Abstract
Spectral sensitivity varies markedly across the center of gaze, in part because of the rapid decline in the density of macular pigment outside the fovea. Yet despite these retinal inhomogeneities, the color appearance of large uniform fields remains very uniform. We explored some of the processes contributing to these stable color percepts by measuring the effects of field size and eccentricity on saturated purples, whose spectra should show the largest biases with macular pigment screening. Small purple fields at 0° and 8° eccentricities differ in appearance but by much less than predicted by the macular screening or by compensation for the average effects of this screening at the two loci. This shows that the compensation is already nearly complete because of local adjustments that filter out the sensitivity variation and confirms that this filtering includes adjustments beyond average gain changes in the cones. In large fields, the appearance is dominated by the local peripheral color. This bias persists when the field edge is fixated or when abrupt edges are removed in Gaussian spots, suggesting that the spreading is not strongly dependent on luminance edges.
1. INTRODUCTION
Spectral sensitivity at the center of gaze is markedly different from the nearby periphery, in large part because of the screening effects of macular pigment. The pigment density peaks in the central fovea but falls rapidly with eccentricity, so that it has relatively little influence beyond a few degrees [1–3]. Because the pigment primarily absorbs short-wavelength light, sensitivity within the fovea to shorter wavelengths is typically much lower than the surrounding retina (for example, an average difference of roughly 0.5 log units at 458 nm, the wave-length where the macular pigment absorption is greatest [4]).
The screening effects of macular pigment can sometimes be seen in entoptic phenomena such as Haidinger’s brushes or Maxwell’s spot [1,5,6]. The former is a percept (of a bar, dumb-bell, etc.) related to the polarization of light, and its appearance relates to chemical properties of the macular pigment [7]. Maxwell’s spot, on the other hand, is typically observed when viewing a flickering purple field composed of short and long wavelengths, which looks darker and redder in the center because of the macular filtering. It also has a large effect on actual measures of spectral sensitivity and is one of the primary sources for the differences in small and large field color matches [8]. Yet what is more intriguing is how seldom we notice it. That is, the color of a stable uniform surface typically appears uniform, even though the spectrum of light reaching the foveal and peripheral photoreceptors is very different.
In this study we explored some of the factors contributing to this stability of color appearance. A number of different processes are thought to play a role. Some involve local adjustments of visual sensitivity. Beer et al. [9] and Webster and Leonard [10] measured the perception of white across the central visual field and found that the appearance of achromatic hue (loci) remained constant between 0° and 8°. Thus the perception of white was completely compensated for by the differences in macular pigment filtering. Webster and Leonard further showed that this sensitivity adjustment occurs at or before the site of short-term chromatic adaptation, suggesting that it reflects a long-term gain adjustment in the cones so that they are each adapted to the average local spectrum they are exposed to [10].
More recently, Webster et al. [11] compared perceived hues at 0° and 8° and found that they also remained highly stable at the two loci, and in fact were more similar than would be predicted by adjusting the sensitivity of each part of the retina only to compensate for the same average white. Specifically, it is well known from the color constancy literature [12,13] that changes in illumination cannot be discounted completely by von Kries adaptation (i.e., independent gain changes) in each of the cones because the relative changes in cone excitations from the illuminant vary depending on the surface reflectance. In the same way, screening the light with a filter like macular pigment could not be compensated only by rescaling the average sensitivity of each cone. The finding that color constancy was actually better than von Kries scaling implies that additional mechanisms contribute to the stability of color appearance across the visual field [11,14] and argues against suggestions from hue cancellation experiments that only some dimensions of color coding are compensated for macular screening [15–17]. Candidate cell types that could promote constancy beyond photoreceptor adaptation, such as double-opponent receptive fields, have been considered in a wide variety of studies (e.g., [18–22]).
Because Webster et al. examined a wide range of only moderately saturated hues, their analysis was not sensitive to small discrepancies in the appearance of individual hues. However, Bompas et al. [14] subsequently focused on comparing the color appearance of purple and more saturated surfaces, where any effects of macular pigment should be greatest. They also found that color appearance was more stable than would be predicted by average gain changes in the foveal and peripheral cones. However, they emphasized that small differences between many of the colors remained, and they suggested that this reflects a failure of a sensory–motor account of perception where the visual system learns to associate constant percepts with constant objects, even if they are viewed with different parts of the retina as the eye samples different locations in the scene [14,23]. Finally, O’Neil et al. also drew attention to weak failures of compensation for shortwave spectra [24]. Their study explored the hypothesis that compensation for the spectral filtering in the eye underlies the Abney effect, a classic color appearance phenomenon in which adding white light to a wavelength changes not only its saturation but also its hue [25,26]. They argued that the visual system was applying an inference that the spectrum is Gaussian (and was thus “fooled” by the Abney stimulus, which is not), and attempting to tie perceived hue to a constant inferred peak in the spectrum even though the relative cone excitations for a given peak are altered as the bandwidth of the spectrum increases [27]. By this account, the visual system would need to apply different corrections in the fovea and periphery since the filtering at the two loci differs. Their results provided partial support for this idea but also drew attention to the residual color appearance differences between shortwave spectra in the fovea and periphery (an example of these differences is shown in Fig. 1 of [24]). In summary, together these studies suggest that purely local calibrations may correct for most of the effects of macular screening on color appearance, and substantially more than predicted by local adaptation to the average spectral stimulus. But they do not correct for all of it.
