Abstract
This paper investigates the light-driven migration of the multi-cellular microorganism Dictyostelium discoideum as a potential bio-actuation mechanism in microsystems. As a platform for slug migration we use microscale confinements, which consist of intersecting microchannels fabricated from solidified agar-water solution. The agar surface provides necessary moisture to the slugs during the experiment while remaining sufficiently stiff to allow effective slug migration. The movements of the slugs in the microchannels are driven and guided by phototaxis via controlling light transmitted through optical fibers. The microchannels impose geometrical confinements on the migrating slugs, improving the spatial precision of the migration. We demonstrate that slugs that form in a microchamber can be driven to migrate through the microchannels, as well as steered to a particular direction at microchannel intersections. Our experimental results indicate that slug movements can be more effectively controlled in microchannels, and potentially useful for bio-actuation applications.
Keywords: Bio-microactuator, microorganism, Dictyostelium discoideum, microchannel, phototaxis
1. Introduction
Controlled movement of microorganisms in microfluidic systems is a subject of active research [1]. In particular, the use of microorganisms as bio-microactuators to generate useful work in a microchip has been receiving increasing attention [2]. Bio-microactuators offer distinct advantages over conventional microactuators. When they are living cells, bio-microactuators are powered directly by biochemical reactions [3], eliminating external energy sources such as batteries. Microorganisms can be self-healing, resulting in higher efficiencies and reliability for microsystems utilizing bio-microactuators that have sustained physical damages [4, 5]. In addition, sensory mechanisms that respond to environmental changes are naturally presented in microorganisms [6], whose responses can hence be simply induced by stimuli such as light, temperature, and chemicals.
Studies have shown the possibility of using various microorganisms as biologically based microactuators, or bio-microactuators. Microorganisms have been used as bio-microactuators to move fluids [6], transport microscale objects [7, 8], drive micro rotors [4], and deliver drugs [9]. For example, flagellated bacteria were able to generate linear and rotational flow patterns, producing sufficient force to move microscale objects [6, 10]. Similarly, a unicellular alga with chemically attached microbeads was driven by phototaxis to carry and release beads in a microchannel [11]. Forces exerted by bacteria were also used to unidirectionally rotate microfabricated gears [12]. Although these works have demonstrated the potential use of microorganisms as actuation components in microsystems, there has been a lack of studies that exploit stimuli responsiveness to precisely control the movements of the microorganisms.
In this paper, we investigate stimuli-responsive migration of multi-cellular microorganisms in microfabricated confinements. Specifically, we explore the light-guided migration (phototaxis) of multi-cellular organisms (slugs) of the social amoeba Dictyostelium discoideum (D. discoideum). Since D. discoideum slugs are phototactic, light stimulation can be used to induce phototaxis, and effect and control their migration in microchannels. To demonstrate this principle, we use a microchip that consists of a microchamber connected to a microchannel, which joins two other microchannels to form a T-junction. An optical fiber, connected to an optical source, is coupled into each of the channels. To ensure a moist and sufficiently stiff surface for the slugs, agar is used as channel material. D. discoideum slugs differentiate in the chamber and migrate through the channels under light stimulation from the optical fibers coupled into the chip. The channels offer geometrical confinements to guide the slug migration. Moreover, the channel intersections provide geometrical bifurcations that enable the migrating slugs to change directions under light stimulation. Experimental results show that migration of slugs can be effectively controlled by manipulating the position of optical fibers in microchannels, demonstrating the potential to exploit stimuli responsiveness of microorganisms to enable innovative bio-microactuators.
2. Design
D. discoideum is a species of amoeba that normally lives in soil and feeds on bacteria. When starved (i.e., deprived of food supply), individual D. discoideum cells stream into aggregates by signaling each other using cyclic adenosine monophosphate. The aggregates further differentiate into multi-cellular organisms that are known as slugs with a length of approximately 1 mm and cross-sectional dimensions of about 100 μm. D. discoideum slugs are responsive to very low intensities of light signals, moving up the light gradients [13, 14]. Thus, by defining a gradient of light intensity in a desired direction, the movement of slugs can be phototaxically controlled. Further control can be obtained by placing the slugs inside microchannels, the geometry of which will constrain their lateral movement.
The phototaxically directed migration of slugs in microfabricated confinements in a microchip is schematically shown in Figure 1. The microchip consists of a triangular microchamber connected to a microchannel (called the main channel), which in turn joins two other microchannels (respectively called left and right channels) to form a T-shaped junction. The microchamber has an equilateral triangle shape (length of each side: 10 mm) converging toward the main channel. The main channel is 30 mm in length, and the right and left channels are both 6 mm in length. All of the channels were 500 μm wide, and had the same height as the chambers, which was also 500 μm. An optical fiber (diameter: 250 μm) is inserted into each channel to transmit light from an external source to the cells. We use optical fibers because they are inexpensively available and compatible with our microchip, although other illumination methods with appropriate wavelengths (below 650 nm for slug phototaxis [15]) could be used.
Figure 1.
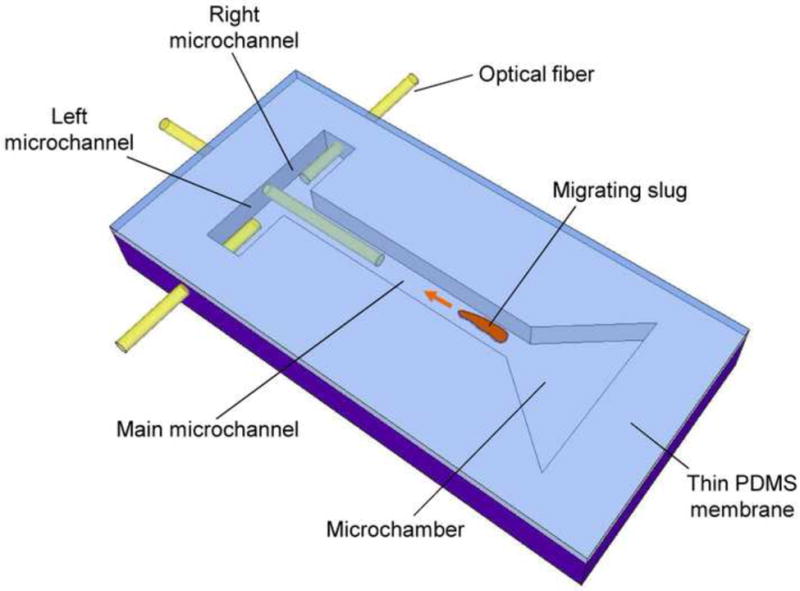
A schematic of phototaxis of a D. discoideum slug in a microchip. Migration of the slug is driven and directed by light transmitted through the optical fibers.
The microchip is fabricated from 4% non-nutrient agar to ensure a moist and nutrient-deprived environment as required for cell viability and slug formation [16]. Also the agar provides a sufficiently stiff support surface on which slugs could migrate without slipping [17]. The microchip is sealed from the top with a sheet of polydimethylsiloxane (PDMS) to vertically confine migrating slugs while providing additional oxygen necessary for cell survival by preventing oxygen depletion due to cell respiration [18].
Multiple D. discoideum amoeba cells are placed in a microchamber and allowed to differentiate into slugs. The slugs are then directed to move through the microchannels in the direction of light from the optical fibers. Under stimulation of light from the optical fiber coupled to the main channel at the distal end, the slug migrates into and along the main channel toward the light. As the slug reaches the T-junction, the light source for the optical fiber in the main channel is turned off, while the light source for the optical fiber in the left or right channel is turned on. Following the change in the light stimulation, the slug turns left or right into either of the side channels accordingly.
3. Experimental
3.1. Materials
The following materials were used in the experiments. Cells of D. discoideum were prepared as follows. The D. discoideum strain NC4 were grown on slime mold (SM) agar plates containing an organic medium on which Klebsiella aerogenes had inoculated [19]. The SM agar plates were stored in a dark room at 24°C for 72 hours. To induce starvation, the cells were collected from the plates and washed in Sorensen’s buffer by centrifugation at 1000 rpm for 5 minutes. Supernatant buffer was discarded and the cells were resuspended in deionized water (concentration: 108 cells/mL).
Four percent non-nutrient water agar (Difco Laboratories) was prepared by adding one gram of agar to 250 mL of purified water filtered from a Milli-Q Plus water purification system (Millipore). The mixture was thoroughly mixed in a glass bottle and then was warmed on a hot-plate until the agar powder was completely dissolved [20]. Bubbles formed in the agar-water mixture were removed by applying vacuum to the bottle.
