Abstract
Many mosquito species take bloodmeals predominantly from either birds or mammals. Other mosquito species are less host-specific and feed readily on both. Furthermore, some species tend to alter their feeding patterns over the course of the year; early in the mosquito season such species may feed primarily on a particular host type, and subsequently take an increasingly larger proportion of their bloodmeals from an alternative host type as the season progresses. We have examined the feeding patterns of the three mosquito species found in Bernalillo County, NM: Culex quinquefasciatus (Say), Culex tarsalis (Coquillett), and Aedes vexans (Meigen). Specifically, we seek to determine if any of these species displays a seasonal shift in terms of its host utilization pattern. Our analysis focuses on these three species because they are all considered to be competent vectors for the West Nile virus (WNV). Our current data for Cx. quinquefasciatus suggest that unlike elsewhere in its range, this species increases its proportion of avian bloodmeals as the season progresses. Alternatively, Ae. vexans feeds primarily on mammals, whereas Cx. tarsalis appears to feed on both mammals and birds throughout the mosquito season. A more complete understanding of the feeding habits of these three mosquito species may help to clarify the transmission dynamics of WNV in Bernalillo County.
Keywords: mosquito, bloodmeal, West Nile virus, seasonality
Many blood-seeking mosquito species are known to have characteristic host utilization patterns (Washino and Tempelis 1983, Molaei and Andreadis 2006, Molaei et al. 2008, Garcia-Rejon et al. 2010). For example, in the midwestern and eastern United States, Culex restuans (Theobald) and Culex pipiens (L.) feed primarily on birds and only occasionally feed on mammals (Andreadis et al. 2001). Alternatively, Culex salinarius (Coquillett) and Coquillettidia perturbans (Walker), also found in these areas, take a larger proportion of their bloodmeals from mammals (Apperson et al. 2002, Molaei et al. 2006). Culex quinquefasciatus (Say) is less host-specific and takes a substantial portion of its bloodmeals from both birds and mammals (Garcia-Rejon et al. 2010). Meanwhile, Aedesvexans (Meigen) takes the majority of its bloodmeals from mammalian hosts and only feeds sporadically on birds (Washino and Tempelis 1983, Molaei and Andreadis 2006, Molaei et al. 2008).
Host utilization is an important component of vector competence for putative mosquito vectors of arboviruses (Turell et al. 2001, Apperson et al. 2002, Kilpatrick et al. 2006, Molaei et al. 2010). Specifically, an understanding of mosquito-feeding patterns can help to elucidate the roles of various mosquito species in viral amplification, maintenance of the enzootic cycle, and as bridge vectors to nonenzootic hosts. However, a clear determination of mosquito host utilization is complicated by the fact that certain mosquito species are known to alter their feeding behavior over the course of the mosquito season (Edman and Taylor 1968, Edman et al. 1974, Kilpatrick et al. 2006, Kent et al. 2007; Molaei et al. 2007). For example, certain Culex species have been found to feed primarily on birds early in the mosquito season, and as the season progresses they take an increasingly larger proportion of their bloodmeals from mammals (Turell et al. 2001, Apperson et al. 2002, Zinser et al. 2004, Molaei et al. 2006, Kent et al. 2009).
Previously we have confirmed that based on mosquitoes collected over the entire mosquito season in central New Mexico, Ae. vexans feeds primarily on mammals, Cx. quinquefasciatus takes the majority of its bloodmeals from avian hosts, and Cx. tarsalis appears to feed regularly on both avian and mammalian hosts (Greenberg et al. 2012, 2013). These three species were selected for analysis because all three have tested positive for the West Nile virus (WNV) in Bernalillo County, NM, and have been implicated as potential vectors of this arbovirus (DiMenna et al. 2006a). All three of these species have been judged to be competent WNV vectors (Turell et al. 2001, Cupp et al. 2004, Kilpatrick et al. 2005, Molaei and Andreadis 2006, DiMenna 2006b), and the roles of both Cx. tarsalis (Kent et al. 2007) and Cx. quinquefasciatus (Samuel et al. 2004, Molaei et al. 2007, Garcia-Rejon et al. 2010) in WNV transmission have been described elsewhere in their range.