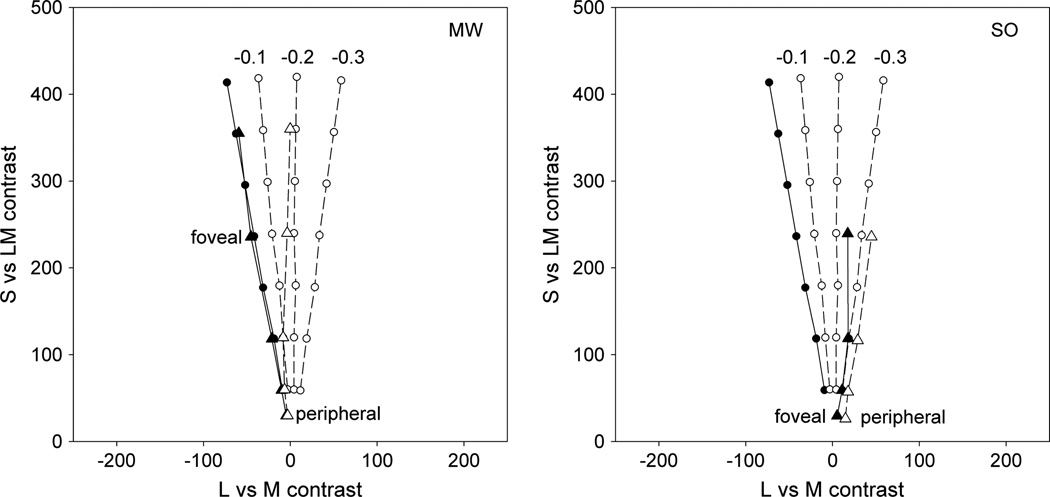
Fig. 1.
Circles: predictions for LvsM and SvsLM contrast settings needed to maintain a balanced purple for full macular pigment (solid line) and for macular pigment reductions of 0.1 to 0.3 log units (dashed lines). Triangles: actual settings by observers MW and SO for foveal settings (solid symbols and solid line) or peripheral settings at 8° (empty symbols and dashed line).
In the present study we further examined these residual color differences and also explored how they are manifest in the color appearance of large fields. If a purple appears more reddish in the fovea and more bluish in the periphery, what is the perceived color of a field that encompasses both locations? Here again there are a number of factors that may come into play. A wide range of studies have explored filling-in processes in perception, where information from one point in the image spreads to influence the percepts at other locations [28,29], such as completion of the blind spot [30,31]. Again these can include a number of distinct mechanisms since they can vary widely in their spatial extent and temporal dynamics (e.g., [32–37]). In color appearance filling-in effects are well known in phenomena such as the Craik–Cornsweet illusion [38], color spreading and neon color effects [39,40], and the water-color illusion [35]. In each the color at the edges spreads into the uniform areas bounded by the edges, emphasizing the potential importance of luminance edges in generating and gating the filled-in percepts [41]. These filling-in effects have also been specifically explored to examine color appearance in the retina. Shortwave cones are absent or are very sparse in the central fovea, leading to small-field tritanopia [42]. This “blue cone scotoma” can again be seen as a small dark spot with blue flickering fields but is normally invisible because of filling in from the periphery [43]. By such accounts, large fields should appear more like the peripheral color and might in particular depend on the color at the edges.
However, there is also evidence for a variety of possible “filling-out” processes, or spreading of the color from the center of gaze. Chromatic sensitivity is best in the fovea [44], and thus it might theoretically be optimal to use the highest quality estimate of the color and apply that to peripheral locations, where the evidence is weaker. An intriguing example of this type of effect was reported by Balas and Sinha [45]. They processed images to gradually reduce the color contrast at increasing distances from the center so that the image was physically grayscale in the periphery. Yet when observers fixated the center, the image appeared uniformly colorful. Thus processes like this might instead predict that the centrally fixated hue should dominate the appearance. To explore these questions, we compared the appearance of small and large purple fields presented at different locations.
2. METHODS
A. Participants
Observers were the two authors. Both have normal color vision as assessed by standard screening tests, and normal visual acuity. Participation was with informed consent and followed protocols approved by the university’s Institutional Review Board.
B. Apparatus
Stimuli were presented on a SONY 20SE CRT monitor controlled by a VSG 2/3 graphics card (Cambridge Research Systems). The display was calibrated and gamma-corrected based on measurements with a Photo Research PR650 spectroradiometer.
C. Stimuli
The stimuli consisted of a single circular spot of uniform chromaticity, displayed on a uniform gray background (20° by 15°). The size of the spot was varied across conditions and ranged from 1° to 16° in diameter. The spot was always displayed in the center of the screen. Fixation during peripheral conditions was controlled by adding a small black fixation cross at various locations in the background. The display was viewed binocularly from a distance of 114 cm in an otherwise dark room. In most cases the spot was equiluminant with the background, and we therefore added small (0.05°) dark edges to demarcate it. In a subset of conditions the luminance of the background was instead varied, or the uniform stimulus was replaced with a tapered Gaussian spot (with no border). These conditions are noted in Section 3.
Chromaticities were defined according to a scaled version of the LvsM and SvsLM chromatic plane:
where rmb, bmb are the coordinates of the color in the MacLeod–Boynton (MB) chromaticity diagram [46]; 2754, 4099 are scaling constants to amplify the distances in MB space so that contrast units corresponded very roughly to multiples of threshold for color changes along the two axes [47]; and 0.6568, 0.01825 are the MB coordinates of the zero contrast background (equivalent to the chromaticity of Illuminant C). The luminance of the spot, and in most cases also the background, was 5 cd/m2 and was defined photometrically. This low luminance was necessary because the displayed colors were all different shades of blue and purple and thus primarily limited by the luminance of the blue phosphor.