3.2. Microchip Fabrication
The fabrication process of the 4% agar microchip is shown in Figure 2. The microchip was fabricated from 4% non-nutrient agar via replica molding. To fabricate the mold, SU-8 2150 photoresist (MicroChem) was spin-coated on a silicon wafer, baked on a hotplate at 95°C for 2 hours, and exposed to UV light through a photomask (Figure 2a). Following the post-exposure bake at 95°C for 30 minutes on a hotplate, the unexposed photoresist was removed in a developer, creating a mold for the microchannels (Figure 2b). Molten 4% non-nutrient agar solution was evenly spread onto the SU-8 mold held in a petridish and placed on a flat surface at room temperature for 10-15 minutes until the agar solution fully solidified (Figure 2c). The solidified agar was removed from the mold to create the microchannel structure (Figure 2d). The resulting 4% agar microchip with a PDMS cover was approximately 5 cm (along the main channel) × 3 cm (along the side channels) in size. In parallel to agar molding, a sheet of PDMS (thickness: 500 μm) was fabricated by spin-coating of a PDMS prepolymer solution of Sylgard 184 (Dow Corning) that was mixed with the manufacturer-supplied curing agent at a volume ratio of 10:1 on a glass substrate. Then the prepolymer was degassed by vacuum for 30 minutes and cured on a hot plate at 75°C for 1 hour. The PDMS sheet then was bonded onto the agar chip by spontaneous adhesion. An optical fiber 250 μm in diameter (The Fiber Optic Store.com) was inserted into each of the microchannels through access holes fabricated within the agar chip.
Figure 2.
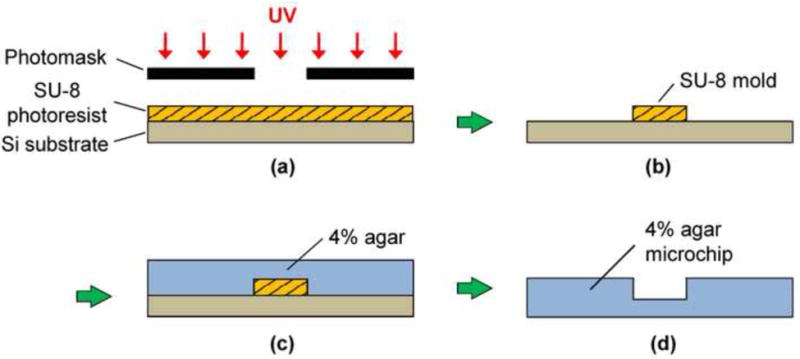
An illustration of the molding procedure of 4% agar microchannels. (a) Spin coated SU-8 photoresist (thickness: 500 μm) is exposed to UV light through a photomask. (b) Development produces a SU-8 mold. (c) Four percent molten agar is pour over the SU-8 mold and cured at room temperature for 10-15 minutes. (d) The solidified 4% agar microchip is then peeled off from the mold.
3.3. Experimental Setup
Phototaxis experiments were performed with the microchip placed in an acrylic plastic enclosure fabricated as follows (Figure 3). Six square sheets of equal size (7 cm long and 0.45 cm thick) were cut out from stock acrylic sheets (McMaster-Carr) using a laser cutter (PLS6, Universal Laser Systems), and assembled into a cubic box using an acrylic adhesive (IPS corporation). Long-pass filters 5 × 5 cm2 in size (cutoff wavelength: 695 nm, Andover) were attached on the top and bottom faces of the enclosure. All of the side faces of the enclosure were covered with aluminum foil and black electric tape. There were holes in the side faces to allow the optical fibers to be fed through the enclosure from external optical sources to the microchip.
Figure 3.
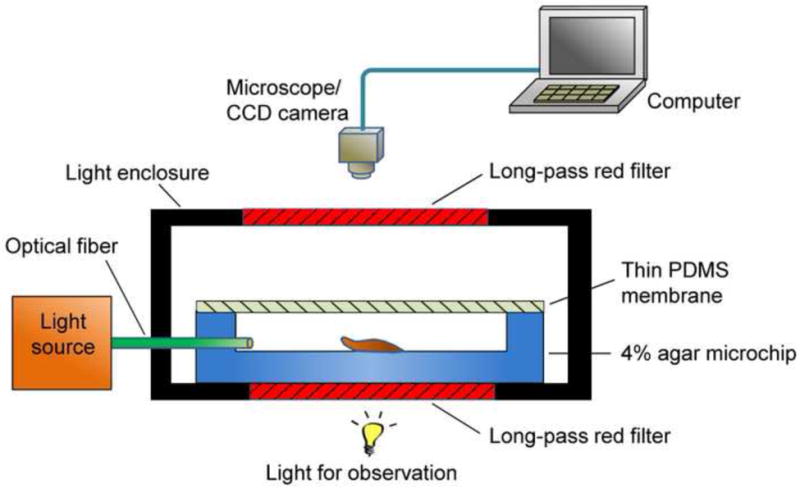
A schematic of a light enclosure for phototaxis experiment of slugs.
The enclosure prevents ambient light from entering the microchip to cause unintended phototaxis of slugs. The long-pass filters allowed observation through the enclosure while filtering out light of shorter wavelengths that could induce phototaxis. To obtain time lapse micrograph images of migrating slugs, the enclosure containing the microchip was placed under a microscope (Nikon) equipped with a charge-coupled device (CCD) camera (PVC 100C, Pixera).
3.4. Experimental Procedure
Phototaxically driven and controlled migration of D. discoideum slugs was carried out as follows. A small drop of concentrated cell (~50 μL) was placed on a filter paper (2 × 2 mm2, Millipore). After the cells settled to the filter paper surface in approximately 20 minutes, the excess buffer was removed. The filter paper was then placed in the triangular microchamber to allow the cells to be transferred to the microchip (and later enter the main channel) which was then placed in the enclosure. The optical fibers were inserted into the microchannels while a thin PDMS membrane was placed on the top of the microchannels. The microchip was kept in a dark room over night to allow slug formation in the microchamber. A micrograph image of the initial position of the slugs in the main channel was then taken.
The experiment then proceeded as follows. After 20 hours of incubation at room temperature (~24°C), slugs formed in the microchamber would start to migrate toward the optical fiber in the main channel. The distance between the light emitting end of the optical fibers and the slug was maintained at 2 to 3 mm by manually adjusting the position of the optical fiber. This ensured that the slugs did not come into contact with a fiber, which would affect their migration. When the slugs reached the end of the main channel, the optical fiber in that channel was removed, while another optical fiber was inserted into the left or right channel to direct the slugs to make an appropriate turn. For experiments in which slug made turns, a shorter main microchannel (length: 2 mm) was used, allowing the slugs to migrate from the microchamber to the T-junction in a relatively short time. During all experiments, migrating slugs were imaged using a CCD camera, at one-hour intervals in the main channel and at ten-minute intervals in the T-junction. The slug position was then determined by analyzing the images using ImageJ software (National Institutes of Health freeware).
4. Results and Discussion
This section presents experimental results from light-directed migration of D. discoideum slugs in the microchip. Migration of slugs in the main microchannel will first be studied, followed by an investigation of slug migration with direction change, when the slugs turn from the main channel into one of the side channels. All results presented in these studies have been verified with multiple (three or more) repeated experiments.
4.1. Slug Migration in the Main Microchannel
To investigate the migration of slugs in the main microchannel, we first conducted a control experiment in which slugs migrated on an open surface of 4% agar, which would allow examination of the effectiveness of the geometrical confinements in directing slug migration. Starved individual D. discoideum cells were inoculated on filter paper. Multiple slugs formed in approximately 20 hours, and migrated on to the open agar surface under stimulation of light transmitted from an optical fiber visible in Figure 4, which shows an image of the migrating slugs. It can be seen that without the presence of a geometrical constraint, the slugs migrated toward the light transmitting optical fiber in an apparently uncontrolled manner.
Figure 4.