In this study, we investigate whether these same three species undergo a similar seasonal shift in terms of host utilization in central New Mexico. Using bloodmeal analysis of mosquitoes trapped throughout the entire mosquito season over a period of 7 yr, we have determined that Ae. vexans undergoes no such shift and continues to rely heavily on mammalian hosts throughout the mosquito season. Cx. tarsalis appears to feed on mammals throughout the season, whereas Cx. quinquefasciatus becomes increasingly likely to feed on avian hosts. The possible implications of these patterns, both for the enzootic WNV cycle and for bridge transmission to mammals in central New Mexico, are discussed.
Materials and Methods
Bernalillo County is located in central New Mexico and includes the greater Albuquerque metropolitan area, which accounts for ≈32% of the state’s population (City of Albuquerque Census 2010). The Rio Grande flows directly through the county from north to south, forming the Rio Grande Valley. Much of the valley retains a rural character. The riverbanks are generally heavily wooded and form a riparian forest known as the Rio Grande Bosque. Outside of Albuquerque, the lands adjacent to the Bosque are often devoted to agriculture or grazing. Most mosquito activity, and consequently most arbovirus transmission, in Bernalillo County occurs in the Rio Grande Valley (DiMenna et al. 2007). More than 50 mosquito species have been collected in New Mexico (Wolff and Nielsen 2007). Our study focuses on Cx. quinquefasciatus, Cx. tarsalis, and Ae. vexans, the three species that have been implicated as WNV vectors in central New Mexico (DiMenna et al. 2006b).
Mosquito Collection and Identification
Mosquitoes were collected weekly throughout the mosquito season (May through October) from 2006 through 2012. Each year, between 18 and 22 trapping sites were established along the Rio Grande Bosque in Bernalillo County. Two types of mosquito traps were used at each site using a procedure that has been previously described (Greenberg et al. 2012). Collected mosquitoes were immediately placed on dry ice and were subsequently stored at −80°C. Mosquitoes were identified to species using dichotomous keys (Carpenter and LaCasse 1955, Pratt and Barnes 1959, Darsie and Ward 1981). Blood-engorged mosquitoes were set aside for bloodmeal analysis. The relative abundance of each mosquito species was calculated by dividing the number of each species collected by the combined total of collected mosquitoes for each collection season.
Bloodmeal Analysis
The abdomens of blood-engorged mosquitoes were removed using microscope slides and sterile razor blades under a dissecting microscope. Genomic DNA from the bloodmeal of each mosquito was subsequently isolated using a DNAzol BD (Molecular Research Center, Cincinnati, OH) procedure modified as described by Molaei et al. (2006).
The source of each bloodmeal was determined by subjecting each sample of genomic DNA to two separate polymerase chain reactions (PCRs), one to identify mammalian DNA and one to identify avian DNA. Mammalian bloodmeals were identified using mammalian-specific primer pairs for a 772-bp portion of the cytochrome bgene (Ngo and Kramer 2003). Likewise, avian bloodmeals were identified by amplifying a 508-bp fragment of the cytochrome bgene with avian-specific primers (Cisneros and Johnson 2001). The 50-μl reactions were carried out using a PCR protocol and purification process previously described (Molaei et al. 2007, Greenberg et al. 2012). Thermocycling conditions have been previously published (Greenberg et al. 2012). A negative water control, lacking template, was included with all PCR reactions. A second negative control consisted of a DNAzol extract lacking mosquito midgut. All reactions also included a positive control containing either mammalian (Mus musculus) or avian (Zenaida macroura) genomic DNA serving as a template. If a sample did not amplify, a second PCR reaction was performed. After two failed amplifications, the sample was excluded from further analysis.
