Abstract
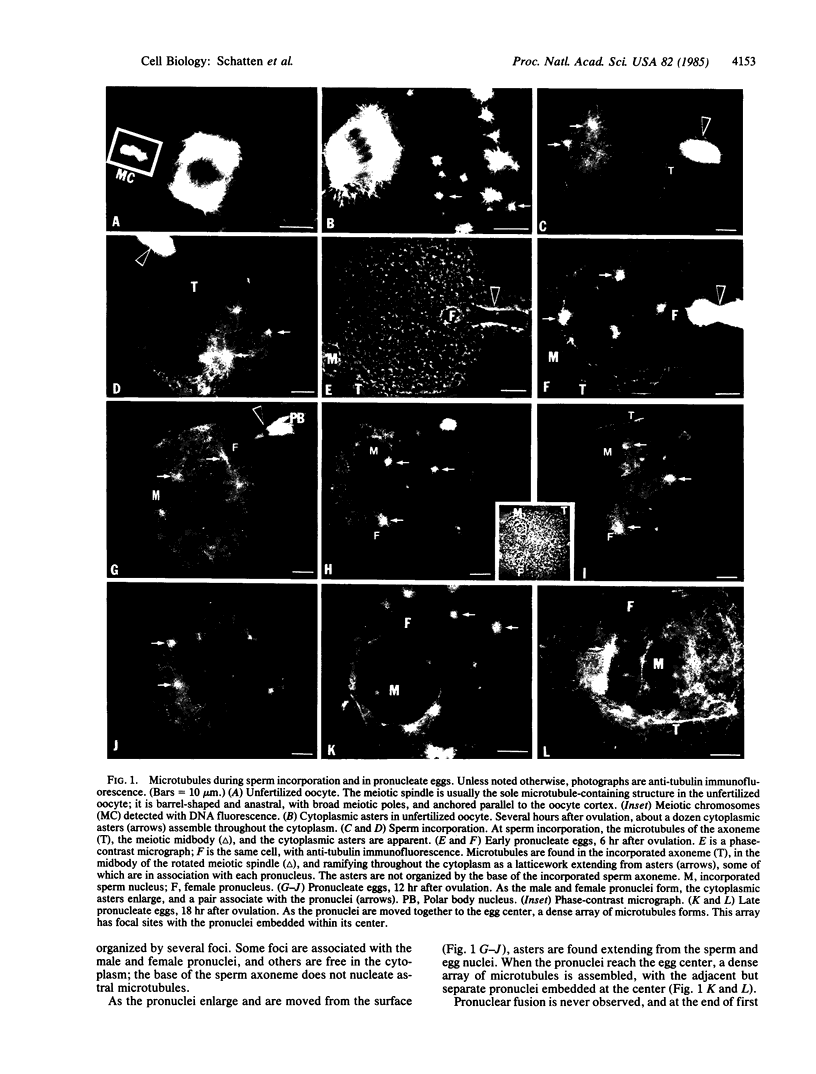
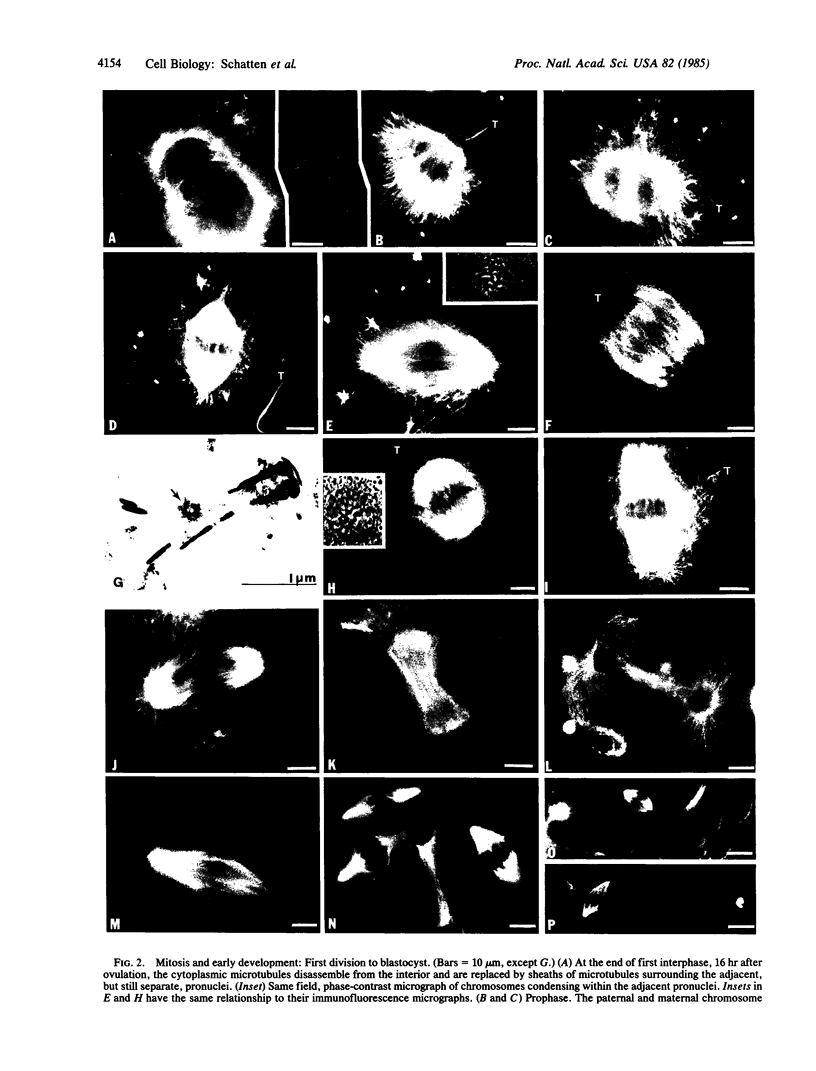
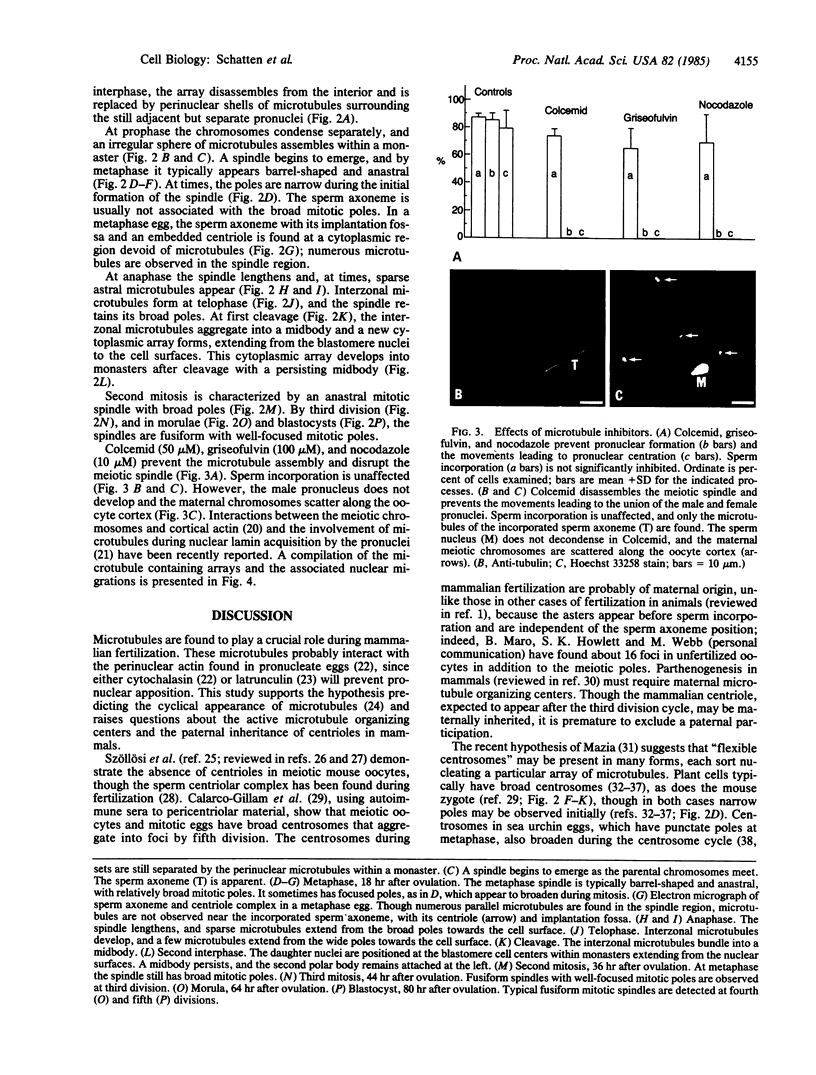
Microtubules forming within the mouse egg during fertilization are required for the movements leading to the union of the sperm and egg nuclei (male and female pronuclei, respectively). In the unfertilized oocyte, microtubules are predominantly found in the arrested meiotic spindle. At the time for sperm incorporation, a dozen cytoplasmic asters assemble, often associated with the pronuclei. As the pronuclei move to the egg center, these asters enlarge into a dense array. At the end of first interphase, the dense array disassembles and is replaced by sheaths of microtubules surrounding the adjacent pronuclei. Syngamy (pronuclear fusion) is not observed; rather the adjacent paternal and maternal chromosome sets first meet at metaphase. The mitotic apparatus emerges from these perinuclear microtubules and is barrel-shaped and anastral, reminiscent of plant cell spindles; the sperm centriole does not nucleate mitotic microtubules. After cleavage, monasters extend from each blastomere nucleus. The second division mitotic spindles also have broad poles, though by third and later divisions the spindles are typical for higher animals, with narrow mitotic poles and fusiform shapes. Colcemid, griseofulvin, and nocodazole inhibit the microtubule formation and prevent the movements leading to pronuclear union; the meiotic spindle is disassembled, and the maternal chromosomes are scattered throughout the oocyte cortex. These results indicate that microtubules forming within fertilized mouse oocytes are required for the union of the sperm and egg nuclei and raise questions about the paternal inheritance of centrioles in mammals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E., Hoppe P. C., Whitten W. K., Lee G. S. In vitro fertilization and early embryogenesis: a cytological analysis. J Ultrastruct Res. 1975 Feb;50(2):231–252. doi: 10.1016/s0022-5320(75)80054-2. [DOI] [PubMed] [Google Scholar]