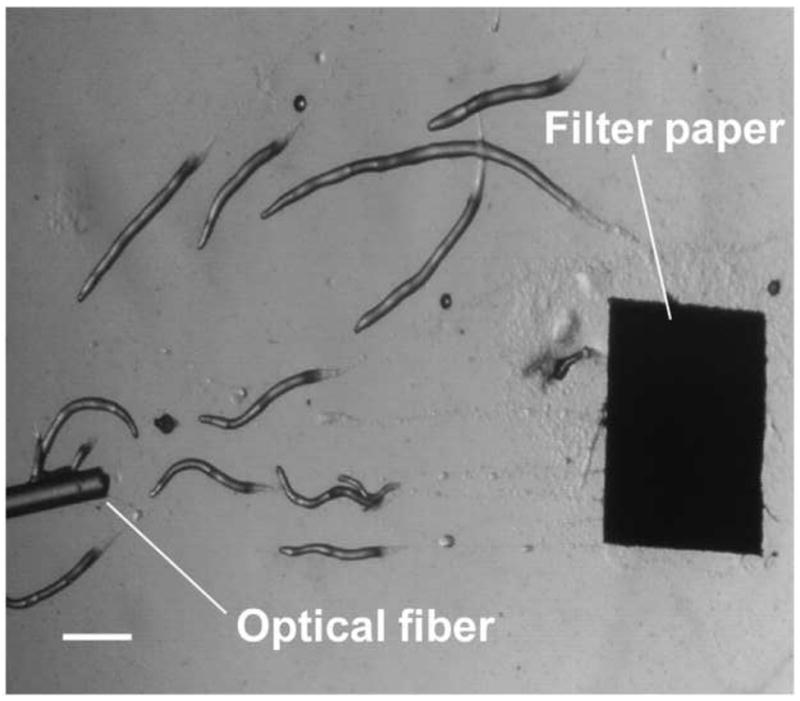
A micrograph image of slugs migrating toward an optical fiber light source on an open 4% agar surface. (Scale bar: 500 μm.)
We next observed the migration of slugs under light stimulation from the microchamber into and through the main channel. Upon introduction into the chamber, slugs were imaged every hour to examine their trajectories and speeds. As shown in Figure 5, two representative slugs, Slugs 1 and 2, were tracked over a 5-hour period, starting at the instant when both Slugs 1 and 2 appeared in the detection region of the microscope (t = 0 hr) (Figure 5a). In one hour (t = 1 hr), Slug 1 had reached near the inlet of the main channel while Slug 2 continued migrating in the chamber (Figure 5b). At this time, the optical fiber was manually moved backward approximately by 2 mm to allow more clearance to the migrating slugs. At t = 2hr, Slug 1 started to migrate through the main channel as more than one half of its body had entered the channel, Slug 2 continued its migration toward the channel inlet (Figure 5c). It was observed that despite the backward movement of the optical fiber in the main channel, Slug 2 could still migrate toward the channel, most likely by sensing the light reflected or transmitted from the side walls of the channel. At time t = 3 hr, Slug 1 was completely in the channel, and Slug 2 reached the channel inlet, with its anterior part gliding on the channel’s side wall (Figure 5d). In another hour, Slug 2 had completely entered the channel (t = 4 hr), and showed a decrease in length due to the resistance to motion of the slug imposed by the upper side wall of the channel. In the mean time, Slug 1 was migrating through the channel in a serpentine pattern, consistent with knowledge from the literature that the slug would sweep its anterior to sense light during phototaxis [21] (Figure 5e). At time t = 5 hr, Slug 2 appeared elongated as it was detached from the upper side wall while continuing to migrate, while Slug 1 became shorter as its anterior glided on the lower side wall (Figure 5f).
Figure 5.
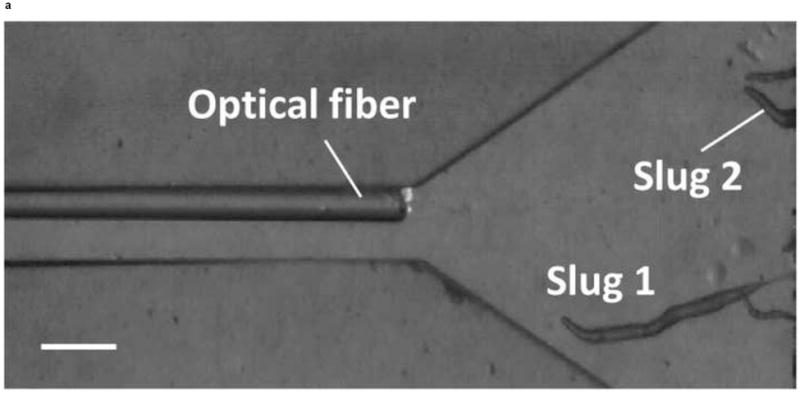
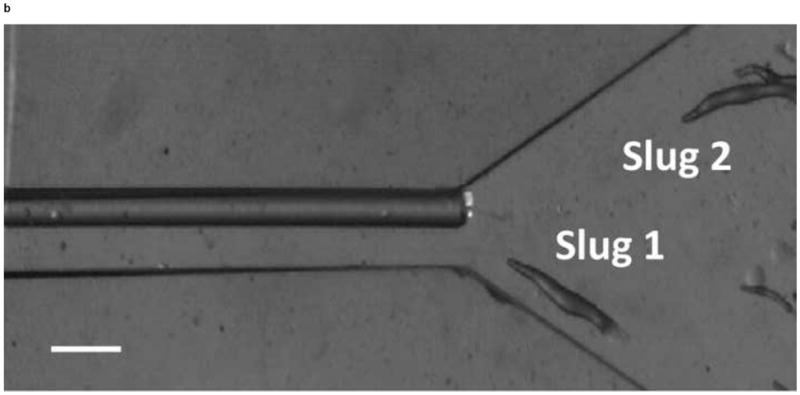
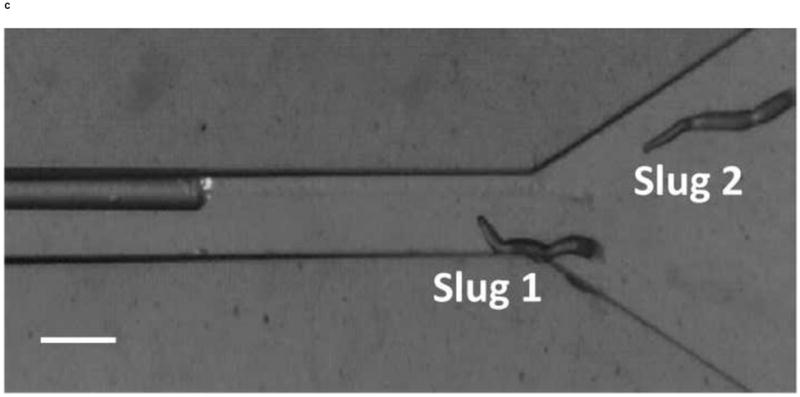
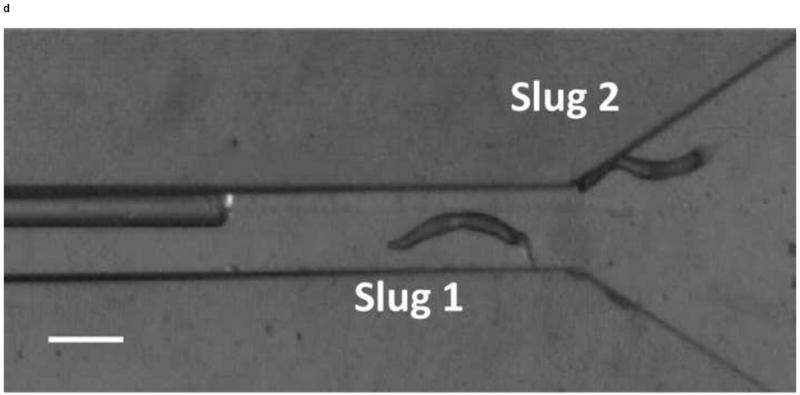
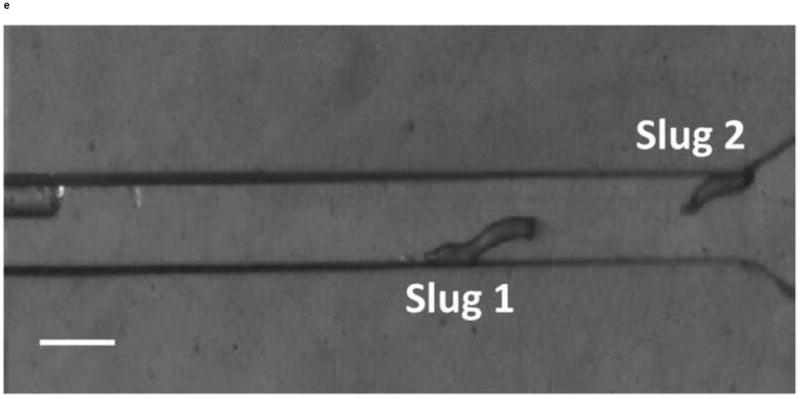
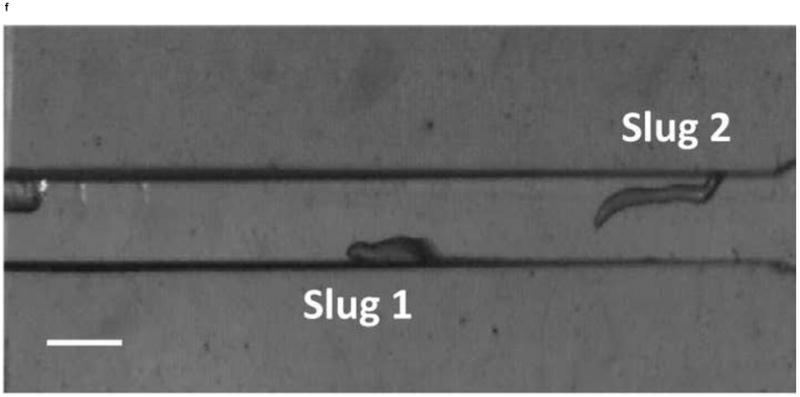
Micrograph images of Slugs 1 and 2 at different times as the slugs migrated from the microchamber into and through the main microchannel: (a) t = 0 hr, (b) t = 1 hr, (c) t = 2 hr, (d) t = 3 hr, (e) t = 4 hr, and (f) t = 5 hr (Scale bar: 500 μm.)
