Abstract
Rapidly adapting mechanoreceptors within the laryngeal mucosa provide the central nervous system with perceptual and proprioceptive afference for a variety of essential yet diverse human functions including voice sound production and airway protection. It is unknown why mechanosensory information that yields a defensive response when an individual breathes may go largely unnoticed when the individual voices. Therefore, a central question is whether there is voice-related modulation of laryngeal mechanosensory detection. Such modulation would be consistent with current models of afferent laryngeal control, and may be important to maintain fluent voice in the presence of potentially distracting sensory input. Therefore, we employed endoscopic assessment of laryngeal mechanosensory detection thresholds in ten healthy adults during tidal breathing and a voice task. We tested the hypothesis that laryngeal mechanosensory detection thresholds would be higher during the voice task. We found that thresholds were significantly higher for all participants during the voice task, and that these changes were significantly more modest in women. Our findings suggest that the laryngeal sensorium may modulate mechanosensory afference to attenuate the potentially distracting influence of sensory input during voice. The finding that women maintain a greater sensitivity during the voice task than men (lower thresholds) may have important implications for the higher prevalence of sensorimotor voice disturbances in women. Our results are consistent with the presence of mechanosensory modulation in other motor systems and with observed sensory differences between women and men. Such modulation has important implications for understanding the underlying neural mechanisms of laryngeal control and how these mechanisms may operate in individuals with laryngeal disturbances.
Keywords: sensory, afference, efference, voice, fluent, breathing, sex
INTRODUCTION
Rapidly adapting mechanoreceptors within the laryngeal mucosa are innervated by the tenth cranial nerve with axons that project through the nodose ganglion and terminate within the solitary tract nucleus, spinal trigeminal nucleus and reticular formation in the lateral medulla (Jurgens 2002; Jurgens and Kirzinger 1985; Simonyan and Jurgens 2005a, b). Most fibers that synapse within the solitary tract nucleus terminate in the parabrachial nuclei within the dorsolateral pons and send projections to the posteromedial nuclei within the ventral thalamus before sending somatotopic projections to the primary somatosensory cortex. Laryngeal mechanoreceptors provide perceptual and proprioceptive afference for a variety of essential human functions including airway protection, breathing, deglutition, speech, and voice. These stretch, touch, and pressure sensitive mechanoreceptors within the laryngeal mucosa appear to be exquisitely arranged for the rapid transmission of a constellation of movement-related afferent information (Andreatta et al. 2002; Jurgens 2002; Jurgens and Kirzinger 1985; Kirchner and Wyke 1965; Loucks et al. 2005; Malannino 1974; Matsuo and Shin 1994; Sampson and Eyzaguirre 1964; Sapir et al. 2000; Shiba et al. 1999; Shiba et al. 1997). Movement-related afference may include those events intrinsic to the larynx, such as position and movement trajectory of the arytenoid cartilages and vocal folds, or those extrinsic to the larynx including the strains, pressures, and movement patterns generated by mechanical and aerodynamic changes of the lower and upper airways (Kogo et al. 2003). Afferent transmission regarding such events would prove particularly useful for laryngeal motor control.
Evidence suggests a relatively strong association of perception, movement generated afference, and the patterns of tissue deformation related to movement (Edin 1992, 2004; Edin and Johansson 1995). For example, imposing tissue strains similar to those that occur during natural movement may result in the perception of movement in the absence of actual movement. The patterns of afferent neural discharge and accompanying perception during movement are strikingly similar to those that occur during simulated tissue deformation. Although this phenomenon remains to be fully explored in the human larynx, it is reasonable to suggest that perception provides an important reflection of movement related afference within the central nervous system. Specific to the larynx, single fiber recordings from laryngeal mechanoreceptor afferents demonstrate that mechanoreceptors are responsive to vocal fold vibration, are associated with the magnitude and direction of respiratory air flow, and influence changes in glottal aperture (Andreatta et al. 2002; Jurgens 2002; Jurgens and Kirzinger 1985; Kirchner and Wyke 1965; Loucks et al. 2005; Malannino 1974; Matsuo and Shin 1994; Sampson and Eyzaguirre 1964; Sapir et al. 2000; Shiba et al. 1999; Shiba et al. 1997). Thus, the patterned distribution of tissue strains across the surface of the face, hand, or larynx may serve to inform the central nervous system of the approximate position, velocity, and higher derivatives of movements. Such afferent input may be useful to plan sequential movement patterns, and to make rapid online corrections in response to unexpected perturbations (Bevan et al. 1994; Cordo et al. 1994). These types of responses may be particularly relevant for laryngeal airway protection and voice control.