- Bajer A. S., Molè-Bajer J. Asters, poles, and transport properties within spindlelike microtubule arrays. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):263–283. doi: 10.1101/sqb.1982.046.01.029. [DOI] [PubMed] [Google Scholar]
- Bavister B. D., Leibfried M. L., Lieberman G. Development of preimplantation embryos of the golden hamster in a defined culture medium. Biol Reprod. 1983 Feb;28(1):235–247. doi: 10.1095/biolreprod28.1.235. [DOI] [PubMed] [Google Scholar]
- Bershadsky A. D., Gelfand V. I., Svitkina T. M., Tint I. S. Microtubules in mouse embryo fibroblasts extracted with Triton X-100. Cell Biol Int Rep. 1978 Sep;2(5):425–432. doi: 10.1016/0309-1651(78)90093-0. [DOI] [PubMed] [Google Scholar]
- Bestor T. H., Schatten G. Anti-tubulin immunofluorescence microscopy of microtubules present during the pronuclear movement of sea urchin fertilization. Dev Biol. 1981 Nov;88(1):80–91. doi: 10.1016/0012-1606(81)90220-7. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Fistel S. H., Marcum J. M., Pardue R. L. Microtubules in cultured cells; indirect immunofluorescent staining with tubulin antibody. Int Rev Cytol. 1980;63:59–95. doi: 10.1016/s0074-7696(08)61757-x. [DOI] [PubMed] [Google Scholar]
- Calarco-Gillam P. D., Siebert M. C., Hubble R., Mitchison T., Kirschner M. Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell. 1983 Dec;35(3 Pt 2):621–629. doi: 10.1016/0092-8674(83)90094-6. [DOI] [PubMed] [Google Scholar]
