Simvastatin
| Gene Names | Ct for vehicle-treated Rats | Ct for LPS-treated Rats | Ct for LPS + Statin-treated Rats | Fold of control by LPS | Fold of control by LPS+ statin | Percentin hibition by statin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | Mean | #1 | #2 | Mean | #1 | #2 | Mean | ||||
| CSF-2 | 29.18 | 29.70 | 29.44 | 27.73 | 27.62 | 27.67 | 28.30 | 28.66 | 28.48 | 3.41 | 1.95 | 60.5% |
| CSF-3 | 29.28 | 29.58 | 29.43 | 27.65 | 27.47 | 27.56 | 28.11 | 28.07 | 28.09 | 3.65 | 2.53 | 42.2% |
| CXCL10 | 28.81 | 28.72 | 28.77 | 27.50 | 27.38 | 27.44 | 27.88 | 28.11 | 27.99 | 2.50 | 1.71 | 52.8% |
| HSP70 | 28.92 | 29.37 | 29.15 | 27.65 | 27.37 | 27.51 | 27.79 | 28.08 | 27.93 | 3.12 | 2.31 | 38% |
| IFNβ1 | 28.96 | 29.55 | 29.25 | 27.78 | 27.64 | 27.71 | 28.05 | 28.15 | 28.10 | 2.91 | 2.23 | 36% |
| IFNγ | 29.66 | 30.23 | 29.95 | 28.43 | 28.17 | 28.30 | 28.79 | 28.75 | 28.77 | 3.12 | 2.26 | 40.7% |
| IL-2 | 28.92 | 29.56 | 29.24 | 27.52 | 27.44 | 27.48 | 28.15 | 27.88 | 28.02 | 3.39 | 2.34 | 44.2% |
| IL-6 | 28.62 | 29.39 | 29.00 | 27.34 | 27.19 | 27.27 | 27.80 | 27.73 | 27.76 | 3.34 | 2.36 | 41.8% |
| TNFα | 28.21 | 29.01 | 28.61 | 27.24 | 27.35 | 27.29 | 27.80 | 27.80 | 27.80 | 2.31 | 1.65 | 50.3% |
| LTA | 28.30 | 28.68 | 28.49 | 26.87 | 26.86 | 26.87 | 26.79 | 27.50 | 27.14 | 3.07 | 2.56 | 25% |
| PPARα | 28.33 | 28.43 | 28.38 | 26.84 | 26.76 | 26.80 | 27.33 | 27.63 | 27.48 | 2.49 | 1.75 | 49.6% |
| COX-2 | 28.39 | 29.30 | 28.85 | 27.31 | 27.22 | 27.26 | 27.73 | 27.81 | 27.77 | 2.99 | 2.11 | 44% |
| RP105 | 28.74 | 28.76 | 28.75 | 26.96 | 26.92 | 26.94 | 27.32 | 27.47 | 27.39 | 3.51 | 2.57 | 37.5% |
| CD80 | 28.69 | 28.93 | 28.81 | 27.03 | 26.91 | 26.97 | 27.37 | 27.38 | 27.37 | 3.61 | 2.71 | 34.5% |
| CD86 | 28.25 | 28.73 | 28.49 | 27.06 | 26.98 | 27.02 | 27.33 | 27.32 | 27.32 | 2.77 | 2.25 | 29% |
| IRAK2 | 28.08 | 28.99 | 28.53 | 27.42 | 27.02 | 27.22 | 27.67 | 27.38 | 27.52 | 2.48 | 2.01 | 32% |
| TICAM-2 | 29.30 | 29.62 | 29.46 | 27.61 | 27.41 | 27.51 | 28.05 | 28.18 | 28.11 | 2.70 | 1.87 | 48.7% |
| NFKB p50 | 26.86 | 27.77 | 27.31 | 26.38 | 26.22 | 26.30 | 26.60 | 26.86 | 26.73 | 2.01 | 1.49 | 51% |
| TLR1 | 28.29 | 28.61 | 28.45 | 26.99 | 26.89 | 26.94 | 27.40 | 27.58 | 27.49 | 2.98 | 1.86 | 56.5% |
| TLR2 | 28.16 | 28.81 | 28.49 | 27.24 | 27.14 | 27.19 | 27.71 | 27.71 | 27.71 | 3.86 | 2.55 | 45.9% |
| TLR3 | 28.30 | 28.69 | 28.50 | 27.15 | 27.20 | 27.18 | 27.56 | 27.66 | 27.61 | 2.84 | 1.95 | 48.6% |
| TLR6 | 28.89 | 29.12 | 29.00 | 27.61 | 27.31 | 27.46 | 27.92 | 27.89 | 27.90 | 2.46 | 1.71 | 51.1% |
| TLR7 | 28.07 | 28.80 | 28.44 | 27.31 | 27.15 | 27.23 | 27.81 | 27.62 | 27.71 | 2.49 | 1.85 | 43.1% |
| TLR9 | 28.21 | 29.01 | 28.61 | 27.24 | 27.35 | 27.29 | 27.80 | 27.80 | 27.80 | 2.93 | 2.14 | 40.6% |
| TLR4 | 27.69 | 28.18 | 27.94 | 27.31 | 27.00 | 27.15 | 27.37 | 27.42 | 27.39 | 1.72 | 1.45 | UD |
| MyD88 | 26.82 | 27.79 | 27.31 | 27.07 | 27.01 | 27.04 | 27.35 | 27.33 | 27.34 | 1.20 | 0.98 | UD |
| CD14 | 26.74 | 28.20 | 27.47 | 26.69 | 26.62 | 26.65 | 27.09 | 27.16 | 27.13 | 1.77 | 1.27 | UD |