Thus, the lateral movements of the slugs inside the main channel were constrained geometrically by the channel’s side walls throughout the experiment. In addition, the slug length decreased when the slug anterior glided along a side wall for an extended period of time as it migrated on the underlying agar surface (Slug 2 in Figure 5e and Slug 1 in Figure 5f). However, if other areas of the slug body touched the side wall, the slug migrated without significant change in its length (Slug 1 in Figure 5c). In fact, the anterior of a slug plays vital roles such as exerting traction force and sensing the external stimulation during migration [22, 23]. Therefore, it appears that it is more difficult for a migrating slug to push rather than pull its body against resistance from a side wall.
The travel distances of the front tips of Slugs 1 and 2 during their migration were obtained by analyzing consecutive images. As shown in Figure 6, Slug 1 migrated approximately 3.94 mm at a nearly constant speed of about 0.81 mm/hr for 5 hours. On the other hand, Slug 2 migrated over a distance of approximately 3.42 mm, or 13% less than that of Slug 1. This could be attributed to Slug 2 needing more time than Slug 1 to turn itself when the light source was relocated. That is, while the light from the optical fiber was always illuminated Slug 1 directly, Slug 2 needed to sense the light reflected from the side walls of the microchannel when it was still in the microchamber between t = 2 and 3 hr. In addition, the speed of Slug 2 decreased from ~0.72 mm/hr to ~0.53 mm/hr for t = 3 to 4 hr, likely because of the resistance from the upper side wall that was in contact with the slug’s anterior. Beyond this time, Slug 2 again migrated at its normal speed (~0.72 mm/hr) as it became detached from the channel’s upper side wall.
Figure 6.
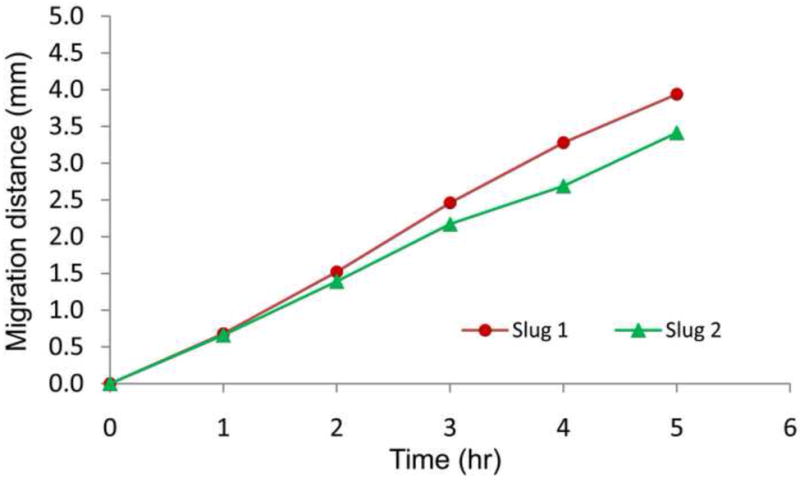
Migration distances of Slugs 1 and 2 in the main microchannel.
Recognizing that the migration speed of a slug is directly related to its length [24], we plot the average speeds of slugs migrating in the microchannel as a function of the lengths of slugs observed in multiple experiments (Figure 7). The slug lengths continually changed during migration [25], thus their average values during the tracking were measured from the slugs’ front tips to their tails. The results show that the average slug speed is approximately proportional to the slug length, in agreement with previously reported results [26, 27].
Figure 7.
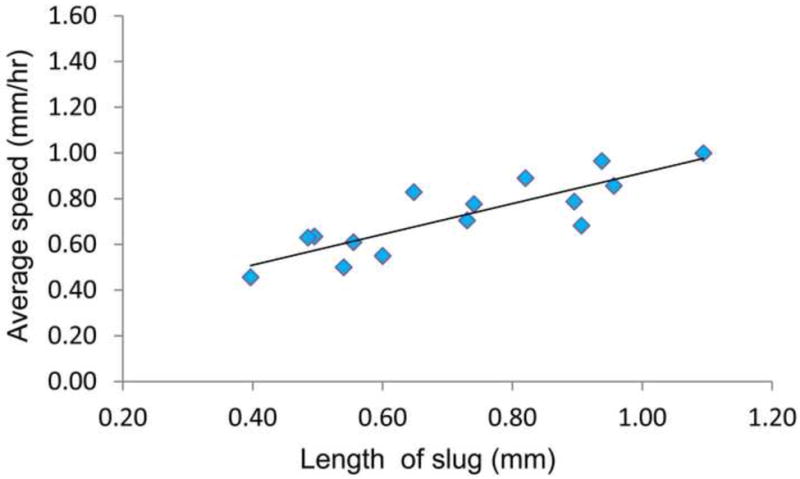
Average speeds of slugs in microchannels as a function of slug length.
4.2. Slug Migration with Direction Change: from the Main Channel to the Left Side Channel
We also demonstrated the ability to steer slugs to turn in a particular direction within the T-junction of the microchannels. We first investigated the migration of slugs into the left channel. For this purpose, when slugs reached the end of the main channel, the optical fiber in that channel was removed, while another optical fiber was inserted in the left channel. Figure 8 shows images of two slugs (Slugs 3 and 4, average lengths: 0.65 mm and 0.90 mm respectively with an equal average thickness 50 μm) as they were guided to turn left. At t = 0 min, the anterior of Slug 3 reached the end of the main channel, while Slug 4 was still migrating inside that channel (Figure 8a). At t = 10 min, the optical fiber in the left channel was pulled back by about 3 mm. At t = 60 min, Slug 3 had exited the main channel completely, turned left, and started to enter the left channel. On the other hand, Slug 4 had turned toward the left channel with an angle of about 45° (Figure 8b). At t = 120 min, Slug 3 had completed entering the left channel, while the anterior of Slug 4 had just reached the entrance of the left channel (Figure 8c). At t = 180 min, Slug 3 was migrating in a serpentine pattern through the left channel and Slug 4 had completely entered the left channel (Figure 8d). It was observed that although Slug 4 occasionally hit the side walls of the microchannels, it moved away quickly and avoided reducing its speed.
Figure 8.