Human voice is typically produced by increases in tracheal air pressure and resultant egressive air flow (Titze 1994). Voice-related changes in translaryngeal air pressure gradients and air flow may stimulate laryngeal mechanoreceptors (Andreatta et al. 2002; Kogo et al. 2003; Matsuo and Shin 1994; Sampson and Eyzaguirre 1964). In addition, as the arytenoid cartilages adduct to draw the vocal folds together for voice sound production, changes in the laryngeal aperture result in mechanical stress and strain to the laryngeal mucosa, and may further stimulate these mechanoreceptors. When an individual breathes, stimulation of mechanoreceptors may yield the perception of potential airway invasion, and may result in rapid closure of the larynx and subsequent ballistic exhalation to defend against potential airway invasion (Sant'Ambrogio and Mathew 1986). Thus, mechanoreceptors provide the central nervous system with rich afference to precisely guide the larynx for voice sound production and airway protection. However, it is interesting that mechanosensory information that yields a defensive response when an individual breathes may go largely unnoticed when the individual voices. Therefore, a central question is whether there is voice-related modulation of laryngeal mechanosensory detection. For many human sensorimotor systems, the central nervous system demonstrates an ability to alter its afferent processing in a task dependent manner (Andreatta and Barlow 2003; Schmidt et al. 1990; Post et al. 1994; Feine et al. 1990). For example, the central nervous system may filter or gate expected movement-generated afferent inputs. Given the variety of centrally patterned behaviors accomplished with the larynx (Davis et al. 1996; Titze et al. 2008; Larson et al. 1994; Chiao et al. 1994; Larson 1991), and the rich afference accompanying such behaviors, the ability of the laryngeal sensorium to gate afferent inputs would be advantageous. Such modulation or gating would be critical to maintain an uninterrupted voice pattern in the presence of potentially distracting sensory input.
Recent experimental evidence and computational modeling suggest an important role for efference copy in voice related laryngeal control (Liu et al. 2010; Larson et al. 2008). Although much of this work has focused on voice auditory feedback, there is growing evidence that motor driven predictions are used to detect and correct for errors in self-generated movements (Liu et al. 2011; Behroozmand and Larson 2011). Such errors may be reflected as a deviation from the expected or predicted sensory input. Relatively small deviations in sensory input may be recognized as self-generated by the central nervous system but may remain undetected by the speaker. However, relatively large deviations in sensory input may be recognized as other-generated stimuli and be more easily detected by the speaker. Thus, modulation of mechanosensory detection thresholds may reflect the presence of sensory gating and efference copy within the cortical and subcortical regions subserving vocalization (Parkinson et al. 2012; Behroozmand et al. 2009).
Potential differences between women and men are also of interest for a number of important reasons. Evidence suggests that women demonstrate increased sensitivity to pain, touch, temperature, dyspnea, and citric acid induced cough, and also exhibit differences in functional connectivity and cortical/subcortical sensory representation (Dicpinigaitis and Rauf 1998; Ide and Aboitiz 2001; Fillingim and Ness 2000; Gui et al. 2010; Harju 2002; Kelsall et al. 2009; Linnman et al. 2012; Matos et al. 2011; Bajaj et al. 2001; Chesterton et al. 2003; Dicpinigaitis et al. 2001; Nishino 2011). Women demonstrate less sensory gating in response to auditory stimuli, and less modulation of pain detection thresholds during dyspnea (Hetrick et al. 1996; Nishino et al. 2008; Patterson et al. 2008; Tomasi et al. 2008). Specific to vocalization, a recent study revealed that women produce significantly smaller and more rapid voice responses to pitch-shifted auditory feedback (Chen et al. 2010). This finding may suggest that women rely more heavily on mechanosensory than auditory afference for voice control (Larson et al. 2008), and therefore may maintain more sensitivity to mechanosensory stimuli during vocalization. Finally, the reported prevalence of voice disturbances is higher in women including those disturbances related to occupational voice use and laryngeal dystonia (Hunter et al. 2011; Smith et al. 1998; Titze 1989; Roy et al. 2005). Therefore, women may be more aware of and sensitive to mechanosensory stimuli during vocalization and the sensory phenomena associated with potential voice disturbances. However, differences in laryngeal mechanosensation between women and men have yet to be explored.
