Abstract
The identification of an infectious or noninfectious uveitis syndrome is important to determine the range of therapeutic and prognostic implications of that disease entity. Diagnostic dilemmas arise with atypical history, atypical clinical presentations, inconclusive diagnostic workup, and persistent or worsened inflammation despite appropriate immunosuppression. More invasive intraocular testing is indicated in these situations particularly in infectious uveitis where a delay in treatment may result in worsening of the patient’s disease and a poor visual outcome. Laboratory analysis of vitreous fluid via diagnostic pars plana vitrectomy is an important technique in the diagnostic armamentarium, but the most important aspects of sample collection include rapid processing, close coordination with an ophthalmic pathology laboratory, and directed testing on this limited collected sample. Culture and staining has utility in bacterial, fungal, and nocardial infection. Polymerase chain reaction (PCR) analysis has shown promising results for bacterial endophthalmitis and infection with mycobacterium tuberculosis whereas PCR testing for viral retinitides and ocular toxoplasmosis has a more established role. Antibody testing is appropriate for toxoplasmosis and toxocariasis, and may be complementary to PCR for viral retinitis. Masquerade syndromes represent neoplastic conditions that clinically appear as infectious or inflammatory conditions and should be considered as part of the differential diagnosis. Diagnostic vitrectomy and chorioretinal biopsy are thus critical tools for the management of patients in whom an infectious etiology of uveitis is suspected.
Introduction
Identifying the etiology of an infectious or noninfectious uveitis syndrome is important for the clinician and patient because of the range of therapeutic and prognostic implications for each disease entity. For the majority of uveitis syndromes, a diagnosis can be made with a combination of history, clinical examination, laboratory and radiologic testing. Diagnostic dilemmas may arise however, when discrepancies are observed in three specific settings – an atypical history, atypical clinical presentation, or an inconclusive diagnostic workup that has implications from a therapy standpoint. The dilemma is further compounded when intraocular inflammation persists or worsens after seemingly appropriate local or systemic immunosuppression, which may then raise concerns for an infectious or neoplastic etiology. In these situations, diagnostic vitrectomy may greatly assist in the diagnosis and guide alternative management strategies.
Experience in the literature for diagnostic pars plana vitrectomy (PPV) has reported overall yields ranging from 12.4% to 64.3%.1–12 In analysis of these case series (Table 1), the yield for diagnostic vitrectomy resulting in a final diagnosis of infectious uveitis in patients treated clinically for infectious uveitis ranged from 27.9% to 77.1%.3, 6, 9, 10 Differences in reported yields have been attributed to patient selection with higher diagnostic yields reported when there is higher clinical suspicion for infection or lymphoma (i.e. higher pre-test probability). Irrespective of the variability in reported yields, diagnostic vitrectomy remains a mainstay in the diagnosis and ultimate management of diagnostic dilemmas in patients with intermediate, posterior and panuveitis.
Table 1.
Diagnostic Vitrectomy Yield from Large Series
| Author | Reference | Overall Yield | Yield for Infectious Conditions* | ||
|---|---|---|---|---|---|
| n | Percentage | n | Percentage | ||
| Carroll et al | 1 | 2/8 | 25 | -- | -- |
| Priem et al | 2 | 10/34 | 29.4 | -- | -- |
| Palexas et al | 3 | 60/215 | 27.9 | 60/215 | 27.9 |
| Verbraeken et al | 4 | 9/28 | 32.1 | -- | -- |
| Akpek et al | 5 | 11/34 | 32.4 | -- | -- |
| Mruthyunjaya et al | 6 | 35/90 | 38.9 | 27/35 | 77.1 |
| Coupland et al | 7 | 12/84 | 14.2 | -- | -- |
| Zaldivar et al | 8 | 9/14 | 64.3 | -- | -- |
| Davis et al | 9 | 48/78 | 61.5 | 34/50 | 68 |
| Margolis et al | 10 | 9/45 | 20 | 2/6 | 33.3 |
| Wittenberg et al | 11 | 126/228 | 55.3 | -- | -- |
| Oahalou et al | 12 | 18/84 | 21.4 | -- | -- |
Yield for infectious conditions calculated only in case series reporting final diagnoses of infectious uveitis.
Technique
Initial Evaluation and Indications for Diagnostic Vitrectomy
Approaching a patient with uveitis requires a comprehensive medical and ophthalmic history. The history of present illness, past medical and social history should be directed at identification of key risk factors and pertinent positives and negatives.
Similarly, the ophthalmic examination should focus on key findings such as laterality, location of the uveitic process per the Standardization of Uveitis Nomenclature classification (SUN) classification system,13 the clinical appearance, and associated ocular and systemic exam findings.
Further systemic evaluation with laboratory testing or imaging should be focused and directed based on the formulated differential diagnosis. In addition, increased pre-test probability of a disease syndrome has been shown to increase the positive predictive value of a disease syndrome when a positive test is identified.14
Indications for more invasive ocular testing including diagnostic vitrectomy arise in the setting of diagnostic dilemmas, particularly in diseases with an acute time course where a delay in diagnosis could worsen the patient’s visual outcome. One situation commonly encountered arises when a patient fails to respond to conventional local or systemic immunosuppressive treatment. Additional indications include significant vitreous inflammation concerning for infectious endophthalmitis, malignancy, or retained foreign body.6, 10, 15 The ultimate goal of intraocular testing is to obtain a diagnosis that may guide or change the course of therapy.
Diagnostic Pars Plana Vitrectomy Technique
Various techniques have been reported to obtain vitreous samples including 20-, 23-, and 25-gauge PPV.1, 5, 6, 8–10, 12 More recently, 27-gauge cutters have been introduced and will likely provide another option for diagnostic PPV. In general, a standard three-port PPV with a wide-angle viewing system allows for adequate visualization to safely harvest a vitreous sample. The phakic or pseudophakic status must be considered prior to surgery, as vitreous debris, hemorrhage, or inflammatory cells may collect on the posterior surface of the lens capsule obscuring the view of posterior segment structures. Poor visualization of the vitreous cavity and retina may ultimately limit the amount of core and peripheral vitrectomy that may be performed and may preclude the identification of iatrogenic retinal breaks. For this reason, it is prudent to harvest the amount of vitreous necessary for laboratory testing, evaluate the retina for any overt pathology and/or iatrogenic injury and instill antibiotics if indicated. Meticulous peripheral vitreous base dissection in the presence of significant media opacity (e.g. corneal edema, lens opacity) is fraught with potential complications and should generally be avoided.
To maximize the diagnostic yield, direct visualization is preferred to ensure that the vitreous cutter remains in the mid-vitreous cavity, avoiding the crystalline lens in phakic patients, and to ensure that the vitreous hand piece is removing vitreous versus infusion fluid.
Specifically, an undiluted vitreous sample is obtained using a 3mL syringe attached to the vitreous cutter. A three-way stopcock closed to the aspiration line can facilitate ease of collection of an undiluted specimen. The use of a three-way stopcock allows the assistant to immediately switch to vacuum aspiration from the machine (vs. manual aspiration). Approximately 1–2 mL of undiluted vitreous specimen may be obtained with this method although careful monitoring of the peripheral retina is needed for choroidal formation, particularly as the intraocular pressure decreases when vitreous is removed.5, 8–10 During collection, some authors propose maintaining the infusion on air or providing digital pressure to the eye wall to maintain steady intraocular pressure.12 Turning the infusion to air is less problematic in pseudophakic patients as removal of air bubbles from the peripheral anterior hyaloid risks injury to the crystalline lens in phakic patients.
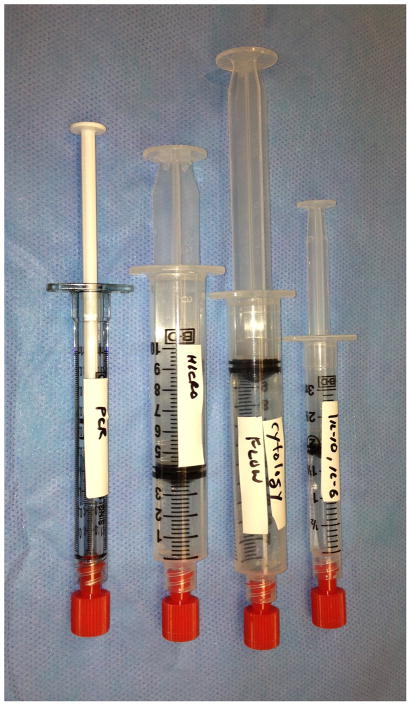
Vitrector speed does not appear to degrade the quality of the sample obtained.16, 17 After the undiluted specimen is collected, fluid infusion is initiated and a second syringe is placed on the vitreous cutter to collect 3–10 mL of a diluted vitreous sample.4, 6, 10 The remaining core vitrectomy, induction of a posterior vitreous detachment, and peripheral vitrectomy may then be performed using a standard approach.8, 12 The vitrectomy cassette may also be sent for laboratory analysis. If there is significant vitreous debris within the mid-peripheral vitreous, sequential 3-, 5- or 10-cc syringes may be used to collect multiple specimens, which still have a high concentration of material for laboratory analysis (Figure 1).
Figure 1.
Diagnostic vitrectomy specimens. Following harvesting a vitreous specimen, diagnostic vitrectomy specimens should be labeled clearly for distribution to molecular diagnostics laboratory (PCR and IL-10/IL-6), microbiology laboratory (MICRO), or ophthalmic pathology laboratory (cytology, flow cytometry). Syringes of varying sizes (1-, 3-, 5- or 10-cc syringes) are used depending on the quantity required from each department conducting the necessary testing.
The undiluted and diluted samples should be sent immediately after collection at room temperature to the ophthalmic pathology laboratory for immediate processing although this may vary based on laboratory preferences.5, 8, 12 Close coordination and discussion with laboratory services is important to ensure proper specimen handling. Details of sample processing in the laboratory have been previously described.8 The undiluted vitreous sample is typically sent for cytological analysis with either Papanicolaou or Hematoxylin-eosin staining and immunohistochemical staining.5, 6, 8–10 The supernatant of the undiluted sample is sent for cytokine analysis and antibody levels.4, 9 If a quantitative polymerase chain reaction (PCR) test or vitreous proteomic or cytokine evaluation is needed, an undiluted vitreous specimen provides the most accurate method of obtaining a DNA concentration. The diluted vitreous sample is typically used for gram stain, culture, flow cytometry, and PCR testing.8, 9 Cytologic analysis may also be performed with the diluted specimen after centrifugation by a pathology laboratory.