| MD-2 | 27.86 | 28.65 | 28.25 | 28.05 | 28.04 | 28.04 | 28.21 | 27.83 | 28.02 | 1.16 | 1.18 | UD |
| c-Jun | 25.09 | 26.34 | 25.71 | 26.70 | 26.50 | 26.60 | 26.71 | 26.79 | 26.75 | 0.54 | 0.49 | UD |
| c-Fos | 27.17 | 27.98 | 27.58 | 27.32 | 27.23 | 27.28 | 27.49 | 27.50 | 27.49 | 1.23 | 1.06 | UD |
RNA was isolated from gingival tissue of rats after the treatments and subjected to PCR array analysis as described in Methods. Two rats in each group were analyzed. Ct = Ct of gene of interest minus Ct of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ahouse keeping gene. The Ctof GAPDH was determined by quantitative real-time PCR using the same RNA for PCR array. Since LPS had lower or no stimulation on some genes, percent inhibition by statin was undetermined (UD). CSF, colony stimulating factor; CXCL10, C-X-C motif ligand 10; HSP70, heat shock protein 70; IFNβ 1, interferon beta 1; LTA, lymphotoxin alpha; PPARα, peroxisome proliferator-activated receptor alpha; COX-2, cyclooxygenase-2; IRAK2, interleukin-1 receptor-associated kinase 2; TICAM-2, toll/interleukin-1 receptor domain-containing adaptor protein; MyD88, myeloid differentiation factor 88; MD-2, myeloid differentiation factor 2.
Figure 3.
Inhibition of LPS-stimulated IL-6 and matrix metalloproteinase (MMP)-9 expression in periodontal tissue. RNA was isolated from rat periodontal tissue after treatments with vehicle (PBS) (n=7), LPS (n=8) or LPS plus simvastatin (n=13), and real-time PCR was performed to quantify IL-6 (A) and MMP-9 expression (B). The data are mean ± SD of the ratios of IL-6 or MMP-9 vs GAPDH mRNA.
Figure 4.
Inhibition of LPS-stimulated MMP-9 protein expression in periodontal tissue. The gingival tissues surrounding the injection sites were collected from rats treated with vehicle PBS (A, n=7), LPS (B, n=8) or LPS plus simvastatin (C, n=13) and sectioned. The sections were immunostained with anti-MMP-9 antibodies and counterstained with hematoxylin. The MMP-9 protein level was quantified as described in Materials and Methods and compared among three groups (D). The horizontal lines indicate the group median levels.
Regulation of TLR and TLR-related Transcription Factors by LPS and Simvastatin
Our gene expression profiling as shown in Table 1 also revealed that LPS stimulated expression of interleukin-1 receptor-associated kinase (IRAK)2 and toll/interleukin-1 receptor (TIR) domain-containing adaptor protein (TICAM)2 by 2.48- and 2.70-fold of the control, respectively. Both IRAK2 and TICAM2 are known to participate in TLR pathways linking to NFκ B signaling (26, 27). In addition, LPS also stimulated NFκ B subunit p50 expression by 2-fold. Interestingly, simvastatin inhibited LPS-stimulated IRAK2, TICAM2 and p50 by 32%, 49% and 51%, respectively. Table 1 also showed that LPS increased expression of several TLRs, but simvastatin inhibited them. Furthermore, our PCR array showed that the stimulation by LPS of the above genes is specific since data showed that LPS has lower or no stimulation on the expression of TLR4, MyD88, CD14, MD-2, c-Jun, and c-Fos.