Previous investigations developed reliable endoscopic methods to determine laryngeal mechanosensory detection thresholds during restful breathing (Hammer 2009). These methods have been useful to examine the important association between sensory detection with speech, swallow, and voice motor control (Hammer and Barlow 2010; Hammer et al. 2013). Previous studies demonstrated task-related modulation of mechanosensory and tactile detection (Andreatta and Barlow 2003; Schmidt et al. 1990; Post et al. 1994; Feine et al. 1990). However, whether laryngeal mechanosensory detection is modulated with voice is unknown. Therefore, the purpose of this study was to measure and compare laryngeal mechanosensory detection thresholds during a baseline condition of tidal breathing and an experimental voice condition. We hypothesized that thresholds would be significantly higher during the voice condition and that women may exhibit less voice related modulation than men.
METHODS
Participants
Data were collected from 10 healthy participants (5 women, 5 men) between 18-23 years old. The institutional ethics committee for the safety of human subjects approved this protocol, and written informed consent was obtained from each participant. Participants were non-smokers and were in good general health, with normal breathing, speech, swallow, and voice, and no history of neurological or psychiatric disease.
Laryngeal Mechanosensory Assessment
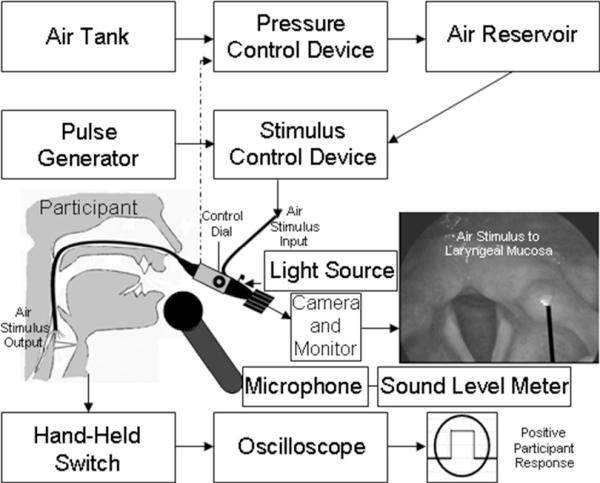
We employed an endoscopic stimulus delivery paradigm to present a pressure-calibrated burst of air to the laryngeal mucosa to determine the threshold pressure at which each participant could detect the stimulus. We previously described the device design and experimental approach (Hammer 2009; Hammer and Barlow 2010; Hammer et al. 2013), and will briefly review the procedures below. Each participant sat comfortably in an exam chair, and a transnasal laryngoscope (Pentax FNL-13RAP) was placed into the most patent naris. A topical decongestant was administered prior to scope placement, and the exam was completed without anesthesia. The fiberoptic light cable of the laryngoscope was coupled to a constant halogen light source (Pentax LH-150PC) to illuminate the laryngeal structures. The eyepiece of the endoscope was coupled to a 3-chip camera (Toshiba IK-C40A) connected to an integrated video monitor (Sony GVD-1000). The air burst port of the laryngoscope was coupled to a three-foot long rigid polyethylene tube. The opposite end of the tube was coupled to the output port of the sensory stimulus delivery device. The device design included a sound attenuating enclosure to prevent potential acoustic cues of the device from imposing a bias on participant responses.
Because the mucosa covering the arytenoid-corniculate cartilages contain the highest density of rapidly adapting mechanoreceptors (Andreatta et al. 2002; Yoshida et al. 2000), visual guidance was used from the display monitor to direct the calibrated stimulus through the distal end of the laryngoscope toward the superior surface of the mucosa surrounding the arytenoid-corniculate cartilage in the posterior region of the larynx. Great care was taken in an attempt to maintain a constant distance of 2 mm between the laryngoscope and the laryngeal mucosa by ensuring that at least 50% of the monitoring screen was occupied by the arytenoid unit during stimulus presentation. This approach was previously verified using excised human adult cadaveric larynges and bench tests that revealed little to no variation in output delivered to targets within 2 to 5 mm from the scope tip (Hammer 2009). Once the target position was reached, an index finger was positioned on the scope tube at the nasal inlet in an attempt to maintain a constant distance from scope tip to arytenoid mucosa of 2 mm during each stimulus presentation. Within this distance, individual blood vessels within the vocal fold became very easy to visualize. Adjustments were made between stimulus presentations as needed.