D. Procedure
As noted, for our experiments we concentrated on the color appearance of purplish spectra because the foveal and peripheral differences in appearance are largest for these stimuli. To judge appearance, observers adjusted the stimulus until it appeared a balanced purple, or an equal mixture of red and blue. This corresponds very roughly to the +S pole or an angle of 90° in the LvsM (reddish-cyan) and SvsLM (purple-yellow/green) cone-opponent space [48] and is a judgment that observers can make with high reliability and which allowed a single color to be shown in isolation. Shifts toward smaller angles appear too reddish, while shifts toward larger angles appear too bluish. The settings were thus done by varying the hue angle of the stimulus within the plane. Observers first adapted to the gray background for 30 s, and the spot was then displayed for 500 ms (or 5 s in some conditions). The observer then made a two-alternative response to indicate whether the perceived hue appeared “too reddish” or “too bluish.” A stair-case varied the hue angle of successive stimuli in steps of 6° initially and then 2° after the fourth reversal. Mean settings were based on the average of the final eight reversals (all from 2° steps). Results reported are based on the average settings from four staircases per condition, with the order of conditions counterbalanced.
3. RESULTS
A. Hue Shifts between the Fovea and Periphery
Figure 1 plots the purple settings for 1° spots presented to the fovea or at 8° in the periphery. Again these were measured by varying the hue angle until the stimulus appeared an equal balance of red and blue and were assessed over a wide range of contrast levels. The measured values thus plot the loci of balanced purple in the LvsM and SvsLM plane. These are roughly linear but differ by an angle of ~10° for the two retinal locations. Specifically, the peripheral settings correspond to a redder hue angle, indicating that the foveal setting appeared too blue when shown in the periphery. This is of course the shift predicted by the differences in shortwave sensitivity. But how big are the hue differences?
To assess this we calculated the hue angles required for constant cone excitations at the two loci for varying differences in the density of macular pigment. These predictions were based on measurements of the gun phosphor spectra for the stimuli and on the Smith and Pokorny cone fundamentals [49] and macular pigment absorption spectrum of Bone and Sparrock [4]. The predictions do not depend critically on the choice of pigment spectra. We arbitrarily chose the stimulus spectra for the foveal purple settings for M. Webster (MW) and then found the change in the stimulus required to yield the equivalent S, M, and L cone responses when the density of macular pigment was progressively decreased. (Note: S. O’Neil (SO) chose a different setting for a balanced purple. This is unlikely to be due to differences in macular pigment between the two observers and is instead consistent with known large individual differences in reports of color appearance [47,48]). Importantly, the cone excitations were first normalized for an equal energy spectrum, and thus the predictions show the settings assuming each part of the retina is adapted to give identical responses to a white stimulus. In Fig. 1 the four predicted curves correspond to the foveal settings (full macular pigment density) or reductions from 0.1 to 0.3 log units. For MW, the observed settings correspond to a predicted difference of roughly 0.2 log units, while for SO the predicted difference is ~0.1.
These shifts in color appearance were highly reliable but are in fact substantially weaker than expected from the actual differences in macular screening at the two locations. These were assessed for the two observers using flicker photometry [50] between 460 and 570 nm stimuli at the two locations and were measured as part of an earlier study [24]. Details of the methods are given in that study. For MW, the estimated density difference between 0° and 8° was 0.72, while for SO it was 0.27. Thus, for both, the observed shifts are only about a third of the difference expected from spectral sensitivity differences, even after von Kries adaptation to equate the white settings at the two loci. Thus these results confirm a very high level of color constancy across the visual field, and specifically that this compensation includes factors above and beyond adaptation to the average stimulus level at the two locations [11,14].
B. Hue Shifts with Background Intensity
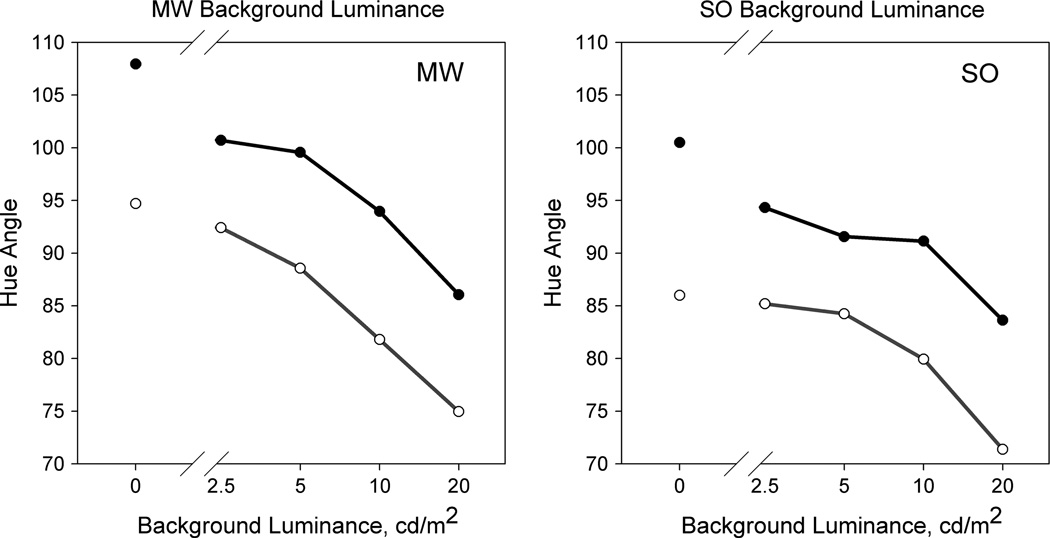
During the study we noted that the color differences between the fovea and periphery appeared largest on an equiluminant background and were difficult to discern when the spots were instead shown on a black background. To evaluate this, we conducted an ancillary experiment where we repeated the settings but with the achromatic background varied from 0 to 4 times the spot luminance (Fig. 2). The hue angle required for purple rotated toward progressively more clockwise (“redder”) angles as the background luminance increased. Yet the angular difference between the foveal and peripheral purple remained constant. Thus the background affected the discriminability of the colors, yet the biases in the purple settings remain similarly small and robust across a wide range of luminance increments and decrements.