Treatment with LPS and Simvastatin did not Change Circulating White Blood Cell Counts
To determine if periodontal LPS injection and simvastatin treatment affect circulating white blood cell counts, the complete blood counts were performed at the end of the study. Results showed that neither LPS injection nor simvastatin treatment significantly changed total white blood cell, neutrophil, lymphocyte and monocyte counts (data not shown).
DISCUSSION
Statins are powerful drugs for treatment of hypercholesterolemia and diabetic complications, and prescribed widely to patients with cardiovascular disease and diabetes (2). Since cardiovascular disease is associated with periodontal disease (28) and patients with diabetes have increased incidence of periodontal disease (29, 30), it is important to elucidate the effect of statins on periodontal disease. In the present study, we have utilized a well-established animal model for periodontal disease and provided evidence that simvastatin treatment reduces LPS-induced periodontal inflammation and alveolar bone loss in the animal model.
Although a large number of in vitro studies from our and other laboratories have demonstrated that statins antagonize the action of LPS on inflammatory gene expression (8–10), no in vivo study using LPS-treated animals has been reported to confirm the in vitro observations. In the present study, we administrated simvastatin in rats at the same time when LPS was injected into the palatal gingiva to induce periodontal disease. This experimental design thus allows us to determine if simvastatin is capable of antagonizing the stimulatory effect of LPS on the expression of inflammatory genes and alveolar bone loss. Therefore, this is the first study to confirm the antagonizing effect of simvastatin on LPS-stimulated inflammatory response in vivo.
In this study, we provided several lines of evidence to support the effectiveness of simvastatin on LPS-induced periodontal disease. First, our analysis on microCT images clearly demonstrated that simvastatin reduced LPS-induced alveolar bone loss. Second, our findings from histology and TRAP staining of alveolar bone tissue showed that simvastatin significantly reduces LPS-induced osteoclastogenesis. Third, our studies using quantitative PCR showed that simvastatin reduces LPS-stimulated expression of pro-inflammatory molecules in periodontal tissue. While the first observation on alveolar bone loss is the most important evidence to support the beneficial effect of simvastatin on periodontal disease, the studies on osteoclastogenesis and inflammatory gene expression revealed the possible mechanisms whereby simvastatin inhibits bone loss.
Recently, Dalcico and coworkers reported that simvastatin reduced gingival inflammatory cytokine expression, oxidative stress, and bone loss (15). In this study, the investigators used a rat model of periodontal disease induced by ligature placement and applied microscopic and histologic examination to quantify alveolar bone loss. Furthermore, they investigated the mechanisms that were potentially involved in the reduction of bone loss by simvastatin by quantifying protein expression of pro-inflammatory cytokines, MMPs, BMP-2, RANKL, and osteoprotegerin using ELISA and immunohistochemistry. In our current study, we used a different rat model of periodontal disease induced by LPS injection and applied microCT to quantify alveolar bone loss. In our investigation of the mechanisms involved in the reduction of LPS-induced bone loss by simvastatin, we quantified mRNA expression of pro-inflammatory cytokines and MMPs using real-time PCR, PCR arrays and immunohistochemistry. In addition, we also quantified osteoclasts using TRAP staining. Clearly, our study using different animal model and different methods has provided novel information about the inhibitory effect of simvastatin on LPS-induced bone loss, osteoclastogenesis and pro-inflammatory gene expression in gingival tissue.
Vaziri et al. reported previously that simvastatin had protective effects against the impact of periodontitis on attachment apparatus and alveolar bone in ovariectomized rats with ligature-induced periodontal disease (31). Furthermore, Price et al. demonstrated that local treatment with simvastatin conjugated with alendronate, a bisphosphonate drug used for osteoporosis and several other bone diseases, had potential to prevent episodes of periodontal bone loss in rats with LPS-induced periodontal disease (32). Compared with the above studies, our investigation was more focused on the effect of simvastatin on LPS-induced alveolar bone loss, osteoclastogenesis and gingival inflammatory gene expression. Overall, all the findings reported from our and other groups have indicated a potential role of statins in the inhibition of experimental periodontal disease and warranted further clinical investigations.