Chorioretinal Biopsy
Chorioretinal biopsy is a surgical consideration in patients with choroidal or retinal disease, but confers a greater risk of iatrogenic morbidity including subretinal hemorrhage, vitreous hemorrhage, and retinal detachment.18, 19 A transscleral approach19–21 was first performed for suspected choroidal malignancy prior to advances in intraocular vitreoretinal surgery, but this technique is used less frequently due to risk of choroidal hemorrhage and extrascleral extension of tumor.22, 23 Use of a pars plana vitrectomy approach has gained favor due to a lower side effect profile.19, 24
Indications for chorioretinal biopsy include a non-revealing workup that may include prior diagnostic PPV, a disease process primarily limited to the choroid and retina with minimal vitreous inflammation, exclusion of malignancy not diagnosed with less invasive means, and progressive macula or vision-threatening process unresponsive to therapy.19, 22, 24
To perform a chorioretinal biopsy, 20- or 23-gauge three-port PPV have been described previously; undiluted and diluted vitreous samples are collected first prior to the chorioretinal biopsy.18, 19, 22–26 Vitreous separation over the biopsy site is performed (with preference for a superotemporal location if possible to assist with post-operative tamponade19) and intraocular diathermy is used to delineate the site of retinal biopsy at the border between the lesion and normal retina.18, 19, 22–26 During excision of a 1 mm × 1 mm or 2 mm × 2 mm size sample with vertical scissors or a diamond blade, the intraocular pressure is raised to 70 mmHg to 90 mmHg temporarily to prevent bleeding.18, 24 Intraocular forceps are then used to grasp the tissue and remove it through the sclerotomy site with enlargement as needed. Endolaser is then applied around the biopsy site with long-acting gas or silicone tamponade at the end of the procedure.18, 19, 23–26
The specimen should be sent immediately after collection to the ophthalmic pathology laboratory for histology, microbiology, electron microscopy, and directed testing if a neoplasm is suspected. 18, 19 Communication with an ophthalmic pathology laboratory is also needed to determine whether the chorioretinal biopsy should be separated in the operating room for microbiologic evaluation and histology or whether the biopsy should be cut in the laboratory.
Special Considerations Based on Infectious Etiology
Bacterial, Fungal, and Atypical Organisms
Bacterial and Fungal
In cases of bacterial and fungal endophthalmitis (Figure 2A–B), gram stain and culture (aerobic, anaerobic, and fungal) of the dilute vitreous sample is performed to identify the causative organism and obtain susceptibilities. Communication with the microbiology laboratory to hold cultures for at least one month for slower-growing organisms such as Propionibacterium acnes and fungi is important to avoid missing a fastidious organism.6, 9, 10
Figure 2.
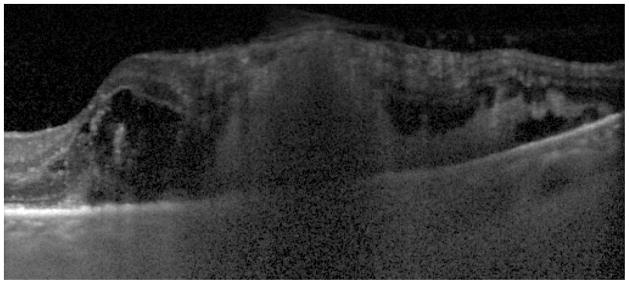
Fundus photograph (A) and spectral domain optical coherence tomography scan (B) of 73-year-old patient on total parenteral nutrition with endogenous Candida endophthalmitis with retinitis. Vitreous cultures and blood cultures were sufficient to diagnosis Candida albicans infection.
The sensitivity of culture after diagnostic vitrectomy for the diagnosis of chronic infectious uveitis has been reported between 16.7 to 96% 3, 9, 10, 27 with higher sensitivity reported in case series were there was a high pre-test probability for chronic endogenous or post-operative endophthalmitis.9 For acute post-operative endophthalmitis, data from the Endophthalmitis Vitrectomy Study showed a yield of 66% for culture and 41% for gram stain for patients undergoing vitrectomy.28 Higher yields are obtained with vitreous rather than aqueous samples.29
In select culture-negative cases of suspected chronic endophthalmitis, targeted PCR testing of patients with high clinical suspicion has led to the diagnosis of ocular Whipple’s disease.9, 30 Development of bacterial PCR analysis of both aqueous and vitreous fluid has been applied in case series of patients with acute and delayed post-operative endophthalmitis with early promising results. In a case series of 25 patients with unambiguous bacterial endophthalmitis, all aqueous and vitreous samples were positive by PCR with aqueous and vitreous cultures yielding the diagnosis in 33% and 68% respectively.29 In one series of patients with post-operative endophthalmitis treated with intravitreal antibiotics, eubacterial PCR (targeting the 16S ribosomal DNA common to all bacteria) of vitreous obtained by PPV identified the causative organism in 10 of 16 patients (62%) while culture only identified three (18%).31 Data from the French Institutional Endophthalmitis Study group showed similar findings with eubacterial PCR of vitreous obtained by PPV identifying the causative organism in 26 of 34 (76.5%) acute postoperative endophthalmitis cases compared with only 2 (5.8%) positive culture results.32 The low culture results in these studies were postulated to be due to pre-treatment with intravitreal antibiotics prior to vitrectomy, but allowed for identification with PCR despite treatment. In contrast, a series of 64 patients with suspected bacterial endophthalmitis prior to antibiotic treatment reported that PCR of vitreous samples identified a bacterial cause in 66% of patients compared with only 34% by culture.33 Although PCR yields were not as high as in previous and smaller series 34, 35, the group had a higher proportion of patients outside the immediate postoperative period with more subtle presentations of intraocular inflammation.33 PCR for bacterial and fungal endophthalmitis continues to be investigated for the role it will play in the evaluation of infectious uveitis.
Atypical Organisms
Mycobacterium Tuberculosis
Intraocular infection with Mycobacterium tuberculosis may have a myriad of presentations including anterior uveitis, posterior uveitis, panuveitis, choroidal granuloma, and retinal vasculitis.36 Diagnosis is arrived at by combining appropriate clinical history with systemic evaluation including chest radiographic imaging, positive Purified Protein Derivative (PPD) tuberculin skin test, Interferon Gamma Release Assays (IGRA) such as the QuantiFERON-TB GOLD (Qiagen ®, Valencia, California, USA) or T-SPOT.TB test (Oxford Immunotec ®, Marlborough, Massachusetts, USA), or analysis of extraocular sites of potential tuberculosis infection.36, 37 A dilemma arises in situations where it is unclear that tuberculosis may be related to the patient’s ocular presentation such as when tuberculosis is endemic, the patient is immunocompromised, systemic tuberculosis infection is not detected, the patient has received the Bacillus Calmette-Guerin (BCG) vaccine within 10 years of evaluation, or if there is unequivocal response to empiric anti-tubercular treatment.36–38 Additionally, PPD skin testing and IGRA testing identifies exposure and cannot delineate between active and latent infection. Further evaluation may be warranted especially in sight threatening situations and to guide initiation of anti-tubercular treatment.36, 37, 39
Intraocular fluid analysis typically has low yields for Ziehl-Neelsen staining for acid fast bacilli and culture on Lowenstein-Jensen medium may take up to 6 to 8 weeks which limits clinical utility.36, 37 Although a small case series reported identifying ocular tuberculosis with smear and culture of aqueous and vitreous fluids, these cases had atypical circumstances such as diffuse iris nodules or large subretinal mass with rupture into the vitreous cavity.38
Experience with aqueous and vitreous PCR testing of patients with presumed ocular tuberculosis by Gupta et al in India has had a yields ranging from 37.7% (20 of 53) up to 72% (13 of 18) 40–42 with a 5.7% false positive rate in controls with non-tubercular uveitis.40 With the exclusion of the largest series of 53 patients in which data is unavailable, 77% to 80% of the PCR positive patients in these series were PPD positive.41, 42 In these series, 90 to 100% of PCR positive patients that pursued anti-tubercular treatment had resolution of inflammation.40–42 This same group also reported Mycobacterium tuberculosis positive PCR from the aqueous and vitreous of 4 patients with presumed tubercular serpiginous-like choroiditis with resolution of disease activity with anti-tubercular treatment.43 Data from a group in Mexico using PCR testing in 22 patients with a known diagnosis of tuberculosis uveitis showed a yield of 77.2% (17 of 22) with 12 of 17 of PCR positive patients (76.5%) having a positive PPD. All patients improved with anti-tubercular treatment with a false positive rate, derived from testing non-tuberculosis uveitis controls, of 8.8% reported in that series.44 Limitations for PCR analysis is that clinical diagnosis is treated as the gold standard for comparison since culture for mycobacterium tuberculosis is difficult and may have low yield from the sample obtained.36, 39, 44
Nocardiosis
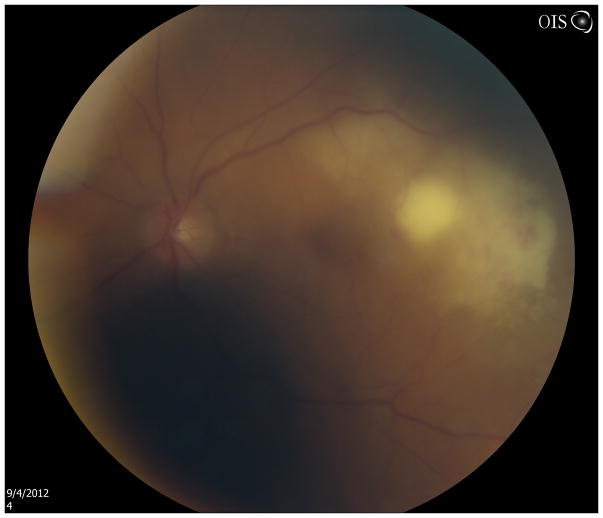
Nocardia species are comprised of a group of Gram-positive, filamentous aerobic bacteria that are ubiquitous in soil, dust, and vegetation.45, 46 Nocardia species may cause either exogenous infections (usually keratitis, scleritis, and post-surgical endophthalmitis) in patients with exposure to agriculture 47, 48 or endogenous ocular Nocardiosis (EON) from hematogenous spread in immunocompromised individuals (Figure 3).49
Figure 3.
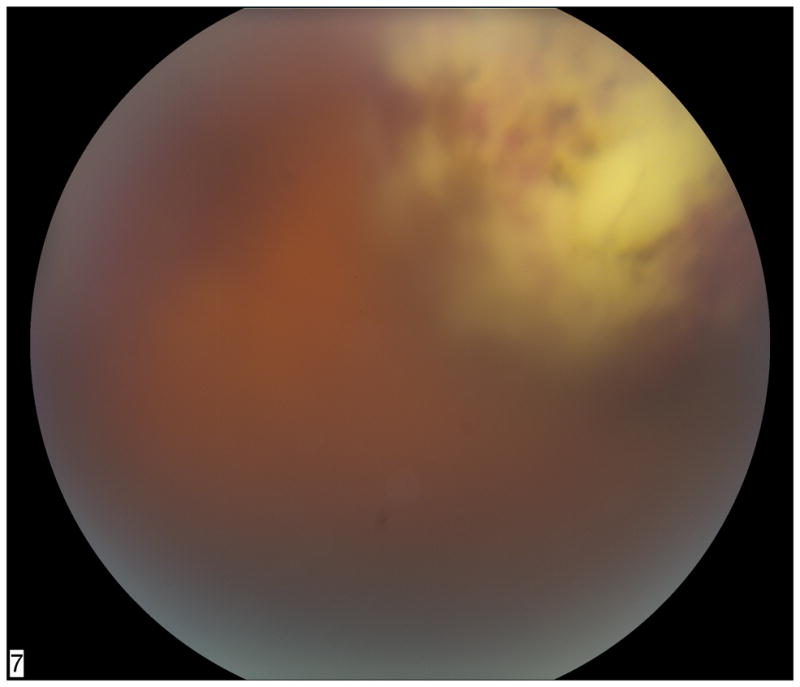
Fundus photograph of superior/superonasal subretinal lesion in a cardiac transplant patient on mycophenolate mofetil, tacrolimus, and prednisone. Diagnostic vitrectomy and subretinal aspiration biopsy showed Nocardia species.