Fig. 2.
Hue angle settings to maintain a balanced purple, as a function of monitor background luminance.
C. Effects of Field Size
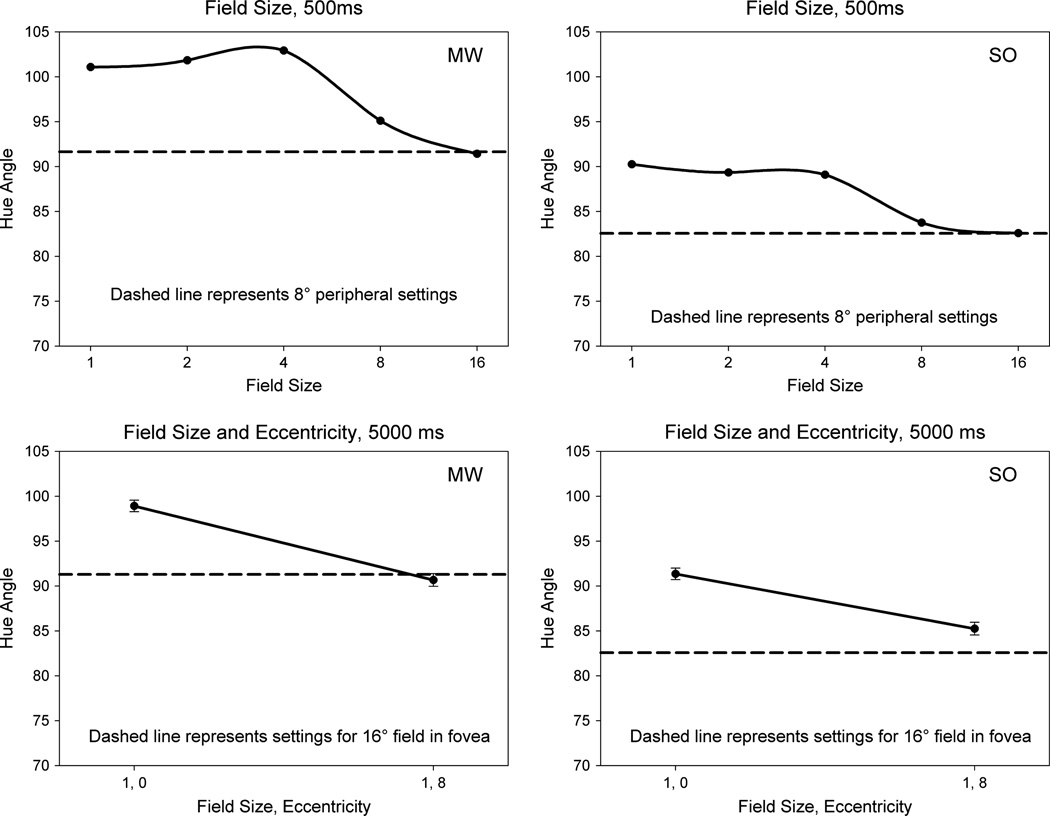
We next explored how the color changed as the field size was varied. In this case the spots were centrally fixated but ranged in diameter from 1° to 16°. Figure 3 again shows the settings for both observers. Perceived hue remained approximately constant up to a diameter of 4° but then shifted toward the peripheral settings and were equivalent to the local (1° spot) setting at 8° in the periphery when the large field itself extended to ±8°. Thus the large-field percept was completely dominated by the peripheral hue. The fields themselves appeared uniform in color. However, there was the possibility that spatial inhomogeneities akin to Maxwell’s spot might be present in the briefly presented, 500 ms stimulus. In that case, the observer’s judgment might be biased more by one part of the field. To control for this, the 1° and 16° settings were repeated for stimuli that were instead shown for 5000 ms, with the judgments based on the color appearance at the end of the interval. These fields again appeared uniform, and the prolonged presentation should have reduced any prevalence of Maxwell’s spot. The settings remained similar to the brief stimulus and again showed that the hue of the large field was equivalent (MW) or strongly shifted (SO) to the value of the local peripheral hue.
Fig. 3.
(Top) Hue angle settings from foveally fixated fields ranging in size from 1° to 16° presented for 500 ms (solid lines) compared to settings for a 1° spot presented at 8° eccentricity (dashed lines) for each observer. (Bottom) Foveal and peripheral settings for a spot presented for 5 s (solid lines) compared to a foveally presented 16° field (dashed line).
D. Effect of Fixation Location
The preceding results showed that the peripheral hue clearly dominated the color appearance of a large field. One way this could arise is simple averaging of the color signals. For example, the 16° stimulus had an area 16 times that of the 4° field, at which the foveal hue instead dominated. However, another possibility is that the color appearance depended primarily on the color appearance at the local edge and thus spread from the peripheral edges into the interior. In the final settings we explored the potential influence of these edges.