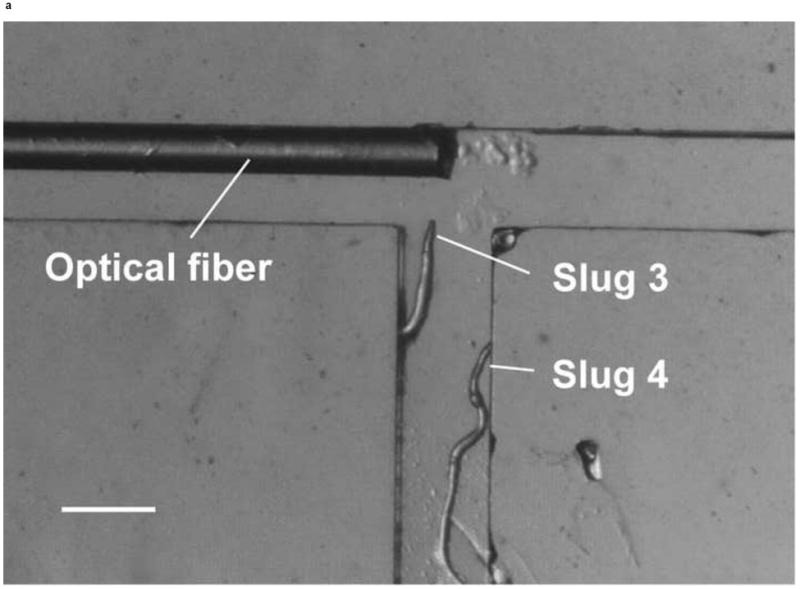
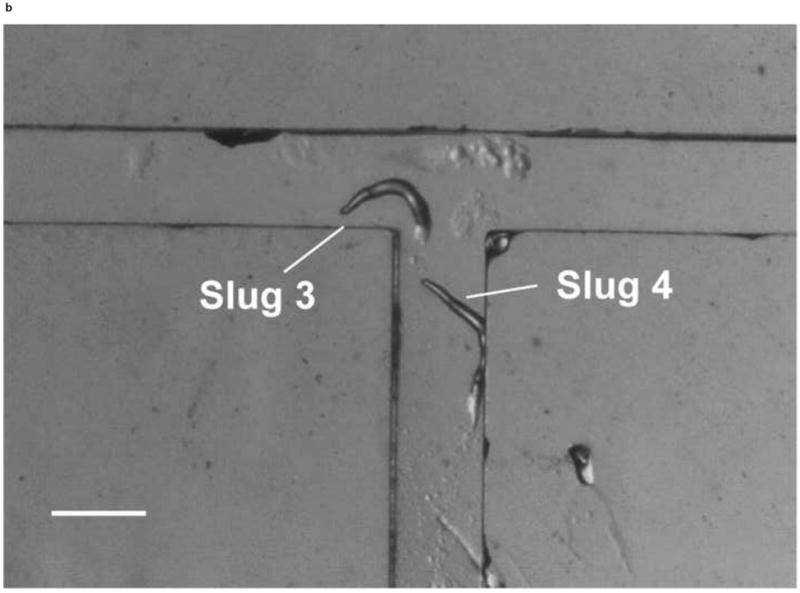
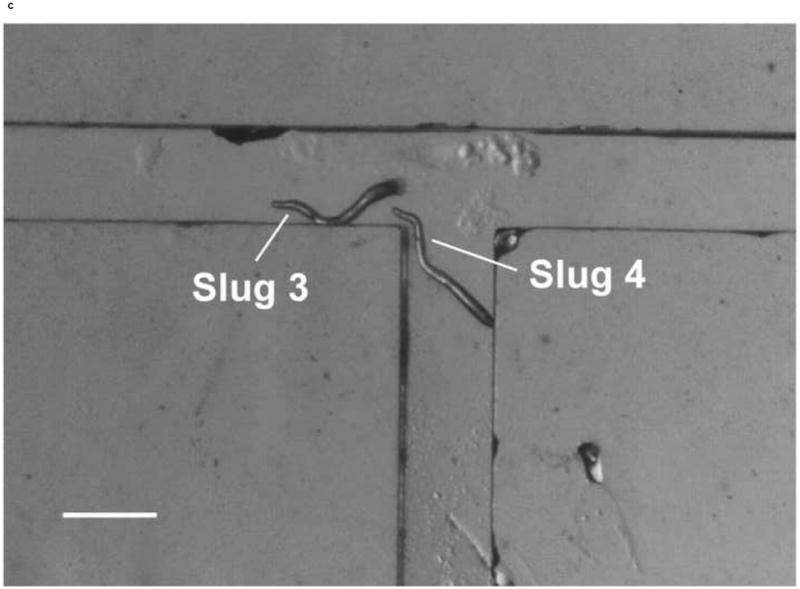
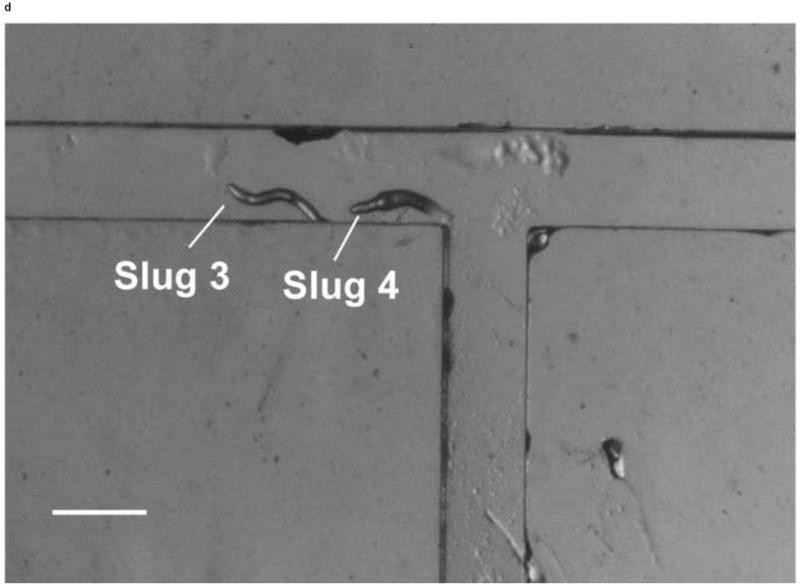
Micrograph images of Slugs 3 and 4 at different times as the slugs turned from the main channel into the left channel: (a) t = 0 min, (b) t = 60 min, (c) t = 120 min, and (d) t = 180 min (Scale bar: 500 μm.)
To determine the trajectories and directions of slug migration, we first analyzed the images of migrating slugs taken at each time step using ImageJ to determine the centroids of the slugs. Then the trajectory of a slug was given by the time-dependent position of the centroid, and the direction of slug migration (left: +90° and right: -90°) was defined by that of the tangent to the trajectory, following common practice in the literature [28, 29].
The trajectories and directions of Slugs 4 and 5 during the left turn (+90°) are plotted in Figure 9. From the trajectories of the centroids of Slugs 3 and 4 (Figure 9a) determined from consecutive images, it can be seen that Slug 3 stayed away from the main channel’s side walls and later migrated close to the left channel’s lower side wall, while Slug 4 first approached and then moved away from the main channel’s right side wall. These observations indicate the active geometric confinement of the channels on the migration of the slugs. The average migration speeds of Slugs 3 and 4 along these trajectories were 0.48 and 0.51 mm/hr, respectively. The radii of curvature of the trajectories of Slugs 3 and 4 are approximately 0.25 mm and 0.92 mm, respectively. That is, Slug 3 made a sharper turn than Slug 4 in the T-junction; the more gradual turn made by Slug 4 reflects that the slug had not yet reached the end of the main channel when the optical fiber was moved backward.
Figure 9.