It is known that the upregulation of the receptor activator of nuclear factor kappa-B ligand (RANKL), a critical osteoclast differentiation factor, in T lymphocytes by LPS plays an essential role in LPS-induced osteoclastogenesis and alveolar bone resorption (33). In studies to elucidate how LPS upregulates RANKL, it was reported that interferon gamma (IFNγ ) stimulated by LPS plays an important role in T cell activation and T cell secretion of RANKL (34). In addition to IFNγ, CSF2 was found to be responsible for fusion of mononuclear osteoclasts into bone-resorbing osteoclasts to form multinucleated osteoclasts and thus enhanced the effect of RANKL on bone resorption (35). These studies have shown a crucial role of chronic inflammation and inflammatory cytokines such as IFNγ and CSF2 in osteoclastogensis. Interestingly, our gene expression profiling data (Table 1) showed that LPS treatment increased IFNγ and CSF2 expression in periodontal tissue by 3.1- and 3.4-fold, but simvastatin effectively attenuated the increase of IFNγ and CSF2 by 41% and 61%, respectively. Thus, the inhibitory action of simvastatin on inflammatory cytokines such as IFNγ and CSF2 may play an important role in simvastatin-lessened alveolar bone loss.
While simvastatin significantly inhibit LPS-induced bone loss, it is noteworthy that the inhibition of the bone loss by 31% is moderate. This finding might be explained partially by our previous report that simvastatin suppresses LPS-induced gene expression in mononuclear cells by inhibiting protein isoprenylation-mediated activation of mitogen-activated protein kinase (MAPK), but not nuclear factory kappa B (NFκ B) pathway (9). Given the important role of LPS-elicited NFκ B signaling in the expression of proinflammatory cytokines, lack of inhibition on the NFκ B pathway by simvastatin may limit the effectiveness of simvastatin on tissue inflammation and alveolar bone loss. In consistence with our observation, Song et al. also demonstrated that simvastatin inhibited LPS-induced activation of MAPK in human monocyte-derived macrophages (36). Interestingly, using RAW 264.7 mouse macrophages, Ahn et al. reported that simvastatin suppressed osteoclastogenesis induced by RANKL through modulation of NFκ B pathway (37). These findings indicate that while simvastatin fails to block LPS-stimulated NFκ B cascade, it does have inhibitory effect on RANKL-stimulated NFκ B signaling. It is possible that simvastatin may inhibit NFκ B signaling activated by stimuli other than LPS. Obviously, more studies are warranted to clarify the action of simvastatin on NFκ B signaling elicited by different stimuli.
In addition to the inhibition on bone resorption, it has been reported that simvastatin also stimulates bone formation (38). Ho et al. reported that simvastatin increased bone volume and osteoblast number in the distal femurs, proximal tibiae and vertebrae and stimulated the expression of osteogenic proteins such as BMP2, collagen type 1 and osteocalcin in vertebral bones of ovariectomized rats (39). However, these studies did not address the effect of simvastatin on bone formation under the influence of LPS and the effect of the interaction between simvastatin and LPS on osteoblastogenesis remains largely unknown.
In conclusion, the present study demonstrated that administration of simvastatin in rats with LPS-induced periodontal disease significantly inhibited periodontal inflammation and osteoclastogenesis and reduced alveolar bone loss, indicating that simvastatin intake may hinder the progression of periodontal disease. While this animal study suggests that statin intake may have beneficial effect on periodontal disease, more clinical studies are necessary to define the effect of statins on human periodontal disease.
Acknowledgments
This work was supported by National Institutes of Health grant DE016353 and the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs (to Y.H.). This project utilized facilities, resources, and/or technical assistance of the Laboratory of the Center for Oral Health Research (L-COHR) that is supported by the National Institute of General Medicine grant P30 GM103331.