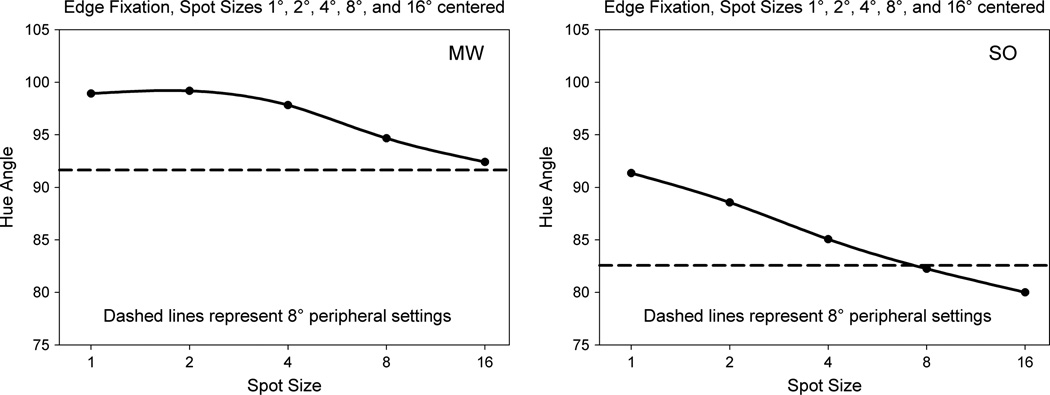
In the first case, the stimulus was again varied in diameter, but fixation was shifted from the center to the leftmost edge. This therefore differed from the prior settings insofar as the most salient edge now fell at the fovea. Filling in from the edges might therefore show a stronger influence of the foveal hue. However, the settings again became increasingly biased toward the peripheral hue as the field size increased, and the effects were roughly similar in magnitude to the shifts for the centrally fixated spots (Fig. 4). This argues against the possibility that a centrally fixated and attended edge might disproportionately influence the settings, though the peripheral bias might again be expected if the percept were based on averaging the hues across all edges.
Fig. 4.
Settings for 1° to 16° spots fixated on the edge (solid lines) compared to settings for a centrally-fixated 1° spot at 8° eccentricity.
E. Color Appearance of Gaussian Spots
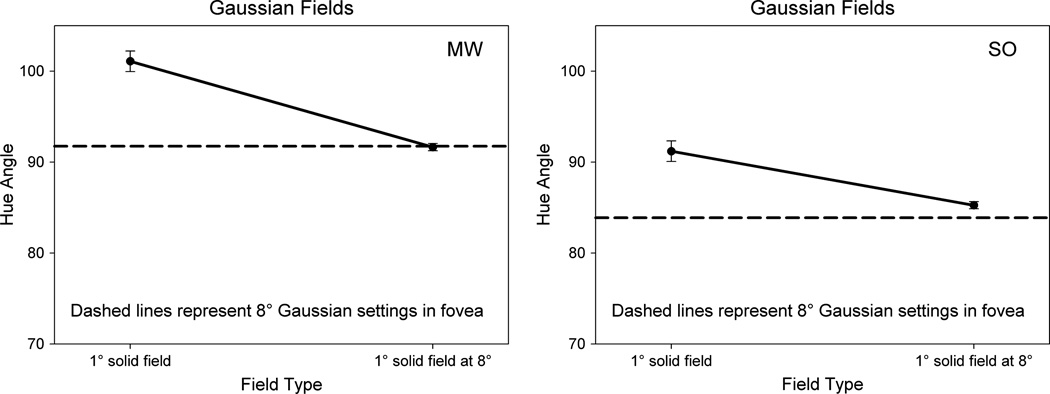
In the final case, we tried to remove all “edges” by presenting the colors as equiluminant Gaussian spots. These do not induce the transient edge responses with local eye movements, as evidenced by their tendency to fade with extended viewing [51]. The tapered spots had a standard deviation of 2.5°, and thus had a large spatial extent, but with the saturation now dominated by the point of central fixation. Surprisingly, even in this stimulus the purple settings were more consistent with the local peripheral appearance (Fig. 5). For MW the hue of the Gaussian matched his peripheral setting, while for SO in this case the biases were even larger than predicted by his local color shifts. Thus clear, discrete edges appeared unnecessary to maintain the dominance of the periphery in the color appearance.
Fig. 5.
Settings for uniform 1° fields at 0° and 8° eccentricity (solid lines) compared to a 8° foveal field with luminance edges replaced by a Gaussian taper (dashed lines).
4. DISCUSSION
In this study we explored some of the factors contributing to stable color percepts near the center of gaze, where sensitivity changes dramatically yet appearance remains nearly constant. Changes in peripheral color perception have been widely investigated, and it is evident that the visual system cannot compensate completely for the changes in sensitivity across the retina. For example, a number of studies have pointed to different rates of peripheral sensitivity change along reddish–greenish and bluish–yellowish axes (with recent examples including [52–57]), consistent with the notion of different zones of peripheral color coding [58]. Yet the suprathreshold appearance of color nevertheless exhibits substantial constancy across the visual field. The processes contributing to this constancy are uncertain and may involve multiple factors ranging from intrinsic coding such as spatial integration [59,60] to adaptation [10] to forms of learning [14,61].