EON typically occurs in organ transplant recipients with systemic immunosuppression (steroids or immunomodulatory therapy) but has been reported in patients with underlying connective tissues disease such as systemic lupus erythematosus. Hematogenous spread leads to seeding of the inner choroid producing a subretinal abscess (35 of 46 clinically described eyes in a literature review) which can extend exteriorly leading to anterior segment manifestations (hypopyon, scleritis, proptosis) or internalize leading to serous retinal detachment and vitritis.49 A dilemma may arise in clinical diagnosis as it may be confused with a neoplastic process in patients with lack of vitritis or confused with fungal infection with Candida or Aspergillus species as the patient demographic is similar. Although systemic manifestations can be severe including pulmonary, brain, and skin involvement, in 53% of the cases reported in the literature, ocular findings preceded the development of systemic symptoms.50 With a 25% mortality rate, establishing the diagnosis is important for both ocular morbidity and patient mortality.50
The mainstay of diagnosis is histologic staining and culture of ocular or extraocular specimens, which allows for identification of the organism with antibiotic susceptibilities. It is important to request special staining as Nocardia is weakly Gram-positive and use of the modified acid-fast Kinyoun stain distinguishes it from other bacteria such as Actinomyces.50 Culture, the gold standard for diagnosis, may result in 2 days to 3 weeks depending on the size of the inoculum and may be performed on standard culture media such as blood agar, chocolate agar, or Sabouraud medium.48, 50, 51
In regards to culture-positive exogenous Nocardia endophthalmitis, a retrospective review by a group in south India found Gram stain and modified Kinyoun staining of aqueous and vitreous specimens detected Nocardia species in 27.5% of cases highlighting the importance of culture.48 Another review of 24 culture-proven cases of exogenous Nocardia endophthalmitis from India found that aqueous specimens had the highest yield for special staining compared to vitreous specimens (93.75% compared with 4.54% respectively).47 Similarly, another group exploring exogenous Nocardia endophthalmitis found that none of the vitreous samples collected had detectable organisms by microscopy.51 It is postulated that higher yields from aqueous fluid are due to minimal posterior segment findings in the face of extensive anterior segment manifestations including grossly infected cataract wounds and yellowish-white nodules on the corneal endothelium and iris.47
In terms of endogenous ocular Nocardiosis (EON), a variety of intraocular sampling techniques have been used to stain and culture for the organism, yet most often the diagnosis is arrived through analysis of samples from extraocular sites.50 In 5 cases, 6 vitreous taps were pursued 46, 52–55 of which 4 were non-diagnostic by either microscopic analysis or culture.46, 52, 54, 55 Pars plana vitrectomy led to identification of Nocardia species by staining and/or culture in 8 of 9 cases.46, 54, 56–61 All cases underwent vitrectomy including retinal biopsy except for two cases 58, 62 of which 1 was non-diagnostic by vitreous fluid analysis.62 Subretinal fine needle aspiration (FNA) was performed in 5 cases all of which were diagnostic of Nocardia species.55, 63–66 A combination of vitrectomy, retinal biopsy, and subretinal aspiration may also be used to identify multiple intraocular sites of Nocardia infiltration, as described in one patient with Nocardia infection involving the vitreous, retina, and subretinal space in the context of systemic immunosuppression subsequent to cardiac transplantation.46
Although PCR has been described to speciate cultured Nocardia from ocular samples,67 the use of PCR testing has not been described in clinical practice for this organism.
Parasitic
Toxoplasma gondii
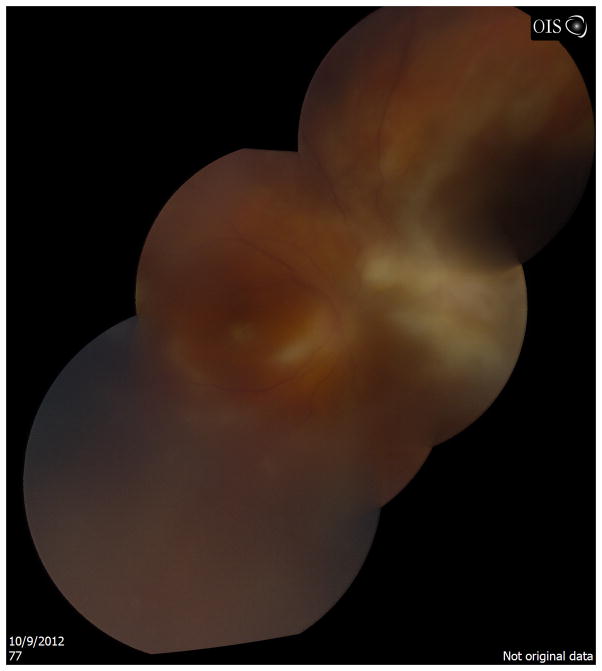
A diagnosis of ocular toxoplasmosis typically is made by a characteristic clinical presentation and evidence of serologic exposure. The clinical findings of retinitis adjacent to a chorioretinal with varying level of overlying vitritis may be sufficient for directing the management options. However, atypical presentations of toxoplasmic retinochoroiditis may mimic viral necrotizing retinitis usually in immunocompromised patients and the elderly prompting the initiation of incorrect therapy.68, 69 Further diagnostic testing for ocular toxoplasmosis is warranted in these instances (Figure 4).
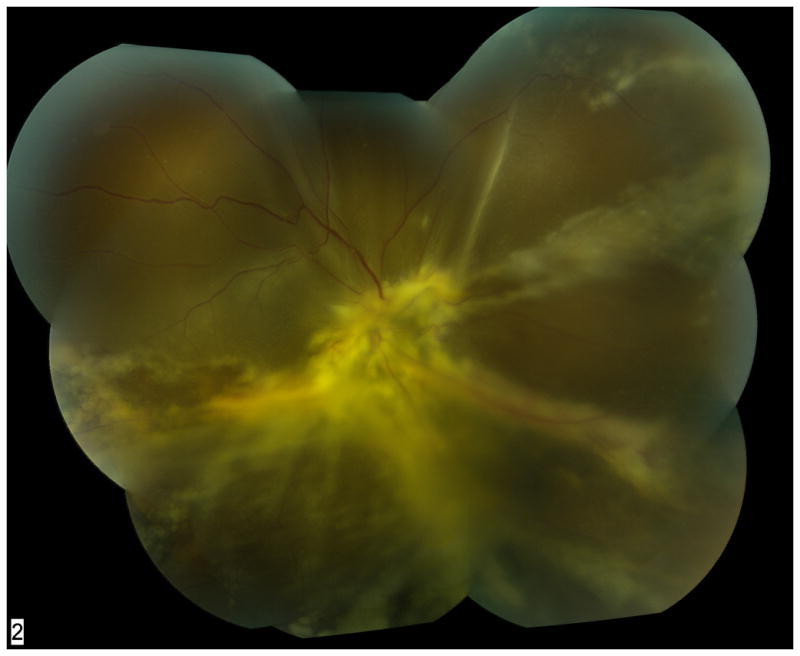
Figure 4.
Fundus photograph montage of patient with dermatomyositis on azathioprine and monthly intravenous immunoglobulin. Anterior chamber paracentesis was positive for toxoplasmosis DNA by PCR testing. The diffuse retinitis mimicked acute retinal necrosis and was atypical for toxoplasmosis, likely secondary to the patient’s immunosuppression.
Although a small series described the successful culture of T. gondii from the vitreous of 5 patients with HIV and/or AIDS exhibiting severe retinochoroiditis, the limitation of this approach is the long detection time ranging from 2 to 23 days for positive cultures.70
The rapid detection of toxoplasmosis DNA using PCR techniques on aqueous fluid has varied in the literature with some reports describing diagnostic yields from 13% to 55% 68, 71–79 with positive PCR results occurring more often in patients with larger chorioretinal lesions,49, 75 in immunosuppressed patients,75, 80 and with active anterior segment inflammation.49
Antibody levels in the aqueous have been used to supplement results from PCR testing by calculating the Goldmann-Witmer Coefficient (GWC) to determine whether local antibody production is occurring.49, 73, 75, 81–83 The GWC is calculated as target IgG in the ocular fluid/total ocular fluid IgG divided by target IgG in the serum/total serum IgG. Theoretically, a GWC greater than 1.0 would indicate local antibody production, but most authors designate a coefficient of 3.0 as indicative of local antibody production to take into account passage of antibody through a disrupted blood-retina or blood-aqueous barrier.81 In one series, calculation of the aqueous GWC for toxoplasmosis antibody prior to and after 3 weeks of clinical manifestation had the highest yield in the delayed sample (57% compared with 70%).73 In the series by Fardeau of aqueous sampling for toxoplasmosis, of the 34 immunocompetent patients with negative PCR testing for toxoplasmosis, 25 of those 34 patients had a positive GWC detecting ocular toxoplasmosis whereas none of the immunocompromised patients exhibited a positive GWC.75 In a similar vein, a series by Rothova showed greatest utility of GWC in the diagnosis of ocular toxoplasmosis in immunocompetent patients (93% with final diagnosis of ocular toxoplasmosis) whereas the GWC was of little utility for immunocompromised patients (57% yield).82 In comparison with PCR, calculation of the GWC had more utility in ocular toxoplasmosis 49, 83, 84 with up to 64% of toxoplasma diagnoses missed had PCR alone been performed 83 and up to 87.5% 49 to 90% 84 of ocular toxoplasmosis diagnoses made with GWC. Despite treatment, it has been observed in a small series that PCR of the aqueous may remain positive.77
PCR and GWC analysis has also been performed on vitreous samples during diagnostic and therapeutic vitrectomy albeit in limited numbers with results seeming to indicate similar to improved yields in vitreous samples for toxoplasmosis PCR though not powered sufficiently to make that distinction.6, 9, 12, 49, 69, 77, 80, 85–87 Yet, the larger amount of undiluted and dilute vitreous collected is thought to allow a larger battery of antibody tests to be performed than on aqueous samples in cases with a large differential diagnosis.
Toxocara canis
Ocular toxocariasis is predominantly a pediatric condition diagnosed in patients with a suggestive history and examination. Detection of acute toxocariasis infection may be difficult but a peripheral granuloma with or without the presence of associated vitreoretinal traction bands is the classical appearance. A posterior pole granuloma may also be observed with ocular toxocariasis. Although a positive serology can assist in evaluation, the high seroprevalence in certain regions of the world may make it less supportive for diagnosis.88 In addition, seropositivity declines with time.89, 90 Furthermore, serum titers have reported to be positive in only 33% to 69% of cases.89–91 A dilemma may arise when ocular toxocariasis presents as a chronic endophthalmitis or if the presence of the granuloma with or without associated traction mimics retinoblastoma or other infectious uveitides in the setting of negative titers.90
Although PPV with biopsy of visible granulomas during retinal detachment repair has led to the identification of the offending helminth in few cases reported in the literature,92–98 most approaches for identification of toxocariasis involve either aqueous or vitreous Toxocara IgG testing. In these situations, the serum ELISA antibody titers are compared to aqueous or vitreous titers with a qualitatively higher ocular fluid ELISA indicating likely presence of ocular toxocariasis (Figure 5A–B).90, 91 In a case series from Japan, of 33 intraocular fluid samples collected (12 aqueous and 21 vitreous), 22 of 33 (66.7%) showed higher titers from ELISA testing in the ocular fluids than in the serum indicating a positive result.91 Case reports and small case series have also shown utility of aqueous and vitreous ELISA testing for Toxocariasis.90, 99–102 In all these case series, total IgG in the serum and ocular fluids was not measured thus precluding the ability for a more quantitative measurement such as GWC testing. It is thought that GWC testing may prevent false positive results by correcting for passive leakage of antibody in a compromised blood-aqueous barrier during inflammation.89
Figure 5.