References
- 1.Gotto AM, Jr, Moon JE. Management of cardiovascular risk: the importance of meeting lipid targets. The American journal of cardiology. 2012;110:3A–14A. doi: 10.1016/j.amjcard.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Sadowitz B, Maier KG, Gahtan V. Basic science review: Statin therapy--Part I: The pleiotropic effects of statins in cardiovascular disease. Vascular and endovascular surgery. 2010;44:241–251. doi: 10.1177/1538574410362922. [DOI] [PubMed] [Google Scholar]
- 3.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends in molecular medicine. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paraskevas KI. Statin treatment for rheumatoid arthritis: a promising novel indication. Clinical rheumatology. 2008;27:281–287. doi: 10.1007/s10067-007-0806-8. [DOI] [PubMed] [Google Scholar]
- 5.Beck JD, Offenbacher S, Williams R, Gibbs P, Garcia R. Periodontitis: a risk factor for coronary heart disease? Annals of periodontology / the American Academy of Periodontology. 1998;3:127–141. doi: 10.1902/annals.1998.3.1.127. [DOI] [PubMed] [Google Scholar]
- 6.Gu Y, Lee HM, Sorsa T, et al. Non-antibacterial tetracyclines modulate mediators of periodontitis and atherosclerotic cardiovascular disease: a mechanistic link between local and systemic inflammation. Pharmacological research: the official journal of the Italian Pharmacological Society. 2011;64:573–579. doi: 10.1016/j.phrs.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Jin J, Sundararaj KP, Samuvel DJ, et al. Different signaling mechanisms regulating IL-6 expression by LPS between gingival fibroblasts and mononuclear cells: seeking the common target. Clinical immunology (Orlando, Fla) 2012;143:188–199. doi: 10.1016/j.clim.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nareika A, Maldonado A, He L, et al. High glucose-boosted inflammatory responses to lipopolysaccharide are suppressed by statin. Journal of periodontal research. 2007;42:31–38. doi: 10.1111/j.1600-0765.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- 9.Sundararaj KP, Samuvel DJ, Li Y, et al. Simvastatin suppresses LPS-induced MMP-1 expression in U937 mononuclear cells by inhibiting protein isoprenylation-mediated ERK activation. Journal of leukocyte biology. 2008;84:1120–1129. doi: 10.1189/jlb.0108064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Methe H, Kim JO, Kofler S, Nabauer M, Weis M. Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:1439–1445. doi: 10.1161/01.ATV.0000168410.44722.86. [DOI] [PubMed] [Google Scholar]
- 11.Pleiner J, Schaller G, Mittermayer F, et al. Simvastatin prevents vascular hyporeactivity during inflammation. Circulation. 2004;110:3349–3354. doi: 10.1161/01.CIR.0000147774.90396.ED. [DOI] [PubMed] [Google Scholar]
- 12.Steiner S, Speidl WS, Pleiner J, et al. Simvastatin blunts endotoxin-induced tissue factor in vivo. Circulation. 2005;111:1841–1846. doi: 10.1161/01.CIR.0000158665.27783.0C. [DOI] [PubMed] [Google Scholar]
- 13.Lindy O, Suomalainen K, Makela M, Lindy S. Statin use is associated with fewer periodontal lesions: A retrospective study. BMC oral health. 2008;8:16. doi: 10.1186/1472-6831-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradeep AR, Thorat MS. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. Journal of periodontology. 2010;81:214–222. doi: 10.1902/jop.2009.090429. [DOI] [PubMed] [Google Scholar]
- 15.Dalcico R, de Menezes AM, Deocleciano OB, et al. Protective Mechanisms of Simvastatin in Experimental Periodontal Disease. Journal of periodontology. 2012 Nov 26; doi: 10.1902/jop.2012.120114. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Nassar PO, Nassar CA, Guimaraes MR, et al. Simvastatin therapy in cyclosporine A-induced alveolar bone loss in rats. Journal of periodontal research. 2009;44:479–488. doi: 10.1111/j.1600-0765.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Yu H, Zinna R, et al. Silencing mitogen-activated protein kinase-activated protein kinase-2 arrests inflammatory bone loss. The Journal of pharmacology and experimental therapeutics. 2011;336:633–642. doi: 10.1124/jpet.110.172395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers JE, Li F, Coatney DD, et al. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. Journal of periodontology. 2007;78:550–558. doi: 10.1902/jop.2007.060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Li Q, Herbert B, et al. Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene therapy. 2011;18:344–353. doi: 10.1038/gt.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemporary topics in laboratory animal science / American Association for Laboratory Animal Science. 2004;43:42–51. [PubMed] [Google Scholar]