For the specific context we considered, the foremost factors contributing to stable color percepts in the vicinity of the fovea involved local compensations in color coding. That is, each local area appeared to almost completely “filter out” the local sensitivity limits of the eye so that these limits were to a large extent discounted from the color percepts. Again, for the saturated purples we examined, this compensation was incomplete. Yet relative to other forms of color constancy, the “index” of constancy across the loci was extremely high. That is, the observed differences are many times smaller than the spectral shifts owing to the differences in macular pigment screening [10,11]. Note that much of this difference is already factored out of the predictions in Fig. 1 because these predictions are already based on normalizing the achromatic settings at the two loci. These gain changes alone account for the bulk of the compensation [10]. However, our results further confirm that the settings in fact remain even more similar than predicted by adjusting each locus to the average spectrum [11], which predicted residual differences roughly three times larger than we observed. The nature of these additional compensatory adjustments remains uncertain [11,14,24]. Bompas et al. [14] argued that they depended on learning the sensory–motor contingencies as the “same” world is sampled with different parts of the eye [23]. They further speculated that the learning might be incomplete if it was restricted to an early critical period during development when the macular pigment density is substantially lower than in adults. However, a problem with this developmental account is that similar higher-order compensations beyond average gain changes are also suggested by the stability of color appearance despite the increasing yellowing of the lens during adult aging [11,62].
The strong contribution of these local adjustments means that there is already comparatively little perceptual inhomogeneity in color appearance across the central 16° of vision we examined. Yet these differences are nevertheless still visible and significant, and a second goal of our study was to explore how they interacted to influence the perception of large fields. The uniform appearance of these fields strongly implicates a nonlocal, color spreading process, while the dominance of the peripheral percept is more consistent with a filling-in of the peripheral color than a filling-out from the foveal percept. However, the nature of these processes again remains uncertain. In color appearance, luminance edges play a dominant role in defining the regions over which spreading occurs [35,39,41,63]. Yet we found similar percepts even when the fields were gradually tapered and nominally equiluminant, so that luminance or edges were largely absent. Because of this we cannot exclude an alternative possibility that the spatial compensation for macular screening is nondirectional –and may involve a simple averaging of the percept within a bounded area which will necessarily include a larger peripheral contribution.
ACKNOWLEDGMENTS
This work was supported by EY-10834.
REFERENCES
- 1.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Investig. Ophthalmol. Vis. Sci. 1984;25:674–685. [PubMed] [Google Scholar]
- 2.Wooten BR, Hammond BR., Jr Spectral absorbance and spatial distribution of macular pigment using heterochromatic flicker photometry. Optom. Vis. Sci. 2005;82:378–386. doi: 10.1097/01.opx.0000162654.32112.a1. [DOI] [PubMed] [Google Scholar]
- 3.Werner JS, Spillmann L. UV-absorbing intraocular lenses: safety, efficacy, and consequences for the cataract patient. Graefe’s Arch. Clin. Exp. Ophthalmol. 1989;227:248–256. doi: 10.1007/BF02172758. [DOI] [PubMed] [Google Scholar]
- 4.Bone RA, Sparrock JM. Comparison of macular pigment densities in human eyes. Vis. Res. 1971;11:1057–1064. doi: 10.1016/0042-6989(71)90112-x. [DOI] [PubMed] [Google Scholar]
- 5.Magnussen S, Spillmann L, Sturzel F, Werner JS. Unveiling the foveal blue scotoma through an afterimage. Vis. Res. 2004;44:377–383. doi: 10.1016/j.visres.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Spencer JA. An investigation of Maxwell’s spot. Brit. J. Physiol. Opt. 1967;24:103–147. [PubMed] [Google Scholar]
- 7.Fairbairn MB. Physical models of Haidinger’s brush. J. R. Astron. Soc. Can. 2001;95:248. [Google Scholar]
- 8.Stockman A, MacLeod DI, Johnson NE. Spectral sensitivities of the human cones. J. Opt. Soc. Am. A. 1993;10:2491–2521. doi: 10.1364/josaa.10.002491. [DOI] [PubMed] [Google Scholar]
- 9.Beer D, Wortman J, Horwitz G, MacLeod D. Compensation of white for macular filtering [Abstract] J. Vision. 2005;5:282a. [Google Scholar]