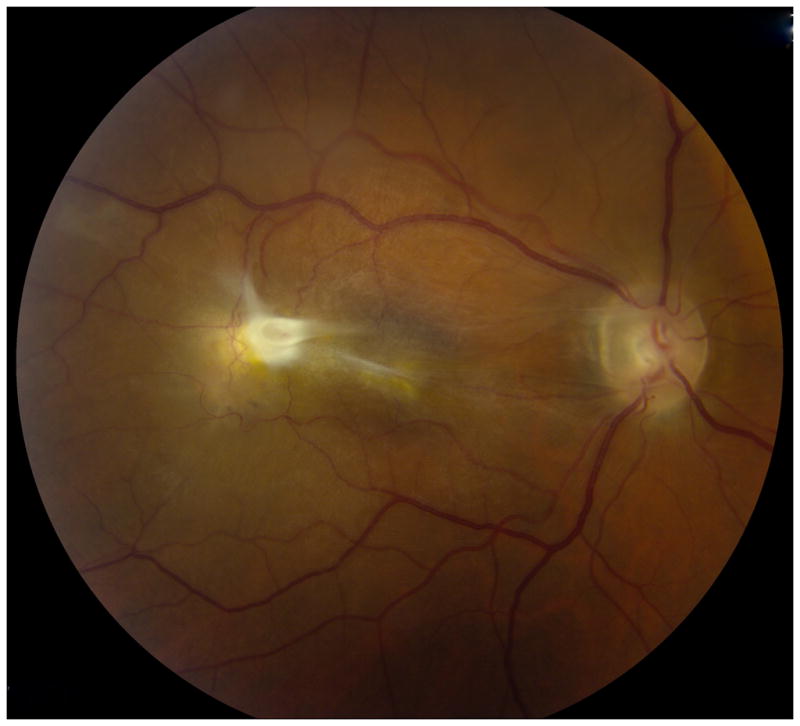
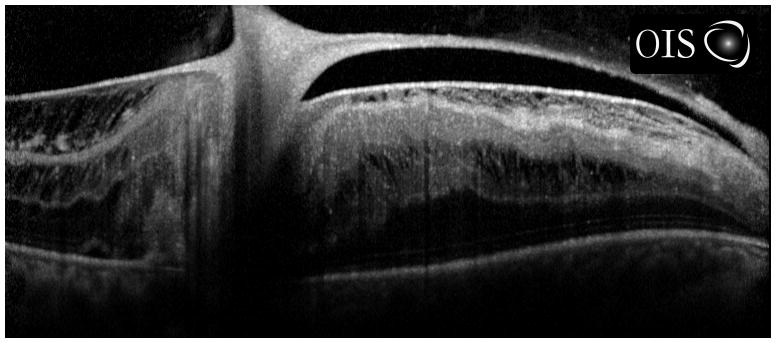
Fundus photograph of patient with posterior pole granuloma (A) and corresponding spectral domain optical coherence tomography scan (B) suggestive of toxocariasis. Diagnostic vitrectomy showed elevated antibody titer to Toxocara canis, establishing the diagnosis of ocular toxocariasis.
In a series that conducted ELISA antibody titers and GWC determination on serum and ocular fluid samples from 37 adults and 12 children with unknown posterior uveitis, 3 of 12 children had revealing aqueous humor analysis for Toxocara canis by ELISA and GWC determination.89 Aqueous humor analysis enabled the diagnosis in these 3 children who were only considered seropositive by including serum titers below the typical screening dilution cut off for serum Toxocara ELISA testing. It has been suggested that testing serum ELISA to dilutions as low as 1:889, 103 or at any detectable dilution 90 may augment Toxocara diagnosis in the appropriate clinical setting.
Although PCR testing for toxocariasis has been attempted, its utility for the diagnosis of this condition is limited.102, 104 In a recent series of patients with posterior uveitis, none of the 15 patients with typical posterior segment findings and seropositivity for Toxocara canis were positive for toxocariasis DNA.104 One report of vitreous PCR testing for Toxocara was similarly non-diagnostic.102 PCR may play a limited role in ocular toxocariasis as it may be unlikely for the helminth to shed DNA into the aqueous or vitreous.88
Viral
Diagnosing an infectious viral retinitis syndrome due to herpes simplex virus (HSV), varicella zoster virus (VZV), and cytomegalovirus (CMV) can be difficult in situations with significant posterior segment inflammation (Figure 6A–B). Ascertaining this diagnosis may also be problematic in immunocompromised patients who may be at risk for both opportunistic and non-opportunistic infections. Culture is of limited utility because of the amount of time needed for a virus to grow in viral culture media; however, PCR testing of aqueous and vitreous fluid plays a prominent diagnostic role because of high-sensitivity, rapidity of the assay, and low false-positive rate.105, 106 Early experiences with viral PCR of the aqueous and vitreous were used to test the concordance of PCR results with the clinical diagnosis of CMV Retinitis 105, 107 as well as identify viral etiologies of Acute Retinal Necrosis (ARN) and Progressive Outer Retinal Necrosis (PORN).107–111
Figure 6.
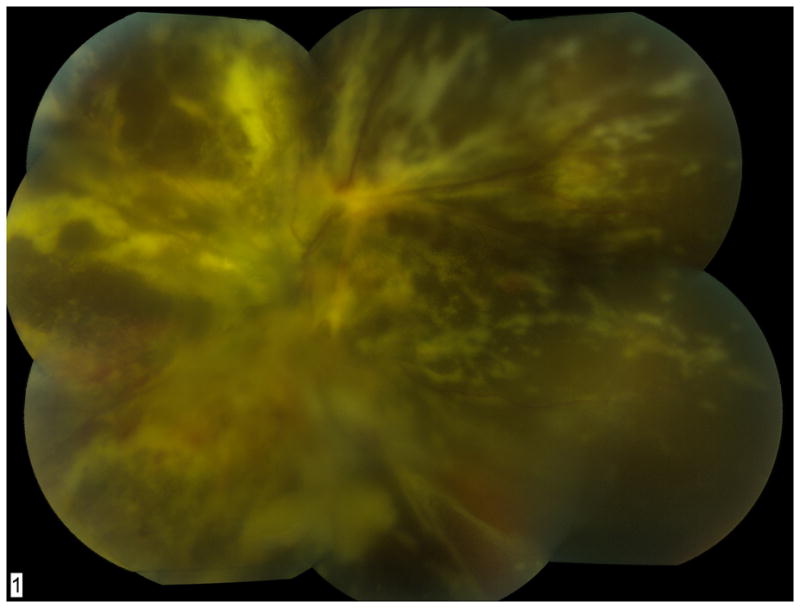
Fundus photograph of diffuse CMV retinitis with underlying retinal detachment in the right eye (A) and left eye (B). The patient was HIV-positive and not taking recommended anti-retroviral medications. His CD4 count was < 5 cells/mm3 on presentation. Qualitative PCR testing for CMV DNA was positive. Quantitative real-time PCR from the left eye showed 207,000 copies/mL of CMV DNA.
The diagnostic yield of PCR testing for viral uveitis syndromes varies in the literature, but is the highest when the pre-test probability is high (i.e. patient with more characteristic clinical features versus atypical findings). In a case series assessing aqueous humor PCR testing for infectious posterior uveitis, PCR testing had a 79% yield for viral infections regardless of patient immune status.82 A more recent large series of patients described by Harper et al showed that viral PCR had a sensitivity of 80.9% and specificity of 97.4% when considering the final clinical diagnosis as the gold standard.87 Conversely, in a series by Matos, aqueous PCR testing for viral infections was non-diagnostic while vitreous PCR showed a 53% yield for all causes of viral uveitis including HSV/VZV acute retinal necrosis or CMV retinitis.85
The use of quantitative real-time PCR of aqueous fluid is also helpful for monitoring viral DNA levels in response to therapy, and moreover, may be used to detect drug-resistance in patients on chronic valganciclovir therapy for recurrent or recalcitrant CMV retinitis or non-ocular involvement.112
To supplement PCR testing, calculation of the GWC for HSV, VZV, and CMV has been undertaken albeit with variable results. In a series by Westeneng of immunocompromised patients with posterior and panuveitis, viral PCR demonstrated superiority to GWC evaluation with viral DNA detected by PCR in 16 of 17 cases (94%) whereas the GWC detected only 3 of those 17 cases (18%).84 Variations in the performance of GWC for detection of viral retinitides has been shown in other series.49 In one series, GWC was instrumental in the identification of 92% of HSV and 87.5% of VZV associated infectious uveitis whereas PCR only identified 54% of HSV and 75% of VZV cases.83 Varying yields of PCR and GWC testing have been postulated to be due in part to timing of ocular fluid analysis – specifically, higher rates of PCR positivity are detected within the first weeks of disease activity while increased GWC positivity later in the disease course may be observed due to a reduction of pathogenic load from increased intraocular IgG production.83, 87 Another hypothesis is that immunosuppressed patients may not be able to mount a local antibody response, thus explaining the low yield of GWC testing in patients with CMV retinitis.107
Although most experience in the literature for viral infectious uveitis involve aqueous humor sampling which can be rapid and easy to perform, diagnostic vitrectomy has a role in situations where the retina cannot be evaluated, vitrectomy can be therapeutic, or malignancy is a diagnostic possibility.82
Masquerade Syndromes
Masquerade syndromes are a group of neoplastic disease processes that mimic inflammatory conditions in presentation leading to a diagnostic dilemma.113, 114 As malignant conditions, they may be life- and vision-threatening. Masquerade syndromes including intraocular lymphoma, melanoma with necrosis, and metastatic disease may mimic infectious uveitis.