Amplicons were prepared for direct sequencing with a Big Dye 3.1 sequencing kit (Invitrogen, Carlsbad, CA) using the big dye step protocol PCR regimen (Platt et al. 2007). Samples were then sequenced on an ABI 3130 DNA Sequencer (Applied Biosystems, Foster City, CA) at the University of New Mexico, Department of Biology Molecular Facility. Sequences were edited using Sequencher version 4.10.1 (Gene Codes, Ann Arbor, MI) and identified to species through a BLAST search comparison with the GenBank DNA database (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Comparisons with an error value <1e–20 were included in our analysis.
Statistical Analysis
Chi-squared tests were used to determine if any of our mosquito species displayed a statistically significant shift in their feeding behavior during the mosquito season. We designated the early season as before 15 July and late season as 15 July onward. This date was selected because it represents the approximate midpoint in the mosquito season. We compared the proportion of mammalian bloodmeals with avian bloodmeals found in mosquitoes for each half of the season. Bloodmeals from all years were pooled for analysis. Tests were completed in JMP 9.0 (SAS Institute Inc., 2013).
Results
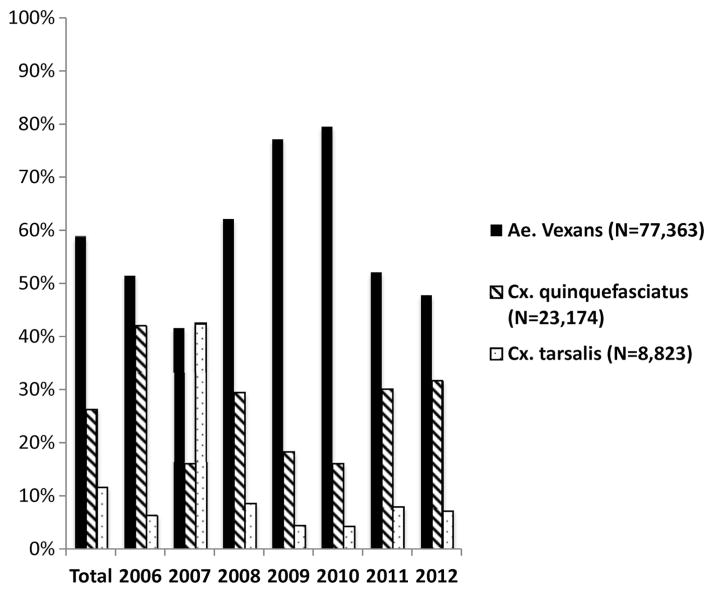
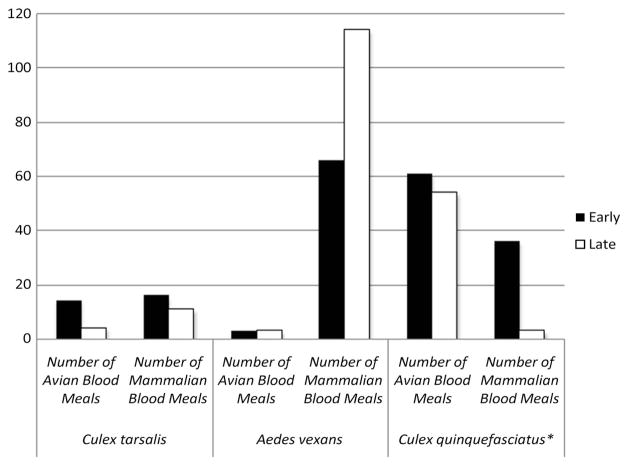
In total, 118,696 mosquitoes were collected from 2006 to 2012. Of these mosquitoes, 77,363 (65.2%) were Ae. vexans, 23,174 (19.5%) were Cx. quinquefasciatus, 8,823 (7.4%) were Cx. tarsalis (Fig. 1), and 9,376 (7.9%) were other mosquito species. Four hundred fifty-two of the mosquitoes were blood-engorged females belonging to one of the three species under investigation. A total of 385 (82.5%) of these bloodmeals were identified to species (Ae. vexans, n = 186; Cx. quinquefasciatus, n = 154; Cx. tarsalis, n = 45). Ae. vexans fed primarily on mammalian hosts throughout the mosquito season, and no significant shift in feeding behavior was detected (χ21 = 0.43, n = 186, P > 0.05; Fig. 2). Only 3 of 69 (4.3%) early season bloodmeals came from avian hosts. During the late season, 3 of 117 (2.6%) bloodmeals were identified as avian. Although Cx. quinquefasciatus feeds primarily on birds throughout the mosquito season, this trend was significantly more pronounced late in the season. Sixty-one of 97 (62.9%) of its early season bloodmeals were from avian hosts. This increased to 54 of 57 (94.7%) in the late season (χ21 = 22.83, n = 154, P < 0.05; Fig. 2). Host utilization patterns by Cx. tarsalis did not differ significantly between the early and late seasons. Fourteen of 30 (46.7%) and 4 of 15 (26.7%) Cx. tarsalis bloodmeals were of avian origin during the early and late seasons, respectively (χ21 = 1.72, n = 45, P > 0.05; Fig. 2).