- De Mey J., Lambert A. M., Bajer A. S., Moeremans M., De Brabander M. Visualization of microtubules in interphase and mitotic plant cells of Haemanthus endosperm with the immuno-gold staining method. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1898–1902. doi: 10.1073/pnas.79.6.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS R. G. Colchicine-induced heteroploidy in the mouse. I. The induction of triploidy by treatment of the gametes. J Exp Zool. 1958 Mar;137(2):317–347. doi: 10.1002/jez.1401370206. [DOI] [PubMed] [Google Scholar]
- Florman H. M., Bechtol K. B., Wassarman P. M. Enzymatic dissection of the functions of the mouse egg's receptor for sperm. Dev Biol. 1984 Nov;106(1):243–255. doi: 10.1016/0012-1606(84)90079-4. [DOI] [PubMed] [Google Scholar]
- Gondos B., Bhiraleus P., Conner L. A. Pronuclear membrane alterations during approximation of pronuclei and initiation of cleavage in the rabbit. J Cell Sci. 1972 Jan;10(1):61–78. doi: 10.1242/jcs.10.1.61. [DOI] [PubMed] [Google Scholar]
- Karsenti E., Newport J., Hubble R., Kirschner M. Interconversion of metaphase and interphase microtubule arrays, as studied by the injection of centrosomes and nuclei into Xenopus eggs. J Cell Biol. 1984 May;98(5):1730–1745. doi: 10.1083/jcb.98.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo F. J., Anderson E. Cytological events leading to the formation of the two-cell stage in the rabbit: association of the maternally and paternally derived genomes. J Ultrastruct Res. 1969 Oct;29(1):86–118. doi: 10.1016/s0022-5320(69)80058-4. [DOI] [PubMed] [Google Scholar]
- Longo F. J. Sperm aster in rabbit zygotes: its structure and function. J Cell Biol. 1976 Jun;69(3):539–547. doi: 10.1083/jcb.69.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro B., Johnson M. H., Pickering S. J., Flach G. Changes in actin distribution during fertilization of the mouse egg. J Embryol Exp Morphol. 1984 Jun;81:211–237. [PubMed] [Google Scholar]