Intraocular Lymphoma
Intraocular lymphoma is regarded as the most common masquerade with one tertiary care uveitis service reporting that intraocular lymphoma represented 68% of their 19 patients with masquerade syndromes.115 Typically, ocular lymphoma presents with vitreous cell and refractory uveitis leading to diagnostic confusion and delay in diagnosis.5 More advanced cases exhibit characteristic yellow-white subretinal infiltrates.113, 114 A dilemma may also arise as corticosteroids can lead to an initial improvement clouding the clinical picture.8, 113, 114 Diagnosis is made definitively by vitreous sampling with pars plana vitrectomy and close coordination with an ophthalmic pathologist. With a high index of suspicion and cautious handling of vitrectomy specimens, a mean of 4 months from symptom onset to diagnosis was achieved in one series compared to a previously reported mean of 21 months to diagnosis.8 Samples are sent for cytology, flow cytometry, and cytokine analysis to assist with evaluation. Cytology is the gold standard for diagnosis allowing differentiation of malignant cells from inflammation with several series requiring cytology alone to identifying intraocular lymphoma.5, 6, 10, 11 Yet, repeat vitreous biopsy and chorioretinal biopsy has been required to establish a diagnosis in intraocular lymphoma 7, 8 which may be due to low yield from the biopsy site, fragility of the cells affected by a delay in immediate sample processing, or cell degradation by steroid treatment.5, 8, 10
Although cytology is the gold standard for diagnosis of intraocular lymphoma, flow cytometry has been used to supplement cytology with its primary utility in identifying cell clonality with specific cell surface markers and to signifying the presence of lymphoma instead of inflammation in comparing the CD4:CD8 ratio.9, 113 Use of flow cytometry in one series identified only 4 of 6 cytology positive cases of intraocular lymphoma demonstrating non-superiority of this modality.8 Another series, in which cytology and flow cytometry were obtained from the undiluted vitreous sample, concluded flow cytometry was superior,116 but the results of this study have been disputed due to equivocal appearing flow cytometry results.8, 113 Other adjunctive tests for intraocular lymphoma have been described including PCR and cytokine analysis but have not replaced cytology in the evaluation of lymphoma.117
Uveal Melanoma
Uveal melanoma does not usually pose a diagnostic dilemma due to its characteristic appearance by clinical exam and ultrasonography. Inflammation in the form of scleritis 118–121, anterior uveitis 122–125, posterior uveitis 126, panuveitis 127, and panophthalmitis 128–130 have been described with 22 (4.9%) of eyes in a series of 450 enucleated for uveal melanoma exhibiting some form of inflammation.113, 131 In a large review of necrotic uveal melanomas from the Armed Forces Institute of Pathology, 75.1% of the 157 totally necrotic uveal melanomas in their registry presented with episcleritis and scleritis.119 In a similar vein, it was noted in the large pathology series by Font that all patients presenting with panophthalmitis had necrotic uveal melanomas.131 In these atypical presentations of uveal melanoma, close follow up, serial photography, ultrasonography, and clinical suspicion are primarily employed.118, 122, 123, 125, 126 In one case of ciliary body melanoma masquerading as chronic uveitis, multiple diagnostic vitrectomies were unrevealing to the diagnosis whereas serial ultrasound of a later identified ciliary body lesion was.127 Tissue biopsy led to the diagnosis of two patients with uveal melanoma masquerading as scleritis.120, 121
Inadvertent vitrectomy for an eye with uveal melanoma may lead to extrascleral extension at the sclerotomy sites.132 In a case report of an eye with untreated uveal melanoma that had undergone vitrectomy, histologic examination of the enucleated eye showed melanoma cells had diffusely spread to all intraocular surfaces.133 Fine Needle Aspiration Biopsy (FNAB) with either a 27 gauge needle passed transvitreally for post-equatorial lesions or 30 gauge needle passed transclerally for pre-equatorial lesions has a reported yield of 97% and 75% respectively for chromosome 3 analysis in a case series by Shields et al.134 Use of a 25-guage sutureless vitrectomy set-up has also been described to obtain a transvitreal retinochoroidal biopsy with the caveat that the vitrector is only used in the substance of the visualized tumor with no further vitrectomy performed once obtained.135 A recent series by Bagger et al using this method reported a 97.3% yield for chromosome 3 analysis with the authors asserting this method allows for improved sampling of more anteriorly located tumors as biopsy is performed under direct visualization.135 All biopsies were followed by either enucleation or plaque placement.134, 135 Although extrascleral extension has not been reported in large case series, a case series of four patients experiencing this complication was reported though it is unclear as to what technique was performed.136
Tumor Metastasis
Tumor metastasis is the most common cause of intraocular malignancy in adults.113 Their typical appearance as well as pre-existing history of cancer rarely makes them a diagnostic dilemma. Obtaining a careful history is paramount to detection.114
Uveal metastasis masquerading as intraocular inflammation has been reported with posterior segment metastasis causing diffuse posterior segment inflammation, anterior segment metastasis causing severe, resistant anterior chamber inflammation,113, 137–146 and in situations where malignancy presents with a viral retinitis type picture.147 In the case reports of metastasis masquerading as anterior uveitis, aqueous sampling for cytology has led to the diagnosis in the few reports it was performed 137–142 with the remaining cases diagnosed with suggestive history and imaging. In patients undergoing diagnostic vitrectomy for uveitis of unknown cause, metastasis was rarely identified directly from vitrectomy cytology results 2–4 with only 1 case reported in each of these series despite uveal metastasis being the most common cause of intraocular malignancy in adults. In a case series of 40 patients with uveitis masquerades, only 1 case of metastatic carcinoma was identified 115 likely due to its infrequency as a diagnostic dilemma. In case reports of patients with the extremely rare occurrence of tumor metastatic to the retina and vitreous, these conditions present as intermediate uveitis, vitreous hemorrhage, or retinal vasculitis with vitreous cytologic sampling and retinal biopsy assisting in diagnosis if no primary malignancy is identified.113, 147, 148
Biopsy of suspected ocular metastasis plays a role in patients with no known primary tumor and in cases of diagnostic uncertainty as sampling may lead to a significant change in ocular and systemic management.149 Fine needle aspiration biopsy in a series of 159 cases by Shields et al led to an adequate sample collection in 88% of cases.150 In a series of 39 patients with uveal metastasis, 25 gauge sutureless vitrectomy had a yield of 100% for cytologic diagnosis.149
Summary
Intraocular sampling including diagnostic vitrectomy and chorioretinal biopsy has a major role in uveitic diagnostic dilemmas. Close coordination with an expert ophthalmic pathology laboratory, if available, is paramount prior to and after diagnostic vitrectomy to ensure proper specimen handling and evaluation. The choice of diagnostic testing should be directed to the differential diagnosis of the patient’s presentation for studies that may include cytology, flow cytometry, gram stain, culture, PCR testing, antibody, and cytokine evaluation. Cultures and appropriate special stains have the greatest utility in bacterial, fungal, and nocardial infection. Appropriate communication with the microbiology laboratory is important in infection with fastidious organisms. Bacterial PCR testing of the vitreous has shown promising results even in cases treated with intravitreal antibiotics. PCR is most appropriate for viral infectious disease, but is also very helpful for suspected cases of toxoplasmosis. Antibody testing is appropriate for toxoplasmosis and toxocariasis, and may be complementary to PCR for viral retinitis. Ocular tuberculosis is primarily identified using supporting history, imaging, PPD skin testing, and IGRA testing in the appropriate clinical setting. Intraocular sampling for mycobacterial PCR has been described from series in endemic countries with success. Masquerade syndromes represent neoplastic conditions that clinically appear as infectious or inflammatory conditions and should be considered as part of the differential diagnosis. Cytology from PPV or retinochoroidal biopsy is pursued if the diagnosis is not obvious with less invasive testing.
Acknowledgments
Sources of Funding
This work was supported in part by an unrestricted departmental grant from Research to Prevent Blindness (New York, NY) to the Emory Eye Center and an NEI Core Grant for Vision Research (P30 EY 006360), and the Knights Templar Educational Foundation of Georgia (SY).
Footnotes
Conflicts of Interest
For the remaining author, none were declared.
References
- 1.Carroll DM, Franklin RM. Vitreous biopsy in uveitis of unknown cause. Retina. 1981;1(3):245–51. doi: 10.1097/00006982-198101030-00022. [DOI] [PubMed] [Google Scholar]
- 2.Priem H, Verbraeken H, de Laey JJ. Diagnostic problems in chronic vitreous inflammation. Graefes Arch Clin Exp Ophthalmol. 1993;231(8):453–6. doi: 10.1007/BF02044231. [DOI] [PubMed] [Google Scholar]
- 3.Palexas GN, Green WR, Goldberg MF, et al. Diagnostic pars plana vitrectomy report of a 21-year retrospective study. Trans Am Ophthalmol Soc. 1995;93:281–308. [PMC free article] [PubMed] [Google Scholar]
- 4.Verbraeken H. Diagnostic vitrectomy and chronic uveitis. Graefes Arch Clin Exp Ophthalmol. 1996;234:S2–7. doi: 10.1007/BF02343040. [DOI] [PubMed] [Google Scholar]
- 5.Akpek EK, Ahmed I, Hochberg FH, et al. Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmology. 1999;106(9):1805–10. doi: 10.1016/S0161-6420(99)90341-X. [DOI] [PubMed] [Google Scholar]
- 6.Mruthyunjaya P, Jumper JM, McCallum R, et al. Diagnostic yield of vitrectomy in eyes with suspected posterior segment infection or malignancy. Ophthalmology. 2002;109(6):1123–9. doi: 10.1016/s0161-6420(02)01033-3. [DOI] [PubMed] [Google Scholar]
- 7.Coupland SE, Bechrakis NE, Anastassiou G, et al. Evaluation of vitrectomy specimens and chorioretinal biopsies in the diagnosis of primary intraocular lymphoma in patients with Masquerade syndrome. Graefes Arch Clin Exp Ophthalmol. 2003;241(10):860–70. doi: 10.1007/s00417-003-0749-y. [DOI] [PubMed] [Google Scholar]