Fig. 1.
The number of Ae. vexans, Cx. quinquefasciatus, and Cx. tarsalis was divided by the total number of identified mosquitoes of all species to determine the percentage of trapped mosquitoes of each of the three species under investigation.
Fig. 2.
Seasonal feeding behavior reported as number of bloodmeals. The total numbers of early and late season bloodmeals from each species is reported. A midpoint of 15 July was used to designate early bloodmeals from late. (*Denotes significant difference in feeding between early and late season.) Ae. vexans and Cx. tarsalis did not shift significantly in their feeding behavior from early to late season.
Discussion
We found that Ae. vexans did not display a significant seasonal shift in feeding behavior and fed almost exclusively on mammals throughout the mosquito season (χ21 = 0.43, P > 0.05). Likewise, no significant alteration in feeding behavior was detected for Cx. tarsalis (χ21 = 1.72, P > 0.05). Alternatively, Cx. quinquefasciatus is significantly more likely to feed on avian hosts as the mosquito season progresses (χ21 = 22.83, P < 0.05).
Our results for Ae. vexans are consistent with those observed elsewhere (Burkot and DeFoliart 1982, Washino and Tempelis 1983, Nasci 1984, Molaei et al. 2006). A shift to a more mammalian-based host utilization pattern has been reported for Cx. tarsalis elsewhere in its range (Kent et al. 2007). Although our results do not suggest such a shift in central New Mexico, small sample sizes, owing to its relative scarcity in our collections, preclude a definitive statement in this regard. In contrast, the feeding behavior of Cx. quinquefasciatus appears to differ from that reported from other locations, where it feeds increasingly on mammalian hosts later in the mosquito season (Tempelis and Washino 1967, Molaei et al. 2007). This shift to mammals has often been attributed to changing host availability over the mosquito season (Zinser et al. 2004, Molaei et al. 2007). Mosquito habitat preference and feeding behavior have also been shown to change in response to environmental factors such as drought (Shaman et al. 2002, Chase and Knight, 2003). Other characteristics of annual weather patterns may also be important factors. For example, in Alabama, it was shown that the severity of the preceding winter can be used to predict the likelihood and the specific timing of a seasonal shift from avian to mammalian hosts by Cx. erratius (Burkett-Cadena et al. 2008). Such a shift may also be, in part, attributable to the relative ease of feeding on nestlings in the early season, before newborn birds are fully fledged. At present, we are unable to account for the different feeding behavior that we observed for Cx. quinquefasciatus in central New Mexico. It is worth noting that 15 July, our selected transition point between the early and late mosquito seasons, corresponds closely to the accepted date for the onset of the summer monsoon season in Bernalillo County (Higgins et al. 1999, Ellis et al. 2004). Whether the inception of these summer rains alters local host availability awaits clarification.