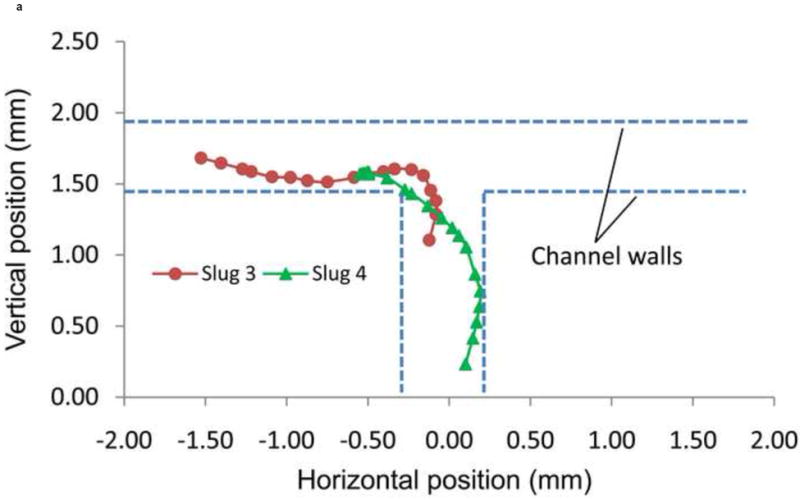
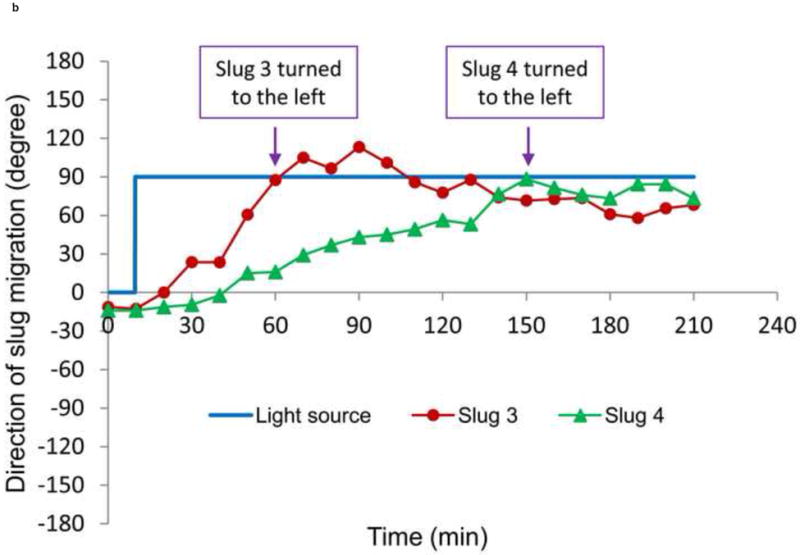
(a) Trajectory, and (b) direction of migration of Slugs 3 and 4 as it was turning to the left during phototaxis.
From their migration direction, it was observed that they were able to turn to the left (+90°) and enter the left channel (Figure 9b). Slugs 3 and 4 turned to the left after approximately 60 and 150 minutes, respectively, which were nearly 60 and 30 minutes before the entire bodies of the slugs had entered the left channel. These results indicate that a migrating slug can be effectively manipulated by phototaxis to enter a particular microchannel at an intersection by changing the positions of a light source, while physical confinements such as microchannel walls make the manipulation more accurate.
4.3. Slug Migration with Direction Change: from the Main Channel to the Right Side Channel
We investigated whether phototactic migration direction changes of D. discoideum in microchannels are biased, as it is known that individual constituent cells in a slug make clockwise rotations when viewed from behind [30]. To determine if slugs are biased to a certain direction, we also steered slugs into the right channel using an optical fiber inserted into that channel. Figure 10 shows the resulting images of a slug (Slug 5, average length: 1.4 mm and average thickness: 95 μm) observed in one representative experiment. At t = 0 min, Slug 5 reached the end of the main channel (Figure 10a). When the optical fiber was pulled back approximately 3 mm along the right channel, the slug continued migrating along the direction of the main channel. At t = 60 min, Slug 5 came in contact with the upper side wall of the T-junction and started to turn to the right (Figure 10b). At t = 90 min, the anterior of Slug 5 had completed the right turn and was gliding on the upper side wall (Figure 10c). The slug had also become thicker and shorter due to the resistance from the wall. At t = 120 min, the slug had completed the right turn, and its whole body was almost in contact with the upper side wall (Figure 10d).
Figure 10.
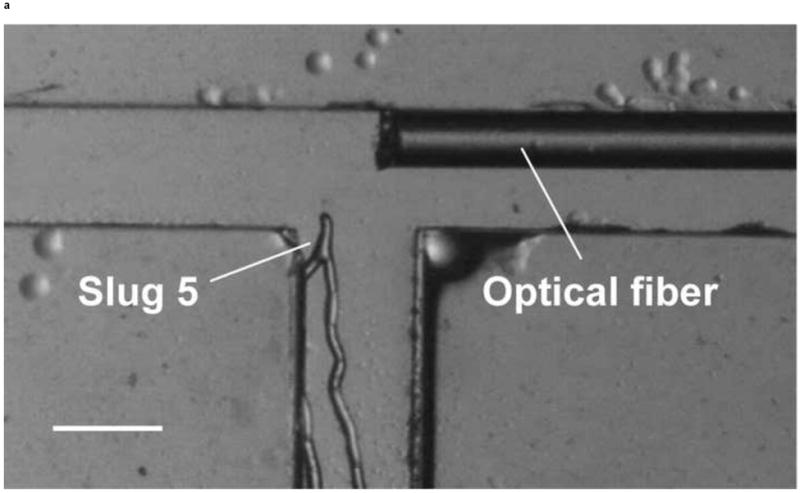
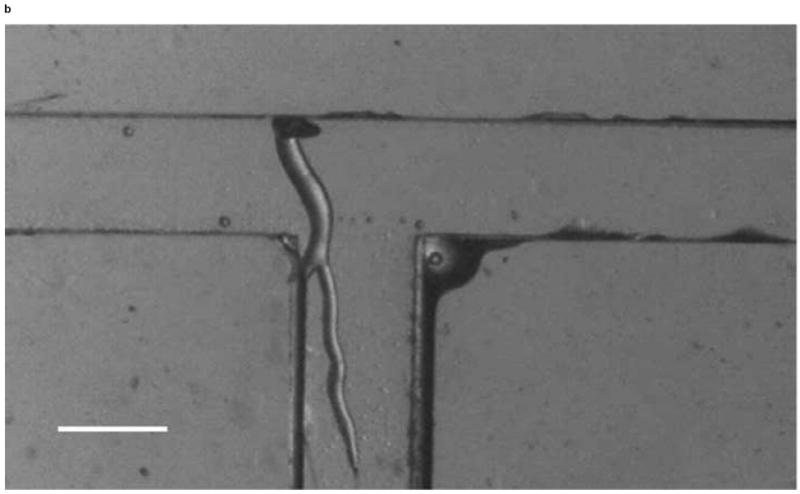
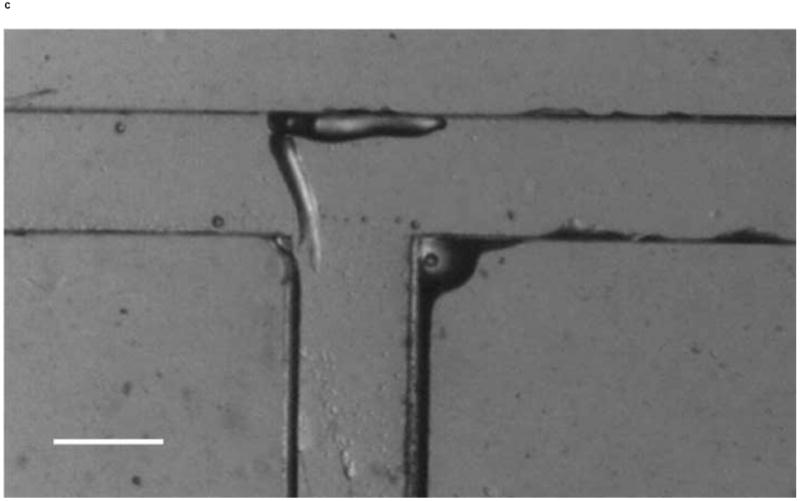
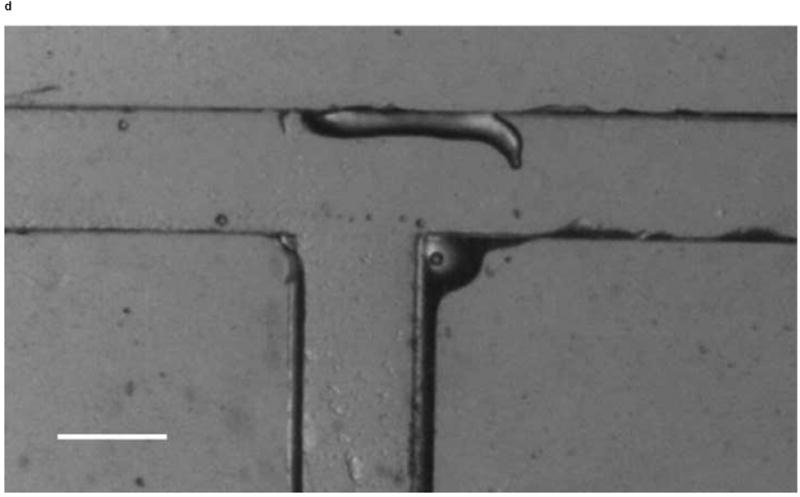
Micrograph images of Slug 5 at different times as the slugs turned from the main channel into the right channel: (a) t = 0 min, (b) t = 60 min, (c) t = 90 min, and (d) t = 120 min (Scale bar: 500 μm.)