- Mazia D. Centrosomes and mitotic poles. Exp Cell Res. 1984 Jul;153(1):1–15. doi: 10.1016/0014-4827(84)90442-7. [DOI] [PubMed] [Google Scholar]
- Mazia D., Schatten G., Sale W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol. 1975 Jul;66(1):198–200. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paweletz N., Mazia D., Finze E. M. The centrosome cycle in the mitotic cycle of sea urchin eggs. Exp Cell Res. 1984 May;152(1):47–65. doi: 10.1016/0014-4827(84)90229-5. [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps J. D., Northcote D. H. Organization of microtubules and endoplasmic reticulum during mitosis and cytokinesis in wheat meristems. J Cell Sci. 1966 Mar;1(1):109–120. doi: 10.1242/jcs.1.1.109. [DOI] [PubMed] [Google Scholar]
- Schatten G. Motility during fertilization. Int Rev Cytol. 1982;79:59–163. doi: 10.1016/s0074-7696(08)61673-3. [DOI] [PubMed] [Google Scholar]
- Szollosi D., Calarco P., Donahue R. P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci. 1972 Sep;11(2):521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Fujiwara K. Immunofluorescent anti-tubulin staining of spindles during meiotic maturation of mouse oocytes in vitro. J Cell Sci. 1978 Feb;29:171–188. doi: 10.1242/jcs.29.1.171. [DOI] [PubMed] [Google Scholar]
- Whittingham D. G. Fertilization of mouse eggs in vitro. Nature. 1968 Nov 9;220(5167):592–593. doi: 10.1038/220592a0. [DOI] [PubMed] [Google Scholar]
- Wick S. M., Seagull R. W., Osborn M., Weber K., Gunning B. E. Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol. 1981 Jun;89(3):685–690. doi: 10.1083/jcb.89.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R., Noda Y. D. Ultrastructural changes in the hamster sperm head during fertilization. J Ultrastruct Res. 1970 Jun;31(5-6):465–485. doi: 10.1016/s0022-5320(70)90163-2. [DOI] [PubMed] [Google Scholar]