Our results may provide insight into the dynamics of local WNV transmission. Only birds maintain a sufficiently high viremia to infect feeding mosquitoes (Hunt et al. 2002, Harrier et al. 2008). Although they can develop significant pathology, mammals are dead-end hosts; mosquitoes feeding on infected mammals do not themselves become infected. Although Ae. vexans has been assessed as a competent WNV vector (Turell et al. 2001, Kilpatrick et al. 2005), it is unlikely to play an important role in WNV transmission, as it rarely feeds on birds. This is confirmed by the low WNV infection rate among Ae. vexans relative to the two Culex species (Kulasekera et al. 2001, Greenberg et al. 2013). Cx. quinquefasciatus, which we found to feed increasingly on birds, may play a crucial role in viral amplification in the enzootic transmission cycle involving avian WNV reservoirs. Later in the season, Cx. tarsalis, which is more likely to feed on mammalian hosts, may serve as the primary bridge vector to humans and other mammals. Although our data did not reveal a significant seasonal shift to mammalian hosts for this species, Cx. tarsalis appears more likely to feed on mammals at any time in the mosquito season than does Cx. quinquefasciatus. This would be especially true late in the season, when Cx. quinquefasciatus shifts increasingly to avian hosts. It is worth noting that Cx. tarsalis accounted for three times the number of late-season mammalian bloodmeals compared with Cx. quinquefasciatus (see Fig. 2). This is despite the relative scarcity of Cx. tarsalis in our collections (see Fig. 1). These data suggest that while Cx. quinquefasciatus is the principal vector responsible for viral amplification in birds, Cx. tarsalis may function as the primary bridge vector, especially in August and September, when the majority of human WNV cases are reported (Eidson et al. 2001, Watson et al. 2004, Rizzo et al. 2009).
Acknowledgments
We thank Goudarz Molaei for his technical help. Laboratory space was provided by Robert Miller at the University of New Mexico. This project was supported in part by a grant from the National Institute of General Medical Sciences award number T34GM00851. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institute of Health. Additional support was provided by the New Mexico Horse Council. Mosquito surveillance was funded in part by the Centers for Disease Control and Prevention funds administered through the New Mexico Department of Health and the taxpayers of Bernalillo County and the City of Albuquerque. Support was also provided by the UNM Center for Evolutionary and Theoretical Immunology (CETI) and by the UNM Molecular Biology Facility, supported by National Institutes of Health grant 1P20RR18754 from the Institute Development Award Program of the National Center for Research Resources.
References Cited
- Andreadis TG, Anderson JF, Vossbrinck CR. Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, and Culiseta melanura. Emerg Infect Dis. 2001;7:670–679. doi: 10.3201/eid0704.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperson CS, Harrison BA, Unnasch TR, Hassan K, Irby WS, Savage HM, Aspen SE, Wesley D, Rueda LM, Engber BR, et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;8:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Burkett-Cadena ND, Graham SP, Hassan HK, Guyer C, Eubanks MD, Katholi CR, Unnasch TR. Blood feeding patterns of potential arbovirus vectors of the genus Culex targeting ectothermic hosts. Am J Trop Med Hyg. 2008;79:809–815. [PMC free article] [PubMed] [Google Scholar]
- Burkot TR, DeFoliart GR. Bloodmeal sources of Aedes triseriatus and Aedes vexans in a southern Wisconsin forest endemic for La Crosse Encephalitis virus. Am J Trop Med Hyg. 1982;31:376–383. doi: 10.4269/ajtmh.1982.31.376. [DOI] [PubMed] [Google Scholar]
- Carpenter SJ, LaCasse WJ. Mosquitoes of North America. Vol. 360. Berkeley. CA: UC Press; 1955. pp. 1–127. [Google Scholar]