- 10.Webster MA, Leonard D. Adaptation and perceptual norms in color vision. J. Opt. Soc. Am. A. 2008;25:2817–2825. doi: 10.1364/josaa.25.002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webster MA, Halen K, Meyers AJ, Winkler P, Werner JS. Colour appearance and compensation in the near periphery. Proc. R. Soc. Lond. B Biol. Sci. 2010;277:1817–1825. doi: 10.1098/rspb.2009.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster DH. Color constancy. Vis. Res. 2011;51:674–700. doi: 10.1016/j.visres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Smithson HE. Sensory, computational and cognitive components of human colour constancy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1329–1346. doi: 10.1098/rstb.2005.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bompas A, Powell G, Sumner P. Systematic biases in adult color perception persist despite lifelong information sufficient to calibrate them. J. Vis. 2013;13 doi: 10.1167/13.1.19. [DOI] [PubMed] [Google Scholar]
- 15.Hibino H. Red–green and yellow–blue opponent-color responses as a function of retinal eccentricity. Vis. Res. 1992;32:1955–1964. doi: 10.1016/0042-6989(92)90055-n. [DOI] [PubMed] [Google Scholar]
- 16.Stringham JM, Hammond BR., Jr Compensation for light loss due to filtering by macular pigment: relation to hue cancellation. Ophthalm. Physiol. Opt. 2007;27:232–237. doi: 10.1111/j.1475-1313.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 17.Stringham JM, Hammond BR, Wooten BR, Snodderly DM. Compensation for light loss resulting from filtering by macular pigment: relation to the S-cone pathway. Optom. Vis. Sci. 2006;83:887–894. doi: 10.1097/01.opx.0000249976.00534.2d. [DOI] [PubMed] [Google Scholar]
- 18.Valberg A, Seim T. Chromatic induction: responses of neurophysiological double opponent units? Biol. Cybern. 1983;46:149–158. doi: 10.1007/BF00339983. [DOI] [PubMed] [Google Scholar]
- 19.Conway BR, Livingstone MS. Spatial and temporal properties of cone signals in alert macaque primary visual cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:10826–10846. doi: 10.1523/JNEUROSCI.2091-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billock VA. The relationship between simple and double opponent cells. Vis. Res. 1991;31:33–42. doi: 10.1016/0042-6989(91)90070-l. [DOI] [PubMed] [Google Scholar]
- 21.Daw NW. Color-coded cells in goldfish, cat, and Rhesus monkey. Investig. Ophthalmol. 1972;11:411–417. [PubMed] [Google Scholar]
- 22.Shapley R, Hawken MJ. Color in the cortex: single- and double-opponent cells. Vis. Res. 2011;51:701–717. doi: 10.1016/j.visres.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Regan JK, Noe A. A sensorimotor account of vision and visual consciousness. Behav. Brain Sci. 2001;24:939–973. doi: 10.1017/s0140525x01000115. discussion 973–1031. [DOI] [PubMed] [Google Scholar]
- 24.O’Neil SF, McDermott KC, Mizokami Y, Werner JS, Crognale MA, Webster MA. Tests of a functional account of the Abney effect. J. Opt. Soc. Am. A. 2012;29:A165–A173. doi: 10.1364/JOSAA.29.00A165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burns SA, Elsner AE, Pokorny J, Smith VC. The Abney effect: chromaticity coordinates of unique and other constant hues. Vis. Res. 1984;24:479–489. doi: 10.1016/0042-6989(84)90045-2. [DOI] [PubMed] [Google Scholar]
- 26.Kurtenbach W, Sternheim CE, Spillmann L. Change in hue of spectral colors by dilution with white light (Abney effect) J. Opt. Soc. Am. 1984;1:365–372. doi: 10.1364/josaa.1.000365. [DOI] [PubMed] [Google Scholar]
- 27.Mizokami Y, Werner JS, Crognale MA, Webster MA. Nonlinearities in color coding: compensating color appearance for the eye’s spectral sensitivity. J. Vision. 2006;6:12–12. doi: 10.1167/6.9.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spillmann L. Fading, filling-in, and the perception of uniform surfaces. Chin. J. Psychol. 2011;53:399–411. [Google Scholar]
- 29.Paradiso MA, Blau S, Huang X, MacEvoy SP, Rossi AF, Shalev G. Lightness, filling-in, and the fundamental role of context in visual perception. Prog. Brain Res. 2006;155:109–123. doi: 10.1016/S0079-6123(06)55007-1. [DOI] [PubMed] [Google Scholar]
- 30.Spillmann L, Otte T, Hamburger K, Magnussen S. Perceptual filling-in from the edge of the blind spot. Vis. Res. 2006;46:4252–4257. doi: 10.1016/j.visres.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran VS. Filling in the blind spot. Nature. 1992;356:115. doi: 10.1038/356115a0. [DOI] [PubMed] [Google Scholar]
- 32.De Valois RL, Webster MA, De Valois KK, Lingelbach B. Temporal properties of brightness and color induction. Vis. Res. 1986;26:887–897. doi: 10.1016/0042-6989(86)90147-1. [DOI] [PubMed] [Google Scholar]
- 33.Blakeslee B, McCourt ME. Spatiotemporal analysis of brightness induction. Vis. Res. 2011;51:1872–1879. doi: 10.1016/j.visres.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi AF, Paradiso MA. Temporal limits of brightness induction and mechanisms of brightness perception. Vis. Res. 1996;36:1391–1398. doi: 10.1016/0042-6989(95)00206-5. [DOI] [PubMed] [Google Scholar]
- 35.Pinna B, Werner JS, Spillmann L. The watercolor effect: a new principle of grouping and figure-ground organization. Vis. Res. 2003;43:43–52. doi: 10.1016/s0042-6989(02)00132-3. [DOI] [PubMed] [Google Scholar]
- 36.Billock VA, Gleason GA, Tsou BH. Perception of forbidden colors in retinally stabilized equiluminant images: an indication of softwired cortical color opponency? J. Opt. Soc. Am. A. 2001;18:2398–2403. doi: 10.1364/josaa.18.002398. [DOI] [PubMed] [Google Scholar]