The trajectory of the centroid of Slug 5 is shown in Figure 11a. This trajectory is seen to be in close proximity of the main channel’s left side wall, again reflecting active geometric confinement of the channel on the slug migration. The radius of curvature of the trajectory was approximately 0.43 mm, and the slug’s average migration speed along the trajectory was about 0.77 mm/hr. These are respectively comparable to the aforementioned radii of curvature and average migration speeds of the left-turning Slugs 3 and 4, and the differences are within the range of slug-to-slug variations in migration behavior.
Figure 11.
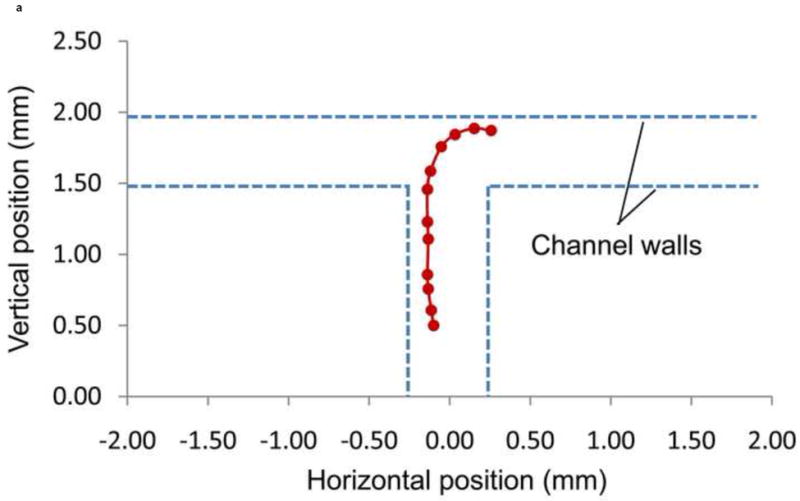
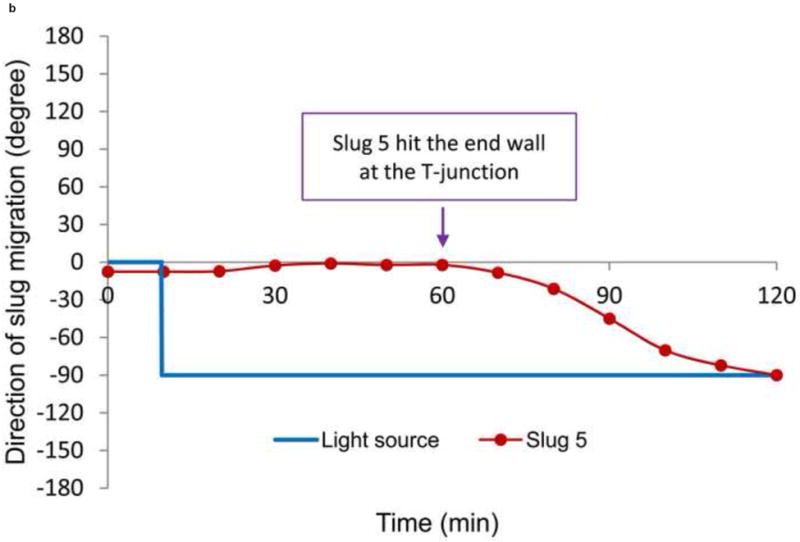
(a) Trajectory, and (b) direction of migration of Slug 5 as it was turning to the right during phototaxis.
The direction of migration of Slug 5 during the right turn (-90°) is shown in Figure 11b. While the light source was positioned in the right channel at t = 10 min, Slug 5 did not start to turn to that direction until approximately t = 60 min, when the slug came in contact with the end wall of the T-junction. It took approximately an hour for Slug 5 to turn itself to the right channel direction. This indicates the microchannel confinements contributed to inducing the slugs to make a right turn at the T-junction.
5. Conclusions
Migration of D. discoideum slugs has been controlled by manipulating the position of light introduced into microscale confinements in a microchip. In the microchip, a microchamber is connected to a microchannel, which joins two other channels to form a T-junction. D. discoideum cells are differentiated into slugs in the chamber. The slugs are then directed to move through the channels in the direction of light from optical fibers. The experiments are performed in a light enclosure to prevent unwanted ambient light from entering the chip. The microchip is fabricated from 4% agar, which provides a moist environment and sufficiently stiff surface for the slugs. The geometrical confinement imposed by microchannels and manipulation of light stimulation using the optical fibers in the microchip enable effective control of the slug migration. Experimental results have demonstrated that slugs were effectively guided through particular channels in the microchip under light stimulation without bias in changing their migration direction.
Based on these results, we can identify several directions of future work to improve the device functionality for potential bio-actuation applications. First, automation and integration of light stimulation can be pursued to more efficiently guide slug migration. This could be accomplished by manipulating the optical fibers using computer-controlled actuators, or integrating miniaturized light sources such as light-emitting diodes (LEDs) within the chip. Second, light can be used to simultaneously guide the migration of multiple slugs at different locations on the microchip, possibly enabled by the use of multiple optical fibers or LEDs within the microchannels. In this case, indirect illumination by reflected or transmitted light may need to be avoided to eliminate crosstalk between different light sources. Note that while indirect illumination benefited the proof-of-concept demonstration of this work, it was not essential and can be eliminated using opaque and non-reflective microchannels, which could be constructed by adding light-absorbing pigments into molten agar solution. Finally, it is of practical interest to demonstrate the use of light-directed slug migration for bio-actuation. For example, in a microfabricated device, micro- or nanoscale objects could be attached to slugs, and transported by the slugs that are photactically actuated and directed to specific locations on a microchip. Upon arrival at the destination, the objects could be released from the slugs by optically or chemically based cleavage. These capabilities could be useful in the assembly of microstructures [31, 32], and could also be extended to ultimately enable innovative drug delivery applications [33].
Acknowledgments
The authors would like to thank Dr. K.A. Yang for help and valuable discussion. We gratefully acknowledge financial support from the National Science Foundation (Award No. CBET-0854030) and the National Institutes of Health (Award Nos. RR025816-02 and CA147925-01). The authors also gratefully acknowledge the support of K. C. Wong Education Foundation, Hong Kong.
Biographies
Jinho Kim is a Ph.D. candidate in Mechanical Engineering at Columbia University. He received B.S. and M.S. degrees in Mechanical Engineering at Temple University, Philadelphia, USA, in 2007 and 2009, respectively. His research interests include development of biosensors for clinical diagnostics as well as applications of computational fluid dynamics (CFD) to all aspects of fluid dynamics and heat transfer.
Herbet L. Ennis was an associate professor of Biochemistry at the University of Tennessee Medical School for many years. Later, he worked for the pharmaceutical company Hoffmann-La Roche until he retired. He did considerable work studying the mechanism of action antibiotics. His recent interest is in the area of Developmental Biology.
ThaiHuu Nguyen received his B.Sci. from the University of Virginia in 2005, majoring in Aerospace Engineering. He received his M.S. and Ph.D. from Columbia University in Mechanical Engineering in 2007 and 2010, respectively. His research focus included integrating aptamers in microfluidic platforms for biochemical and biomedical related applications. He is currently a Naval Reactors Engineer for the Departments of Energy and the Navy at Naval Reactors Headquarters, Washington D.C.
Xuye Zhuang received his B.S. from Southwest Petroleum University in 2004 and Ph.D. from Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences in 2009. He is now an assistant researcher in Institute of Optics and Electronics, Chinese Academy of Sciences. His research interests are optical fiber biochemical sensors and MEMS.
Ji Luo received her BS from Shan Dong Jian Zhu University in 2009. She is currently a Ph.D. student at the State Kay Lab of Optical technologies for Microfabrication, Institute of Optics and Electronic, Chinese Academy of Sciences. Her research interests are optical fiber biochemical sensors and MEMS.
Jun Yao received his Ph.D. from the Physics Department of Sichuan University in 2001. From 2001 to 2007, he worked on MEMS, micro-optics, and adaptive optics at Strathclyde University, Durham University and University College London, respectively. Currently, Dr. Yao is a professor in MOEMS at the State Kay Lab of Optical technologies for Microfabrication, Chinese Academy of Sciences. His research interests include optical MEMS, micro-optics, and micro-system integration.