- 8.Zaldivar RA, Martin DF, Holden JT, et al. Primary intraocular lymphoma: clinical, cytologic, and flow cytometric analysis. Ophthalmology. 2004;111(9):1762–7. doi: 10.1016/j.ophtha.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Davis JL, Miller DM, Ruiz P. Diagnostic testing of vitrectomy specimens. Am J Ophthalmol. 2005;140(5):822–9. doi: 10.1016/j.ajo.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Margolis R, Brasil OF, Lowder CY, et al. Vitrectomy for the diagnosis and management of uveitis of unknown cause. Ophthalmology. 2007;114(10):1893–7. doi: 10.1016/j.ophtha.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Wittenberg LA, Maberley DA, Ma PE, et al. Contribution of vitreous cytology to final clinical diagnosis fifteen-year review of vitreous cytology specimens from one institution. Ophthalmology. 2008;115(11):1944–50. doi: 10.1016/j.ophtha.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Oahalou A, Schellekens PA, De Groot-Mijnes JD, et al. Diagnostic pars plana vitrectomy and aqueous analyses in patients with uveitis of unknown cause. Retina. 2013 doi: 10.1097/IAE.0b013e31828e6985. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbaum JT, Wernick R. The utility of routine screening of patients with uveitis for systemic lupus erythematosus or tuberculosis. A Bayesian analysis. Arch Ophthalmol. 1990;108(9):1291–3. doi: 10.1001/archopht.1990.01070110107034. [DOI] [PubMed] [Google Scholar]
- 15.Davis JL. Diagnostic dilemmas in retinitis and endophthalmitis. Eye (Lond) 2012;26(2):194–201. doi: 10.1038/eye.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conlon MR, Craig I, Harris JF, et al. Effect of vitrectomy and cytopreparatory techniques on cell survival and preservation. Can J Ophthalmol. 1992;27(4):168–71. [PubMed] [Google Scholar]
- 17.Margolis R. Diagnostic vitrectomy for the diagnosis and management of posterior uveitis of unknown etiology. Curr Opin Ophthalmol. 2008;19(3):218–24. doi: 10.1097/ICU.0b013e3282fc261d. [DOI] [PubMed] [Google Scholar]
- 18.Bechrakis NE, Foerster MH, Bornfeld N. Biopsy in indeterminate intraocular tumors. Ophthalmology. 2002;109(2):235–42. doi: 10.1016/s0161-6420(01)00931-9. [DOI] [PubMed] [Google Scholar]
- 19.Johnston RL, Tufail A, Lightman S, et al. Retinal and choroidal biopsies are helpful in unclear uveitis of suspected infectious or malignant origin. Ophthalmology. 2004;111(3):522–8. doi: 10.1016/j.ophtha.2002.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Peyman GA, Juarez CP, Raichand M. Full-thickness eye-wall biopsy: long-term results in 9 patients. Br J Ophthalmol. 1981;65(10):723–6. doi: 10.1136/bjo.65.10.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin DF, Chan CC, de Smet MD, et al. The role of chorioretinal biopsy in the management of posterior uveitis. Ophthalmology. 1993;100(5):705–14. doi: 10.1016/s0161-6420(93)31585-x. [DOI] [PubMed] [Google Scholar]
- 22.Cassoux N, Charlotte F, Rao NA, et al. Endoretinal biopsy in establishing the diagnosis of uveitis: a clinicopathologic report of three cases. Ocul Immunol Inflamm. 2005;13(1):79–83. doi: 10.1080/09273940590909149. [DOI] [PubMed] [Google Scholar]
- 23.Kvanta A, Seregard S, Kopp ED, et al. Choroidal biopsies for intraocular tumors of indeterminate origin. Am J Ophthalmol. 2005;140(6):1002–6. doi: 10.1016/j.ajo.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Lim LL, Suhler EB, Rosenbaum JT, et al. The role of choroidal and retinal biopsies in the diagnosis and management of atypical presentations of uveitis. Trans Am Ophthalmol Soc. 2005;103:84–92. [PMC free article] [PubMed] [Google Scholar]
- 25.Cole CJ, Kwan AS, Laidlaw DA, et al. A new technique of combined retinal and choroidal biopsy. Br J Ophthalmol. 2008;92(10):1357–60. doi: 10.1136/bjo.2008.141697. [DOI] [PubMed] [Google Scholar]
- 26.Seregard S, All-Ericsson C, Hjelmqvist L, et al. Diagnostic incisional biopsies in clinically indeterminate choroidal tumours. Eye (Lond) 2013;27(2):115–8. doi: 10.1038/eye.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bispo PJ, Melo GB, d’Azevedo PA, et al. Culture proven bacterial endophthalmitis: a 6-year review. Arq Bras Oftalmol. 2008;71(5):617–22. doi: 10.1590/s0004-27492008000500002. [DOI] [PubMed] [Google Scholar]
- 28.Han DP, Wisniewski SR, Kelsey SF, et al. Microbiologic yields and complication rates of vitreous needle aspiration versus mechanized vitreous biopsy in the Endophthalmitis Vitrectomy Study. Retina. 1999;19(2):98–102. doi: 10.1097/00006982-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Okhravi N, Adamson P, Carroll N, et al. PCR-based evidence of bacterial involvement in eyes with suspected intraocular infection. Invest Ophthalmol Vis Sci. 2000;41(11):3474–9. [PubMed] [Google Scholar]
- 30.Chan RY, Yannuzzi LA, Foster CS. Ocular Whipple’s disease: earlier definitive diagnosis. Ophthalmology. 2001;108(12):2225–31. doi: 10.1016/s0161-6420(01)00818-1. [DOI] [PubMed] [Google Scholar]
- 31.Chiquet C, Lina G, Benito Y, et al. Polymerase chain reaction identification in aqueous humor of patients with postoperative endophthalmitis. J Cataract Refract Surg. 2007;33(4):635–41. doi: 10.1016/j.jcrs.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Chiquet C, Maurin M, Thuret G, et al. Analysis of diluted vitreous samples from vitrectomy is useful in eyes with severe acute postoperative endophthalmitis. Ophthalmology. 2009;116(12):2437–41.e1. doi: 10.1016/j.ophtha.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Joseph CR, Lalitha P, Sivaraman KR, et al. Real-time polymerase chain reaction in the diagnosis of acute postoperative endophthalmitis. Am J Ophthalmol. 2012;153(6):1031–7. doi: 10.1016/j.ajo.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Sugita S, Shimizu N, Watanabe K, et al. Diagnosis of bacterial endophthalmitis by broad-range quantitative PCR. Br J Ophthalmol. 2011;95(3):345–9. doi: 10.1136/bjo.2009.171504. [DOI] [PubMed] [Google Scholar]
- 35.Bispo PJ, de Melo GB, Hofling-Lima AL, et al. Detection and gram discrimination of bacterial pathogens from aqueous and vitreous humor using real-time PCR assays. Invest Ophthalmol Vis Sci. 2011;52(2):873–81. doi: 10.1167/iovs.10-5712. [DOI] [PubMed] [Google Scholar]
- 36.Gupta V, Gupta A, Rao NA. Intraocular tuberculosis--an update. Surv Ophthalmol. 2007;52(6):561–87. doi: 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Vasconcelos-Santos DV, Zierhut M, Rao NA. Strengths and weaknesses of diagnostic tools for tuberculous uveitis. Ocul Immunol Inflamm. 2009;17(5):351–5. doi: 10.3109/09273940903168688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas J, Madhavan HN, Gopal L, et al. Intraocular tuberculosis. Clinicopathologic study of five cases. Retina. 1995;15(6):461–8. [PubMed] [Google Scholar]
- 39.Yeh S, Sen HN, Colyer M, et al. Update on ocular tuberculosis. Curr Opin Ophthalmol. 2012;23(6):551–6. doi: 10.1097/ICU.0b013e328358ba01. [DOI] [PubMed] [Google Scholar]
- 40.Arora SK, Gupta V, Gupta A, et al. Diagnostic efficacy of polymerase chain reaction in granulomatous uveitis. Tuber Lung Dis. 1999;79(4):229–33. doi: 10.1054/tuld.1999.0210. [DOI] [PubMed] [Google Scholar]
- 41.Gupta V, Arora S, Gupta A, et al. Management of presumed intraocular tuberculosis: possible role of the polymerase chain reaction. Acta Ophthalmol Scand. 1998;76(6):679–82. doi: 10.1034/j.1600-0420.1998.760609.x. [DOI] [PubMed] [Google Scholar]
- 42.Gupta A, Gupta V, Arora S, et al. PCR-positive tubercular retinal vasculitis: clinical characteristics and management. Retina. 2001;21(5):435–44. doi: 10.1097/00006982-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Gupta V, Gupta A, Arora S, et al. Presumed tubercular serpiginouslike choroiditis: clinical presentations and management. Ophthalmology. 2003;110(9):1744–9. doi: 10.1016/S0161-6420(03)00619-5. [DOI] [PubMed] [Google Scholar]
- 44.Ortega-Larrocea G, Bobadilla-del-Valle M, Ponce-de-Leon A, et al. Nested polymerase chain reaction for Mycobacterium tuberculosis DNA detection in aqueous and vitreous of patients with uveitis. Arch Med Res. 2003;34(2):116–9. doi: 10.1016/S0188-4409(02)00467-8. [DOI] [PubMed] [Google Scholar]
- 45.Lalitha P, Rajagopalan J, Prakash K, et al. Postcataract endophthalmitis in South India incidence and outcome. Ophthalmology. 2005;112(11):1884–9. doi: 10.1016/j.ophtha.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Scott M, Mehta S, Rahman HT, et al. Nocardia veterana endogenous endophthalmitis in a cardiac transplant patient. J Ophthalmic Inflamm Infect. 2013;3(1):44. doi: 10.1186/1869-5760-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haripriya A, Lalitha P, Mathen M, et al. Nocardia endophthalmitis after cataract surgery: clinicomicrobiological study. Am J Ophthalmol. 2005;139(5):837–46. doi: 10.1016/j.ajo.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Manikandan P, Bhaskar M, Revathi R, et al. Isolation and antimicrobial susceptibility pattern of Nocardia among people with culture-proven ocular infections attending a tertiary care eye hospital in Tamilnadu, South India. Eye (Lond) 2007;21(8):1102–8. doi: 10.1038/sj.eye.6702513. [DOI] [PubMed] [Google Scholar]
- 49.Errera MH, Goldschmidt P, Batellier L, et al. Real-time polymerase chain reaction and intraocular antibody production for the diagnosis of viral versus toxoplasmic infectious posterior uveitis. Graefes Arch Clin Exp Ophthalmol. 2011;249(12):1837–46. doi: 10.1007/s00417-011-1724-7. [DOI] [PubMed] [Google Scholar]
- 50.Eschle-Meniconi ME, Guex-Crosier Y, Wolfensberger TJ. Endogenous ocular nocardiosis: an interventional case report with a review of the literature. Surv Ophthalmol. 2011;56(5):383–415. doi: 10.1016/j.survophthal.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 51.DeCroos FC, Garg P, Reddy AK, et al. Optimizing diagnosis and management of nocardia keratitis, scleritis, and endophthalmitis: 11-year microbial and clinical overview. Ophthalmology. 2011;118(6):1193–200. doi: 10.1016/j.ophtha.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 52.Davitt B, Gehrs K, Bowers T. Endogenous Nocardia endophthalmitis. Retina. 1998;18(1):71–3. doi: 10.1097/00006982-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Azap A, Arslan H, Ergin F, et al. Disseminated Nocardia asteroides and coinfection with Trichophyton rubrum in a renal transplant recipient. Transpl Infect Dis. 2002;4(4):223–5. doi: 10.1034/j.1399-3062.2002.02006.x. [DOI] [PubMed] [Google Scholar]
- 54.Ng EW, Zimmer-Galler IE, Green WR. Endogenous Nocardia asteroides endophthalmitis. Arch Ophthalmol. 2002;120(2):210–3. [PubMed] [Google Scholar]