- 37.Hamburger K, Prior H, Sarris V, Spillmann L. Filling-in with colour: different modes of surface completion. Vis. Res. 2006;46:1129–1138. doi: 10.1016/j.visres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Wachtler T, Wehrhahn C. The Craik–O’Brien–Cornsweet illusion in colour: quantitative characterisation and comparison with luminance. Perception. 1997;26:1423–1430. doi: 10.1068/p261423. [DOI] [PubMed] [Google Scholar]
- 39.Broerse J, Vladusich T, O’Shea RP. Colour at edges and colour spreading inMcCollough effects. Vis. Res. 1999;39:1305–1320. doi: 10.1016/s0042-6989(98)00231-4. [DOI] [PubMed] [Google Scholar]
- 40.Bressan P, Mingolla E, Spillmann L, Watanabe T. Neon color spreading: a review. Perception. 1997;26:1353–1366. doi: 10.1068/p261353. [DOI] [PubMed] [Google Scholar]
- 41.Devinck F, Delahunt PB, Hardy JL, Spillmann L, Werner JS. The watercolor effect: quantitative evidence for luminance-dependent mechanisms of long-range color assimilation. Vis. Res. 2005;45:1413–1424. doi: 10.1016/j.visres.2004.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams DR, MacLeod DI, Hayhoe MM. Foveal tritanopia. Vis. Res. 1981;21:1341–1356. doi: 10.1016/0042-6989(81)90241-8. [DOI] [PubMed] [Google Scholar]
- 43.Magnussen S, Spillmann L, Sturzel F, Werner JS. Filling in of the foveal blue scotoma. Vis. Res. 2001;41:2961–2967. doi: 10.1016/s0042-6989(01)00178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abramov I, Gordon J. Color appearance: on seeing red—or yellow, or green, or blue. Annu. Rev. Psychol. 1994;45:451–485. doi: 10.1146/annurev.ps.45.020194.002315. [DOI] [PubMed] [Google Scholar]
- 45.Balas B, Sinha P. ‘Filling-in’ colour in natural scenes. Vis. Cogn. 2007;15:765–778. [Google Scholar]
- 46.MacLeod DI, Boynton RM. Chromaticity diagram showing cone excitation by stimuli of equal luminance. J. Opt. Soc. Am. 1979;69:1183–1186. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- 47.Webster MA, Miyahara E, Malkoc G, Raker VE. Variations in normal color vision. II. unique hues. J. Opt. Soc. Am. A. 2000;17:1545–1555. doi: 10.1364/josaa.17.001545. [DOI] [PubMed] [Google Scholar]
- 48.Malkoc G, Kay P, Webster MA. Variations in normal color vision. IV. binary hues and hue scaling. J. Opt. Soc. Am. A. 2005;22:2154–2168. doi: 10.1364/josaa.22.002154. [DOI] [PubMed] [Google Scholar]
- 49.Smith VC, Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vis. Res. 1975;15:161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- 50.Wooten BR, Hammond BR, Jr, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Investig. Ophthalmol. Vis. Sci. 1999;40:2481–2489. [PubMed] [Google Scholar]
- 51.Krauskopf J. Heterochromatic stabilized images: a classroom demonstration. Am. J. Psychol. 1967;80:634–637. [PubMed] [Google Scholar]
- 52.Mullen KT, Kingdom FA. Losses in peripheral colour sensitivity predicted from ‘hit and miss’ post-receptoral cone connections. Vis. Res. 1996;36:1995–2000. doi: 10.1016/0042-6989(95)00261-8. [DOI] [PubMed] [Google Scholar]
- 53.Mullen KT, Kingdom FA. Differential distributions of red–green and blue–yellow cone opponency across the visual field. Vis. Neurosci. 2002;19:109–118. doi: 10.1017/s0952523802191103. [DOI] [PubMed] [Google Scholar]
- 54.McKeefry DJ, Murray IJ, Parry NR. Perceived shifts in saturation and hue of chromatic stimuli in the near peripheral retina. J. Opt. Soc. Am. A. 2007;24:3168–3179. doi: 10.1364/josaa.24.003168. [DOI] [PubMed] [Google Scholar]
- 55.Murray IJ, Parry NR, McKeefry NR. Cone opponency in the near peripheral retina. Vis. Neurosci. 2006;23:503–507. doi: 10.1017/S0952523806233315. [DOI] [PubMed] [Google Scholar]
- 56.Parry NR, McKeefry DJ, Murray IJ. Variant and invariant color perception in the near peripheral retina. J. Opt. Soc. Am. A. 2006;23:1586–1597. doi: 10.1364/josaa.23.001586. [DOI] [PubMed] [Google Scholar]
- 57.Nerger JL, Volbrecht VJ, Ayde CJ. Unique hue judgments as a function of test size in the fovea and at 20-deg temporal eccentricity. J. Opt. Soc. Am. A. 1995;12:1225–1232. doi: 10.1364/josaa.12.001225. [DOI] [PubMed] [Google Scholar]
- 58.Moreland JD. Peripheral color vision. In: Jameson J, Hurvich LM, editors. Handbook of Sensory Physiology. Springer; 1972. pp. 517–536. [Google Scholar]
- 59.Abramov I, Gordon J, Chan H. Color appearance in the peripheral retina: effects of stimulus size. J. Opt. Soc. Am. A. 1991;8:404–414. doi: 10.1364/josaa.8.000404. [DOI] [PubMed] [Google Scholar]
- 60.Volbrecht VJ, Shrago EE, Schefrin BE, Werner JS. Spatial summation in human cone mechanisms from 0 degrees to 20 degrees in the superior retina. J. Opt. Soc Am. A. 2000;17:641–650. doi: 10.1364/josaa.17.000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bompas A, O’Regan JK. Evidence for a role of action in colour perception. Perception. 2006;35:65–78. doi: 10.1068/p5356. [DOI] [PubMed] [Google Scholar]
- 62.Schefrin BE, Werner JS. Loci of spectral unique hues throughout the life span. J. Opt. Soc. Am. A. 1990;7:305–311. doi: 10.1364/josaa.7.000305. [DOI] [PubMed] [Google Scholar]
- 63.van Lier R, Vergeer M, Anstis S. Filling-in afterimage colors between the lines. Curr. Biol. 2009;19:R323–R324. doi: 10.1016/j.cub.2009.03.010. [DOI] [PubMed] [Google Scholar]