Richard H. Kessin obtained his Ph.D. at Brandeis University in 1971 and has been a professor at Harvard and, for the past 28 years, at Columbia University. His specialty is in microbiology and particularly the simple amoeba Dictyostelium, which has served as an experimental organism for many decades.
Milan Stojanovic obtained his B.Sc. at Beogradski Univerzitet, Serbia and Ph.D. in organic chemistry at Harvard University, USA. After a postdoctoral fellowship at Columbia University, Department of Medicine, he remained there as a faculty member.
Qiao Lin received his Ph.D. in Mechanical Engineering from the California Institute of Technology in 1998 with thesis research in robotics. He conducted postdoctoral research in microelectromechanical systems (MEMS) at the Caltech Micromachining Laboratory from 1998 to 2000, and was an assistant professor of Mechanical Engineering at Carnegie Mellon University from 2000 to 2005. He has been an associate professor of Mechanical Engineering at Columbia University since 2005. His research interests are in designing and creating integrated micro/nanosystems, in particular MEMS and microfluidic systems, for biomedical applications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weibel D, DiLuzio W, Whitesides G. Microfabrication meets microbiology. Nature Reviews Microbiology. 2007;5:209–218. doi: 10.1038/nrmicro1616. [DOI] [PubMed] [Google Scholar]
- 2.Steager E, Kim C, Patel J, Bith S, Naik C, Reber L, Kim M. Control of microfabricated structures powered by flagellated bacteria. Applied Physics Letters. 2007;90:263901. [Google Scholar]
- 3.Behkam B, Sitti M. Bacterial flagella assisted propulsion of patterned latex particles: Effect of particle size. IEEE. 2008:723–727. [Google Scholar]
- 4.Hiratsuka Y, Miyata M, Uyeda T. Living microtransporter by uni-directional gliding of Mycoplasma along microtracks. Biochemical and biophysical research communications. 2005;331:318–324. doi: 10.1016/j.bbrc.2005.03.168. [DOI] [PubMed] [Google Scholar]
- 5.Hiratsuka Y, Miyata M, Tada T, Uyeda T. A microrotary motor powered by bacteria. Proceedings of the National Academy of Sciences. 2006;103:13618. doi: 10.1073/pnas.0604122103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darnton N, Turner L, Breuer K, Berg H. Moving fluid with bacterial carpets. Biophysical journal. 2004;86:1863–1870. doi: 10.1016/S0006-3495(04)74253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behkam B, Sitti M. Bacterial flagella-based propulsion and on/off motion control of microscale objects. Applied Physics Letters. 2007;90:023902. [Google Scholar]
- 8.Kim Y, Dalhaimer P, Christian D, Discher D. Polymeric worm micelles as nanocarriers for drug delivery. Nanotechnology. 2005;16:S484. doi: 10.1088/0957-4484/16/7/024. [DOI] [PubMed] [Google Scholar]
- 9.Sitti M. Miniature devices: Voyage of the microrobots. Nature. 2009;458:1121–1122. doi: 10.1038/4581121a. [DOI] [PubMed] [Google Scholar]
- 10.Behkam B, Sitti M. Bacterial propulsion of chemically patterned micro-cylinders. IEEE. 2009:753–757. [Google Scholar]
- 11.Weibel D, Garstecki P, Ryan D, DiLuzio W, Mayer M, Seto J, Whitesides G. Microoxen: Microorganisms to move microscale loads. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11963–11967. doi: 10.1073/pnas.0505481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Leonardo R, Angelani L, Dell’Arciprete D, Ruocco G, Iebba V, Schippa S, Conte M, Mecarini F, De Angelis F, Di Fabrizio E. Bacterial ratchet motors. Proceedings of the National Academy of Sciences. 2010;107:9541–9545. doi: 10.1073/pnas.0910426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annesley SJ, Fisher PR. Dictyostelium discoideum—a model for many reasons. Molecular and cellular biochemistry. 2009;329:73–91. doi: 10.1007/s11010-009-0111-8. [DOI] [PubMed] [Google Scholar]
- 14.Kessin RH. Dictyostelium - Evolution, cell biology, and the development of multicellularity. Cambridge Univ. Press; Cambridge, UK: 2001. [Google Scholar]
- 15.Francis DW. Some studies on phototaxis of Dictyostelium. Journal of Cellular and Comparative Physiology. 1964;64:131–138. doi: 10.1002/jcp.1030640113. [DOI] [PubMed] [Google Scholar]
- 16.Fisher P, Annesley S. Slug phototaxis, thermotaxis, and spontaneous turning behavior. METHODS IN MOLECULAR BIOLOGY-CLIFTON THEN TOTOWA. 2006;346:137. doi: 10.1385/1-59745-144-4:137. [DOI] [PubMed] [Google Scholar]
- 17.Odell G, Bonner J. How the Dictyostelium discoideum grex crawls, Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1986;312:487–525. [Google Scholar]
- 18.Zanzotto A, Szita N, Boccazzi P, Lessard P, Sinskey A, Jensen K. Membrane aerated microbioreactor for high throughput bioprocessing. Biotechnology and bioengineering. 2004;87:243–254. doi: 10.1002/bit.20140. [DOI] [PubMed] [Google Scholar]
- 19.Fey P, Kowal A, Gaudet P, Pilcher K, Chisholm R. Protocols for growth and development of Dictyostelium discoideum. Nature Protocols. 2007;2:1307–1316. doi: 10.1038/nprot.2007.178. [DOI] [PubMed] [Google Scholar]
- 20.Poff KL, Skokut M. Thermotaxis by pseudoplasmodia of Dictyostelium discoideum. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:2007. doi: 10.1073/pnas.74.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Häder D. Negative phototaxis of Dictyostelium discoideum pseudoplasmodia in UV radiation. Photochemistry and Photobiology. 1985;41:225–228. [Google Scholar]
- 22.Smith E, Williams K. Evidence for tip control of the ‘slug/fruit’switch in slugs of Dictyostelium discoideum. Journal of embryology and experimental morphology. 1980;57:233. [PubMed] [Google Scholar]
- 23.Rieu J, Saito T, Delanoë-Ayari H, Sawada Y, Kay R. Migration of Dictyostelium slugs: Anterior-like cells may provide the motive force for the prespore zone. Cell Motility and the Cytoskeleton. 2009;66:1073–1086. doi: 10.1002/cm.20411. [DOI] [PubMed] [Google Scholar]
- 24.Inouye K, Takeuchi I. Analytical studies on migrating, movement of the pseudoplasmodium of Dictyostelium discoideum. Protoplasma. 1979;99:289–304. [Google Scholar]
- 25.Miura K, Siegert F. Light affects cAMP signaling and cell movement activity in Dictyostelium discoideum. Science’s STKE. 2000;97:2111. doi: 10.1073/pnas.040554497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonner J. Migration in Dictyostelium polycephalum. Mycologia. 2006;98:260. doi: 10.3852/mycologia.98.2.260. [DOI] [PubMed] [Google Scholar]
- 27.Breen E, Vardy P, Williams K. Movement of the multicellular slug stage of Dictyostelium discoideum: an analytical approach. Development. 1987;101:313. [Google Scholar]
- 28.Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nature cell biology. 2007;9:193–200. doi: 10.1038/ncb1536. [DOI] [PubMed] [Google Scholar]
- 29.Luo L, Gershow M, Rosenzweig M, Kang KJ, Fang-Yen C, Garrity PA, Samuel ADT. Navigational decision making in Drosophila thermotaxis. The Journal of Neuroscience. 2010;30:4261. doi: 10.1523/JNEUROSCI.4090-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegert F, Weijer C. Three-dimensional scroll waves organize Dictyostelium slugs. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6433. doi: 10.1073/pnas.89.14.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martel S, Mohammadi M. Using a swarm of self-propelled natural microrobots in the form of flagellated bacteria to perform complex micro-assembly tasks. IEEE. 2010:500–505. [Google Scholar]
- 32.Chung SE, Park W, Shin S, Lee SA, Kwon S. Guided and fluidic self-assembly of microstructures using railed microfluidic channels. Nature materials. 2008;7:581–587. doi: 10.1038/nmat2208. [DOI] [PubMed] [Google Scholar]
- 33.Angelani L, Leonardo RD. Geometrically biased random walks in bacteria-driven micro-shuttles. New Journal of Physics. 2010;12:113017. [Google Scholar]