- 21.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Simvastatin suppresses the progression of experimentally induced cerebral aneurysms in rats. Stroke; a journal of cerebral circulation. 2008;39:1276–1285. doi: 10.1161/STROKEAHA.107.503086. [DOI] [PubMed] [Google Scholar]
- 22.Park CH, Abramson ZR, Taba M, Jr, et al. Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. Journal of periodontology. 2007;78:273–281. doi: 10.1902/jop.2007.060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehr HA, Mankoff DA, Corwin D, Santeusanio G, Gown AM. Application of photoshop-based image analysis to quantification of hormone receptor expression in breast cancer. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1997;45:1559–1565. doi: 10.1177/002215549704501112. [DOI] [PubMed] [Google Scholar]
- 24.Ramamurthy NS, Rifkin BR, Greenwald RA, et al. Inhibition of matrix metalloproteinase-mediated periodontal bone loss in rats: a comparison of 6 chemically modified tetracyclines. Journal of periodontology. 2002;73:726–734. doi: 10.1902/jop.2002.73.7.726. [DOI] [PubMed] [Google Scholar]
- 25.Branco-de-Almeida LS, Franco GC, Castro ML, et al. Fluoxetine inhibits inflammatory response and bone loss in a rat model of ligature-induced periodontitis. Journal of periodontology. 2012;83:664–671. doi: 10.1902/jop.2011.110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keating SE, Maloney GM, Moran EM, Bowie AG. IRAK-2 participates in multiple toll-like receptor signaling pathways to NFkappaB via activation of TRAF6 ubiquitination. The Journal of biological chemistry. 2007;282:33435–33443. doi: 10.1074/jbc.M705266200. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan C, Postlethwait JH, Lage CR, Millard PJ, Kim CH. Evidence for evolving Toll-IL-1 receptor-containing adaptor molecule function in vertebrates. Journal of immunology (Baltimore, Md: 1950) 2007;178:4517–4527. doi: 10.4049/jimmunol.178.7.4517. [DOI] [PubMed] [Google Scholar]
- 28.Persson GR, Persson RE. Cardiovascular disease and periodontitis: an update on the associations and risk. Journal of clinical periodontology. 2008;35:362–379. doi: 10.1111/j.1600-051X.2008.01281.x. [DOI] [PubMed] [Google Scholar]
- 29.Pontes Andersen CC, Flyvbjerg A, Buschard K, Holmstrup P. Relationship between periodontitis and diabetes: lessons from rodent studies. Journal of periodontology. 2007;78:1264–1275. doi: 10.1902/jop.2007.060491. [DOI] [PubMed] [Google Scholar]
- 30.Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaziri H, Naserhojjati-Roodsari R, Tahsili-Fahadan N, et al. Effect of simvastatin administration on periodontitis-associated bone loss in ovariectomized rats. Journal of periodontology. 2007;78:1561–1567. doi: 10.1902/jop.2007.060480. [DOI] [PubMed] [Google Scholar]
- 32.Price U, Le HO, Powell SE, et al. Effects of local simvastatin-alendronate conjugate in preventing periodontitis bone loss. Journal of periodontal research. 2012 Dec 30; doi: 10.1111/jre.12036. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. Journal of periodontology. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Grassi F, Ryan MR, et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. The Journal of clinical investigation. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MS, Kim HS, Yeon JT, et al. GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. Journal of immunology (Baltimore, Md: 1950) 2009;183:3390–3399. doi: 10.4049/jimmunol.0804314. [DOI] [PubMed] [Google Scholar]
- 36.Song JX, Ren JY, Chen H. Simvastatin reduces lipoprotein-associated phospholipase A2 in lipopolysaccharide-stimulated human monocyte-derived macrophages through inhibition of the mevalonate-geranylgeranyl pyrophosphate-RhoA-p38 mitogen-activated protein kinase pathway. Journal of cardiovascular pharmacology. 2011;57:213–222. doi: 10.1097/FJC.0b013e31820376ac. [DOI] [PubMed] [Google Scholar]
- 37.Ahn KS, Sethi G, Chaturvedi MM, Aggarwal BB. Simvastatin, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, suppresses osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand through modulation of NF-kappaB pathway. International journal of cancer Journal international du cancer. 2008;123:1733–1740. doi: 10.1002/ijc.23745. [DOI] [PubMed] [Google Scholar]
- 38.Hwang R, Lee EJ, Kim MH, et al. Calcyclin, a Ca2+ ion-binding protein, contributes to the anabolic effects of simvastatin on bone. The Journal of biological chemistry. 2004;279:21239–21247. doi: 10.1074/jbc.M312771200. [DOI] [PubMed] [Google Scholar]
- 39.Ho ML, Chen YH, Liao HJ, et al. Simvastatin increases osteoblasts and osteogenic proteins in ovariectomized rats. European journal of clinical investigation. 2009;39:296–303. doi: 10.1111/j.1365-2362.2009.02092.x. [DOI] [PubMed] [Google Scholar]