- 55.de Silva T, Evans C, Mudhar HS, et al. Isolated endogenous endophthalmitis secondary to Nocardia spp in an immunocompetent adult. J Clin Pathol. 2006;59(11):1226. doi: 10.1136/jcp.2005.036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sher NA, Hill CW, Eifrig DE. Bilateral intraocular Nocardia asteroides infection. Arch Ophthalmol. 1977;95(8):1415–8. doi: 10.1001/archopht.1977.04450080125015. [DOI] [PubMed] [Google Scholar]
- 57.Ishibashi Y, Watanabe R, Hommura S, et al. Endogenous Nocardia asteroides endophthalmitis in a patient with systemic lupus erythematosus. Br J Ophthalmol. 1990;74(7):433–6. doi: 10.1136/bjo.74.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelayes DE, Colombero D, Gioino JM, et al. Endogenous Nocardia asteroides endophthalmitis in a patient with systemic lupus erythematosus. Medicina (B Aires) 2004;64(2):146–8. [PubMed] [Google Scholar]
- 59.Dodds EM, Echandi LV, Puente SI, et al. Subretinal abscess due to Nocardia farcinica resistant to trimethoprim- sulfamethoxazole in a patient with systemic lupus erythematosus. Ocul Immunol Inflamm. 2006;14(4):249–51. doi: 10.1080/09273940600760514. [DOI] [PubMed] [Google Scholar]
- 60.Zaatreh M, Alabulkarim W. Images in clinical medicine. Disseminated central nervous system nocardiosis. N Engl J Med. 2006;354(26):2802. doi: 10.1056/NEJMicm990952. [DOI] [PubMed] [Google Scholar]
- 61.Chen LY, Kesen MR, Ghafourian A, et al. Isolated endogenous Nocardia endophthalmitis after immunosuppression. J Ophthalmic Inflamm Infect. 2012;2(3):141–3. doi: 10.1007/s12348-011-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu E, Laughlin S, Kassel EE, et al. Nocardial endophthalmitis and subretinal abscess: CT and MR imaging features with pathologic correlation: a case report. AJNR Am J Neuroradiol. 2005;26(5):1220–2. [PMC free article] [PubMed] [Google Scholar]
- 63.Gregor RJ, Chong CA, Augsburger JJ, et al. Endogenous Nocardia asteroides subretinal abscess diagnosed by transvitreal fine-needle aspiration biopsy. Retina. 1989;9(2):118–21. doi: 10.1097/00006982-198909020-00009. [DOI] [PubMed] [Google Scholar]
- 64.Knouse MC, Lorber B. Early diagnosis of Nocardia asteroides endophthalmitis by retinal biopsy: case report and review. Rev Infect Dis. 1990;12(3):393–8. doi: 10.1093/clinids/12.3.393. [DOI] [PubMed] [Google Scholar]
- 65.Phillips WB, Shields CL, Shields JA, et al. Nocardia choroidal abscess. Br J Ophthalmol. 1992;76(11):694–6. doi: 10.1136/bjo.76.11.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bozbeyoglu S, Yilmaz G, Akova YA, et al. Choroidal abscess due to nocardial infection in a renal allograft recipient. Retina. 2004;24(1):164–6. doi: 10.1097/00006982-200402000-00027. [DOI] [PubMed] [Google Scholar]
- 67.Yin X, Liang S, Sun X, et al. Ocular nocardiosis: HSP65 gene sequencing for species identification of Nocardia spp. Am J Ophthalmol. 2007;144(4):570–3. doi: 10.1016/j.ajo.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 68.Johnson MW, Greven GM, Jaffe GJ, et al. Atypical, severe toxoplasmic retinochoroiditis in elderly patients. Ophthalmology. 1997;104(1):48–57. doi: 10.1016/s0161-6420(97)30362-5. [DOI] [PubMed] [Google Scholar]
- 69.Moshfeghi DM, Dodds EM, Couto CA, et al. Diagnostic approaches to severe, atypical toxoplasmosis mimicking acute retinal necrosis. Ophthalmology. 2004;111(4):716–25. doi: 10.1016/j.ophtha.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Miller D, Davis J, Rosa R, et al. Utility of tissue culture for detection of Toxoplasma gondii in vitreous humor of patients diagnosed with toxoplasmic retinochoroiditis. J Clin Microbiol. 2000;38(10):3840–2. doi: 10.1128/jcm.38.10.3840-3842.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Figueroa MS, Bou G, Marti-Belda P, et al. Diagnostic value of polymerase chain reaction in blood and aqueous humor in immunocompetent patients with ocular toxoplasmosis. Retina. 2000;20(6):614–9.1. doi: 10.1097/00006982-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Bou G, Figueroa MS, Marti-Belda P, et al. Value of PCR for detection of Toxoplasma gondii in aqueous humor and blood samples from immunocompetent patients with ocular toxoplasmosis. J Clin Microbiol. 1999;37(11):3465–8. doi: 10.1128/jcm.37.11.3465-3468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garweg JG, Jacquier P, Boehnke M. Early aqueous humor analysis in patients with human ocular toxoplasmosis. J Clin Microbiol. 2000;38(3):996–1001. doi: 10.1128/jcm.38.3.996-1001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Labalette P, Delhaes L, Margaron F, et al. Ocular toxoplasmosis after the fifth decade. Am J Ophthalmol. 2002;133(4):506–15. doi: 10.1016/s0002-9394(02)01324-7. [DOI] [PubMed] [Google Scholar]
- 75.Fardeau C, Romand S, Rao NA, et al. Diagnosis of toxoplasmic retinochoroiditis with atypical clinical features. Am J Ophthalmol. 2002;134(2):196–203. doi: 10.1016/s0002-9394(02)01500-3. [DOI] [PubMed] [Google Scholar]
- 76.Lee SE, Hong SH, Lee SH, et al. Detection of ocular Toxoplasma gondii infection in chronic irregular recurrent uveitis by PCR. Korean J Parasitol. 2012;50(3):229–31. doi: 10.3347/kjp.2012.50.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Montoya JG, Parmley S, Liesenfeld O, et al. Use of the polymerase chain reaction for diagnosis of ocular toxoplasmosis. Ophthalmology. 1999;106(8):1554–63. doi: 10.1016/S0161-6420(99)90453-0. [DOI] [PubMed] [Google Scholar]
- 78.Fekkar A, Bodaghi B, Touafek F, et al. Comparison of immunoblotting, calculation of the Goldmann-Witmer coefficient, and real-time PCR using aqueous humor samples for diagnosis of ocular toxoplasmosis. J Clin Microbiol. 2008;46(6):1965–7. doi: 10.1128/JCM.01900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Talabani H, Asseraf M, Yera H, et al. Contributions of immunoblotting, real-time PCR, and the Goldmann-Witmer coefficient to diagnosis of atypical toxoplasmic retinochoroiditis. J Clin Microbiol. 2009;47(7):2131–5. doi: 10.1128/JCM.00128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santos FF, Commodaro AG, Souza AV, et al. Real-time PCR in infectious uveitis as an alternative diagnosis. Arq Bras Oftalmol. 2011;74(4):258–61. doi: 10.1590/s0004-27492011000400006. [DOI] [PubMed] [Google Scholar]
- 81.Uy HS, Foster CS. Diagnostic vitrectomy and uveitis. Int Ophthalmol Clin. 1999;39(1):223–35. doi: 10.1097/00004397-199903910-00020. [DOI] [PubMed] [Google Scholar]
- 82.Rothova A, de Boer JH, Ten Dam-van Loon NH, et al. Usefulness of aqueous humor analysis for the diagnosis of posterior uveitis. Ophthalmology. 2008;115(2):306–11. doi: 10.1016/j.ophtha.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 83.De Groot-Mijnes JD, Rothova A, Van Loon AM, et al. Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol. 2006;141(2):313–8. doi: 10.1016/j.ajo.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 84.Westeneng AC, Rothova A, de Boer JH, et al. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and Goldmann-Witmer coefficient in aqueous analysis. Am J Ophthalmol. 2007;144(5):781–5. doi: 10.1016/j.ajo.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 85.Matos K, Muccioli C, Belfort R, Junior, et al. Correlation between clinical diagnosis and PCR analysis of serum, aqueous, and vitreous samples in patients with inflammatory eye disease. Arq Bras Oftalmol. 2007;70(1):109–14. doi: 10.1590/s0004-27492007000100020. [DOI] [PubMed] [Google Scholar]
- 86.Sugita S, Shimizu N, Watanabe K, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92(7):928–32. doi: 10.1136/bjo.2007.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harper TW, Miller D, Schiffman JC, et al. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol. 2009;147(1):140–7. doi: 10.1016/j.ajo.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schneier AJ, Durand ML. Ocular toxocariasis: advances in diagnosis and treatment. Int Ophthalmol Clin. 2011;51(4):135–44. doi: 10.1097/IIO.0b013e31822d6a5a. [DOI] [PubMed] [Google Scholar]
- 89.de Visser L, Rothova A, de Boer JH, et al. Diagnosis of ocular toxocariasis by establishing intraocular antibody production. Am J Ophthalmol. 2008;145(2):369–74. doi: 10.1016/j.ajo.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 90.Stewart JM, Cubillan LD, Cunningham ET., Jr Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005;25(8):1005–13. doi: 10.1097/00006982-200512000-00009. [DOI] [PubMed] [Google Scholar]
- 91.Yokoi K, Goto H, Sakai J, et al. Clinical features of ocular toxocariasis in Japan. Ocul Immunol Inflamm. 2003;11(4):269–75. doi: 10.1076/ocii.11.4.269.18266. [DOI] [PubMed] [Google Scholar]
- 92.Acar N, Kapran Z, Utine CA, et al. Pars plana vitrectomy revealed Toxocara canis organism. Int Ophthalmol. 2007;27(4):277–80. doi: 10.1007/s10792-007-9078-1. [DOI] [PubMed] [Google Scholar]
- 93.Gonvers M, Mermoud A, Uffer S, et al. Ocular toxocara canis in a 30-year-old adult. Klin Monbl Augenheilkd. 1992;200(5):522–4. doi: 10.1055/s-2008-1045814. [DOI] [PubMed] [Google Scholar]
- 94.Amin HI, McDonald HR, Han DP, et al. Vitrectomy update for macular traction in ocular toxocariasis. Retina. 2000;20(1):80–5. doi: 10.1097/00006982-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 95.Maguire AM, Green WR, Michels RG, et al. Recovery of intraocular Toxocara canis by pars plana vitrectomy. Ophthalmology. 1990;97(5):675–80. doi: 10.1016/s0161-6420(90)32528-9. [DOI] [PubMed] [Google Scholar]
- 96.Werner JC, Ross RD, Green WR, et al. Pars plana vitrectomy and subretinal surgery for ocular toxocariasis. Arch Ophthalmol. 1999;117(4):532–4. doi: 10.1001/archopht.117.4.532. [DOI] [PubMed] [Google Scholar]
- 97.Meyer-Riemann W, Petersen J, Vogel M. An attempt to extract an intraretinal nematode located in the papillomacular bundle. Klin Monbl Augenheilkd. 1999;214(2):116–9. doi: 10.1055/s-2008-1034761. [DOI] [PubMed] [Google Scholar]
- 98.de Souza EC, Nakashima Y. Diffuse unilateral subacute neuroretinitis. Report of transvitreal surgical removal of a subretinal nematode. Ophthalmology. 1995;102(8):1183–6. doi: 10.1016/s0161-6420(95)30892-5. [DOI] [PubMed] [Google Scholar]
- 99.Felberg NT, Shields JA, Federman JL. Antibody to Toxocara canis in the aqueous humor. Arch Ophthalmol. 1981;99(9):1563–4. doi: 10.1001/archopht.1981.03930020437005. [DOI] [PubMed] [Google Scholar]
- 100.Benitez del Castillo JM, Herreros G, Guillen JL, et al. Bilateral ocular toxocariasis demonstrated by aqueous humor enzyme-linked immunosorbent assay. Am J Ophthalmol. 1995;119(4):514–6. doi: 10.1016/s0002-9394(14)71241-3. [DOI] [PubMed] [Google Scholar]
- 101.Yoshida M, Shirao Y, Asai H, et al. A retrospective study of ocular toxocariasis in Japan: correlation with antibody prevalence and ophthalmological findings of patients with uveitis. J Helminthol. 1999;73(4):357–61. doi: 10.1017/s0022149x99000608. [DOI] [PubMed] [Google Scholar]