- Chase JM, Knight TM. Drought-induced mosquito outbreaks in wetlands. Ecol Lett. 2003;6:1017–1024. [Google Scholar]
- City of Albuquerque Census. Welcome to the City of Albuquerque. 2010 ( http://www.cabq.gov/)
- Cupp EW, Tennessen KJ, Oldland WK, Hassan HK, Hill GE, Katholi CR, Unnasch TR. Mosquito and arbovirus activity during 1997–2002 in a wetland in northeastern Mississippi. J Med Entomol. 2004;41:495–501. doi: 10.1603/0022-2585-41.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. American Mosquito Control Association; Fresno, CA: 1981. [Google Scholar]
- DiMenna MA, Bueno R, Jr, Parmenter RR, Norris DE, Sheyka JM, Molina JL, Glass GE. Comparison of mosquito trapping method efficacy for West Nile virus surveillance in New Mexico. J Am Mosquito Control Assoc. 2006a;22:246–253. doi: 10.2987/8756-971x(2006)22[246:comtme]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMenna MA, Bueno R, Jr, Parmenter RR, Norris DE, Sheyka JM, Molina JL, Glass GE. Emergence of West Nile virus in mosquito (Diptera: Culicidae) communities of the New Mexico Rio Grande Valley. J Med Entomol. 2006b;43:594–599. doi: 10.1603/0022-2585(2006)43[594:eownvi]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMenna MA, Bueno R, Jr, Parmenter RR, Norris DE, Sheyka JM, Molina JL, Glass GE. Urban habitat evaluation for West Nile virus surveillance in mosquitoes in Albuquerque, New Mexico. J Am Mosq Control Assoc. 2007;23:153–160. doi: 10.2987/8756-971x(2007)23[153:uhefwn]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman JD, Taylor DJ. Culex nigripalpus: seasonal shift in the bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science. 1968;161:67–68. doi: 10.1126/science.161.3836.67. [DOI] [PubMed] [Google Scholar]
- Edman JD, Webber LA, Schmid AA. Effect of host defenses on the feeding pattern of Culex nigripalpus when offered a choice of blood sources. J Parasit. 1974;60:874–883. [PubMed] [Google Scholar]
- Ellis AW, Saffell EM, Hawkins TW. A method for defining monsoon onset and demise in the southwestern USA. Int J Climatol. 2004;24:247–265. [Google Scholar]
- Eidson M, Komar N, Sorhage F, Nelson R, Talbot T, Mostashari F, McLean R. Crow deaths as a sentinel surveillance system for West Nile virus in the northeastern United States, 1999. Emerg Infect Dis. 2001;7:61–66. doi: 10.3201/eid0704.010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rejon JE, Blitvich BJ, Farfan-Ale JA, Loroño-Pino MA, Chi Chim WA, Flores-Flores LF, Beaty BJ. Host-feeding preference of the mosquito, Culex quinquefasciatus, in Yucatan State, Mexico. J Insect Sci. 2010;10:32. doi: 10.1673/031.010.3201. ( insectscience.org/10.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JA, DiMenna MA, Hanelt B, Hofkin BV. Analysis of post-blood meal flight distances in mosquitoes utilizing zoo animal blood meals. J Vector Ecol. 2012;37:83–89. doi: 10.1111/j.1948-7134.2012.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JA, Lujan DA, DiMenna MA, Wearing HJ, Hofkin BV. Identification of blood meal sources in Aedes vexans and Culex quinquefasciatus in Bernalillo County, New Mexico. J Insect Sci. 2013;13:75. doi: 10.1673/031.013.7501. ( insetscience.org/13.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins RW, Chen Y, Douglas AV. Interannual variability of the North American warm season precipitation regime. J Climate. 1999;12:653–680. [Google Scholar]
- Kent R, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal blood-feeding behavior of Culextarsalis (Diptera: Culicidae) in Weld county, Colorado, 2007. J Med Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- Kent RJ, Harrington LC, Norris DE. Genetic differences between Culex pipiens f. molestus and Culex pipiens pipiens (Diptera: Culicidae) in New York. J Med Entomol. 2007;44:50–59. doi: 10.1603/0022-2585(2007)44[50:gdbcpf]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis. 2005;11:425–434. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:82–90. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasekera VL, Kramer L, Nasci RS, Mostashari F, Cherry B, Trock SC, Miller JR. West Nile virus infection in mosquitoes, birds, horses, and humans, Staten Island, New York, 2000. Emerg Infect Dis. 2001;7:722–731. doi: 10.3201/eid0704.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG. Identification of avian-and mammalian-derived bloodmeals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile Virus transmission in Connecticut, USA. J Med Entomol. 2006;43:1088–1093. doi: 10.1603/0022-2585(2006)43[1088:IOAAMB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. Host Feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Bueno R, Jr, Dennett JA, Real SV, Tesh RB. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile Virus in Harris County, Texas. Am J Trop Med Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Bueno R, Jr, Diuk-Wasser M. Host-feeding patterns of potential mosquito vectors in Connecticut, USA: molecular analysis of bloodmeals from 23 species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. J Med Entomol. 2008;45:1143–1151. doi: 10.1603/0022-2585(2008)45[1143:hpopmv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Molaei G, Cummings RF, Su T, Armstrong PM, Williams GA, Cheng ML, Andreadis TG. Vector-host interactions governing epidemiology of West Nile Virus in Southern California. Am J Trop Med Hyg. 2010;83:1269–1277. doi: 10.4269/ajtmh.2010.10-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasci RS. Variations in the blood-feeding patterns of Aedes vexans and Aedes trivittatus (Diptera: Culicidae) J Med Entomol. 1984;21:95–99. doi: 10.1093/jmedent/21.1.95. [DOI] [PubMed] [Google Scholar]
- Platt AR, Woodhall RW, George AL. Improved DNA sequencing quality and efficiency using an optimized fast cycle sequencing protocol. Biotechniques. 2007;43:58–60. doi: 10.2144/000112499. [DOI] [PubMed] [Google Scholar]
- Pratt HD, Barnes RC. CDC training guide. US Department of Health, Education and Welfare. Public Health Service; Atlanta, GA: 1959. Identification keys for common mosquitoes of United States. [Google Scholar]
- Rizzo C, Vescio F, Declich S, Finarelli AC, Macini P, Mattivi A, Rezza G. West Nile virus transmission with human cases in Italy, August–September 2009. Euro Surveill. 2009;14:40–47. [PubMed] [Google Scholar]
- Samuel PP, Arunachalam N, Hiriyan J, Thenmozhi V, Gajanana A, Satyanarayana K. Host-feeding pattern of Culex quinquefasciatus Say and Mansonia annulifera (Theobald) (Diptera: Culicidae), the major vectors of filariasis in a rural area of south India. J Med Entomol. 2004;41:442–446. doi: 10.1603/0022-2585-41.3.442. [DOI] [PubMed] [Google Scholar]
- SAS Institute. JMP version 9.0. SAS Institute; Cary, NC: 2013. [Google Scholar]
- Shaman J, Day FJ, Stieglitz M. Drought-induced amplification of St. Louis encephalitis virus, Florida. Emerg Infec Dis. 2002;8:575–580. doi: 10.3201/eid0806.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempelis GH, Washino RK. Host-feeding patterns of Culex tarsalis in the Sacramento Valley, California, with notes on other species. J Med Entomol. 1967;4:315–318. doi: 10.1093/jmedent/4.3.315. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Washino RK, Tempelis CH. Mosquito host bloodmeal identification: methodology and data analysis. Annu Rev Entomol. 1983;28:179–201. doi: 10.1146/annurev.en.28.010183.001143. [DOI] [PubMed] [Google Scholar]
- Watson JT, Jones RC, Gibbs K, Paul W. Dead crow reports and location of human West Nile virus cases, Chicago, 2002. Emerg Infect Dis. 2004;10:938–944. doi: 10.3201/eid1005.030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff TA, Nielsen LT. The Mosquitoes of New Mexico. UNM Press; Albuquerque, NM: 2007. [Google Scholar]
- Zinser M, Ramberg F, Willott E. Culex quinquefasciatus(Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: blood meal analysis indicates feeding on both humans and birds. J Insect Sci. 2004;4:20. doi: 10.1093/jis/4.1.20. ( insectscience.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]