- 102.Alabiad CR, Albini TA, Santos CI, et al. Ocular Toxocariasis in a Seronegative Adult. Ophthalmic Surg Lasers Imaging. 2010:1–3. doi: 10.3928/15428877-20100325-06. [DOI] [PubMed] [Google Scholar]
- 103.Hagler WS, Pollard ZF, Jarrett WH, et al. Results of surgery for ocular Toxocara canis. Ophthalmology. 1981;88(10):1081–6. doi: 10.1016/s0161-6420(81)80039-5. [DOI] [PubMed] [Google Scholar]
- 104.Lim SJ, Lee SE, Kim SH, et al. Prevalence of Toxoplasma gondii and Toxocara canis among Patients with Uveitis. Ocul Immunol Inflamm. 2013 doi: 10.3109/09273948.2013.839798. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 105.Fox GM, Crouse CA, Chuang EL, et al. Detection of herpesvirus DNA in vitreous and aqueous specimens by the polymerase chain reaction. Arch Ophthalmol. 1991;109(2):266–71. doi: 10.1001/archopht.1991.01080020112054. [DOI] [PubMed] [Google Scholar]
- 106.Pendergast SD, Werner J, Drevon A, et al. Absence of herpesvirus DNA by polymerase chain reaction in ocular fluids obtained from immunocompetent patients. Retina. 2000;20(4):389–93. doi: 10.1097/00006982-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 107.Abe T, Tsuchida K, Tamai M. A comparative study of the polymerase chain reaction and local antibody production in acute retinal necrosis syndrome and cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol. 1996;234(7):419–24. doi: 10.1007/BF02539407. [DOI] [PubMed] [Google Scholar]
- 108.Pavesio CE, Mitchell SM, Barton K, et al. Progressive outer retinal necrosis (PORN) in AIDS patients: a different appearance of varicella-zoster retinitis. Eye (Lond) 1995;9:271–6. doi: 10.1038/eye.1995.53. [DOI] [PubMed] [Google Scholar]
- 109.Itoh N, Matsumura N, Ogi A, et al. High prevalence of herpes simplex virus type 2 in acute retinal necrosis syndrome associated with herpes simplex virus in Japan. Am J Ophthalmol. 2000;129(3):404–5. doi: 10.1016/s0002-9394(99)00391-8. [DOI] [PubMed] [Google Scholar]
- 110.Ganatra JB, Chandler D, Santos C, et al. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2000;129(2):166–72. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
- 111.Tran TH, Rozenberg F, Cassoux N, et al. Polymerase chain reaction analysis of aqueous humour samples in necrotising retinitis. Br J Ophthalmol. 2003;87(1):79–83. doi: 10.1136/bjo.87.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yeh S, Fahle G, Forooghian F, et al. Polymerase chain reaction-based ganciclovir resistance testing of ocular fluids for cytomegalovirus retinitis. Arch Ophthalmol. 2012;130(1):113–5. doi: 10.1001/archophthalmol.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Read RW, Zamir E, Rao NA. Neoplastic masquerade syndromes. Surv Ophthalmol. 2002;47(2):81–124. doi: 10.1016/s0039-6257(01)00305-8. [DOI] [PubMed] [Google Scholar]
- 114.Tsai T, O’Brien JM. Masquerade syndromes: malignancies mimicking inflammation in the eye. Int Ophthalmol Clin. 2002;42(1):115–31. doi: 10.1097/00004397-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 115.Rothova A, Ooijman F, Kerkhoff F, et al. Uveitis masquerade syndromes. Ophthalmology. 2001;108(2):386–99. doi: 10.1016/s0161-6420(00)00499-1. [DOI] [PubMed] [Google Scholar]
- 116.Davis JL, Viciana AL, Ruiz P. Diagnosis of intraocular lymphoma by flow cytometry. Am J Ophthalmol. 1997;124(3):362–72. doi: 10.1016/s0002-9394(14)70828-1. [DOI] [PubMed] [Google Scholar]
- 117.Davis JL. Intraocular lymphoma: a clinical perspective. Eye (Lond) 2013;27(2):153–62. doi: 10.1038/eye.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yap EY, Robertson DM, Buettner H. Scleritis as an initial manifestation of choroidal malignant melanoma. Ophthalmology. 1992;99(11):1693–7. doi: 10.1016/s0161-6420(92)31744-0. [DOI] [PubMed] [Google Scholar]
- 119.Moshari A, Cheeseman EW, McLean IW. Totally necrotic choroidal and ciliary body melanomas: associations with prognosis, episcleritis, and scleritis. Am J Ophthalmol. 2001;131(2):232–6. doi: 10.1016/s0002-9394(00)00783-2. [DOI] [PubMed] [Google Scholar]
- 120.Kafkala C, Daoud YJ, Paredes I, et al. Masquerade scleritis. Ocul Immunol Inflamm. 2005;13(6):479–82. doi: 10.1080/09273940591004133. [DOI] [PubMed] [Google Scholar]
- 121.Palamar M, Thangappan A, Shields CL, et al. Necrotic choroidal melanoma with scleritis and choroidal effusion. Cornea. 2009;28(3):354–6. doi: 10.1097/ICO.0b013e3181875463. [DOI] [PubMed] [Google Scholar]
- 122.Martin B. Diffuse malignant melanoma of the iris. Trans Ophthalmol Soc U K. 1973;93(0):473–5. [PubMed] [Google Scholar]
- 123.al-Haddab S, Hidayat A, Tabbara KF. Ciliary body melanoma with optic nerve invasion. Br J Ophthalmol. 1990;74(2):123–4. doi: 10.1136/bjo.74.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mathew B, Brownstein S, Kertes PJ, et al. Clinically unsuspected diffuse uveal melanoma presenting as recurrent iritis. Can J Ophthalmol. 2004;39(4):464–7. doi: 10.1016/s0008-4182(04)80021-8. [DOI] [PubMed] [Google Scholar]
- 125.Kearns C, Boyer S, Gay D. Two differing presentations, treatments, and outcomes of malignant choroidal melanoma. Optometry. 2008;79(7):365–70. doi: 10.1016/j.optm.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 126.Nagpal A, Biswas J. Pseudouveitis--analysis of cases misdiagnosed as posterior uveitis. Ocul Immunol Inflamm. 2006;14(1):13–20. doi: 10.1080/09273940500545627. [DOI] [PubMed] [Google Scholar]
- 127.Nguyen QD, Foster CS. Ciliary body melanoma masquerading as chronic uveitis. Ocul Immunol Inflamm. 1998;6(4):253–6. doi: 10.1076/ocii.6.4.253.4031. [DOI] [PubMed] [Google Scholar]
- 128.Sood NN, Ratnaraj A. Malignant melanoma of choroid presenting as panophthalmitis. J All India Ophthalmol Soc. 1968;16(2):67–9. [PubMed] [Google Scholar]
- 129.Kline LB, Bright M, Brownstein S. Uveal melanoma presenting as post-traumatic choroidal hemorrhage and panophthalmitis. Can J Ophthalmol. 1977;12(3):226–9. [PubMed] [Google Scholar]
- 130.Pizzuto D, deLuise V, Zimmerman N. Choroidal malignant melanoma appearing as acute panophthalmitis. Am J Ophthalmol. 1986;101(2):249–51. doi: 10.1016/0002-9394(86)90606-9. [DOI] [PubMed] [Google Scholar]
- 131.Fraser DJ, Jr, Font RL. Ocular inflammation and hemorrhage as initial manifestations of uveal malignant melanoma. Incidence and prognosis. Arch Ophthalmol. 1979;97(7):1311–4. doi: 10.1001/archopht.1979.01020020053012. [DOI] [PubMed] [Google Scholar]
- 132.Kavanagh MC, Everman KR, Opremcak EM, et al. Uveal melanoma with massive extrascleral extension via pars plana vitrectomy sites. Ophthal Plast Reconstr Surg. 2008;24(4):334–6. doi: 10.1097/IOP.0b013e31817e91ce. [DOI] [PubMed] [Google Scholar]
- 133.Sakuma T, Iseki R, Mimura A, et al. Rapid cytologic diagnosis of choroidal malignant melanoma by vitreous smear. J Fr Ophtalmol. 2012;35(7):535, e1–4. doi: 10.1016/j.jfo.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 134.Shields CL, Ganguly A, Materin MA, et al. Chromosome 3 analysis of uveal melanoma using fine-needle aspiration biopsy at the time of plaque radiotherapy in 140 consecutive cases. Trans Am Ophthalmol Soc. 2007;105:43–53. [PMC free article] [PubMed] [Google Scholar]
- 135.Bagger M, Tebering JF, Kiilgaard JF. The Ocular Consequences and Applicability of Minimally Invasive 25-Gauge Transvitreal Retinochoroidal Biopsy. Ophthalmology. 2013;120(12):2565–72. doi: 10.1016/j.ophtha.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 136.Schefler AC, Gologorsky D, Marr BP, et al. Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA Ophthalmol. 2013;131(9):1220–4. doi: 10.1001/jamaophthalmol.2013.2506. [DOI] [PubMed] [Google Scholar]
- 137.Talegaonkar SK. Anterior uveal tract metastasis as the presenting feature of bronchial carcinoma. Br J Ophthalmol. 1969;53(2):123–6. doi: 10.1136/bjo.53.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Morgan WE, 3rd, Malmgren RA, Albert DM. Metastatic carcinoma of the ciliary body simulating uveitis. Diagnosis by cytologic examination of aqueous humor. Arch Ophthalmol. 1970;83(1):54–8. doi: 10.1001/archopht.1970.00990030056010. [DOI] [PubMed] [Google Scholar]
- 139.Denslow GT, Kielar RA. Metastatic adenocarcinoma to the anterior uvea and increased carcinoembryonic antigen levels. Am J Ophthalmol. 1978;85(3):363–7. doi: 10.1016/s0002-9394(14)77731-1. [DOI] [PubMed] [Google Scholar]
- 140.Scholz R, Green WR, Baranano EC, et al. Metastatic carcinoma to the iris. Diagnosis by aqueous paracentesis and response to irradiation and chemotherapy. Ophthalmology. 1983;90(12):1524–7. doi: 10.1016/s0161-6420(83)34375-x. [DOI] [PubMed] [Google Scholar]
- 141.Takahashi T, Oda Y, Chiba T, et al. Metastatic carcinoma of the iris and ciliary body simulating iridocyclitis. Ophthalmologica. 1984;188(4):266–72. doi: 10.1159/000309373. [DOI] [PubMed] [Google Scholar]
- 142.Woog JJ, Chess J, Albert DM, et al. Metastatic carcinoma of the iris simulating iridocyclitis. Br J Ophthalmol. 1984;68(3):167–73. doi: 10.1136/bjo.68.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Michelson JB, Grossman KR, Lozier JR, et al. Iridocyclitis masquerade syndrome. Surv Ophthalmol. 1986;31(2):125–30. doi: 10.1016/0039-6257(86)90080-9. [DOI] [PubMed] [Google Scholar]
- 144.Lieb WE, Shields JA, Shields CL, et al. Mucinous adenocarcinoma metastatic to the iris, ciliary body, and choroid. Br J Ophthalmol. 1990;74(6):373–6. doi: 10.1136/bjo.74.6.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kesen MR, Edward DP, Ulanski LJ, et al. Pulmonary metastasis masquerading as anterior uveitis. Arch Ophthalmol. 2008;126(4):572–4. doi: 10.1001/archopht.126.4.572. [DOI] [PubMed] [Google Scholar]
- 146.Iannetti L, Corsi C, Iafrate F, et al. Bilateral uveitis with hypopyon as a presenting symptom of metastatic peritoneal carcinomatosis. Eur J Ophthalmol. 2010;20(5):948–51. doi: 10.1177/112067211002000522. [DOI] [PubMed] [Google Scholar]
- 147.Payne JF, Rahman HT, Grossniklaus HE, et al. Retinal metastasis simulating cytomegalovirus retinitis. Ophthalmic Surg Lasers Imaging. 2012;43:e90–3. doi: 10.3928/15428877-20120823-06. [DOI] [PubMed] [Google Scholar]
- 148.Jaissle GB, Szurman P, Rohrbach JM, et al. A case of cutaneous melanoma metastatic to the vitreous cavity: possible pathomechanism and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2007;245(5):733–40. doi: 10.1007/s00417-006-0469-1. [DOI] [PubMed] [Google Scholar]
- 149.Konstantinidis L, Rospond-Kubiak I, Zeolite I, et al. Management of patients with uveal metastases at the Liverpool Ocular Oncology Centre. Br J Ophthalmol. 2013 doi: 10.1136/bjophthalmol-2013-303519. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 150.Shields JA, Shields CL, Ehya H, et al. Fine-needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology. 1993;100(11):1677–84. doi: 10.1016/s0161-6420(93)31418-1. [DOI] [PubMed] [Google Scholar]