During tidal breathing, stimulus presentation was triggered by the initiation of the expiratory phase of respiration as transduced using inductance plethysmography (Respiratory Monitoring, Inc.). During the onset of expiration, a transistor-transistor logic signal triggered a control pulse resulting in event-related triggering of a 135 ms air burst stimulus. Based on results from an earlier study (Hammer 2009), the more salient 135 ms stimulus was selected to reduce the potential influence of temporal summation on sensory threshold estimates. During the voice task, stimulus presentation was similarly triggered after an initial 1.5 s of sustained voice. Each participant was instructed to voice on the vowel “ee” as in the word “bee” for 6 s at a constant and comfortable pitch and loudness. To ensure that the participant maintained the same constant pitch and loudness, a pitch pipe and a sound level meter were employed to provide real-time feedback to the participant. The microphone of the sound level meter was maintained at a constant distance of 5 cm from the participant's mouth.
The primary signals of interest for assessing mechanosensory function were the pressure (mm Hg) of the air burst stimulus applied to the laryngeal mucosa overlying the superior surface of the arytenoid cartilage and the participant's response to the stimulus pulse. Each participant was presented with an initial suprathreshold stimulus to orient to the response task and was instructed to press a custom designed hand-held switch as soon as they detected the stimulus. All participants found the hand-switch easy to use. Pressing the switch resulted in a +5V signal displayed on an oscilloscope (Tektronix TDS 2004). A “positive response” was defined as a +5V response by the participant occurring within a 2.5 second window beginning at the midpoint of the control signal. The time interval between stimulus presentations was randomized, with a minimum of 10 seconds between stimuli. The stimulus level of the air burst source was decreased by 1.00 mm Hg until the participant could no longer feel the stimulus. At this point, the stimulus level was increased by 0.50 mm Hg until the participant yielded a “positive response.” Then, the stimulus was decreased by 0.10 mmHg. This process continued until the participant reached the threshold level. The laryngeal mechanosensory detection threshold (LMDT) was defined as the level of stimulus pressure at which the participant responded 50% of the time following six crossings of the same stimulus level.
Statistical Analyses
We utilized a mixed model ANOVA to examine differences in mechanosensory detection between baseline tidal breathing and voice conditions and to test for differences between women and men. We hypothesized that thresholds would be significantly higher during the voice condition. We also hypothesized that women may exhibit less voice related modulation than men.
RESULTS
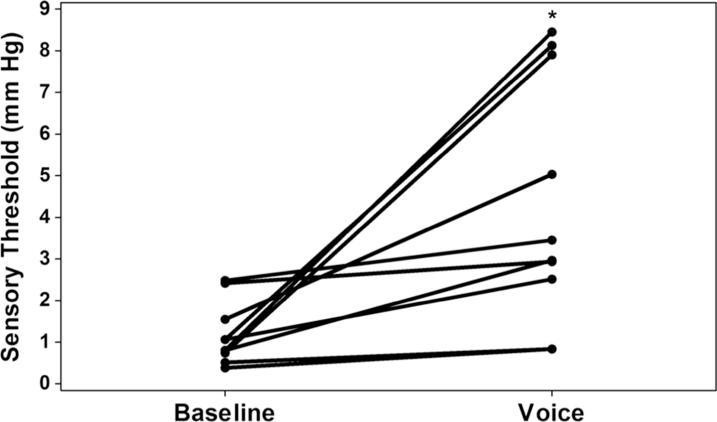
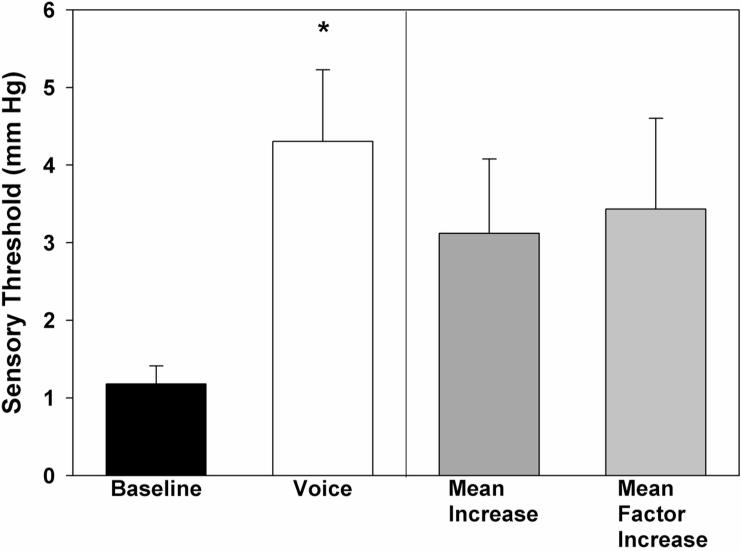
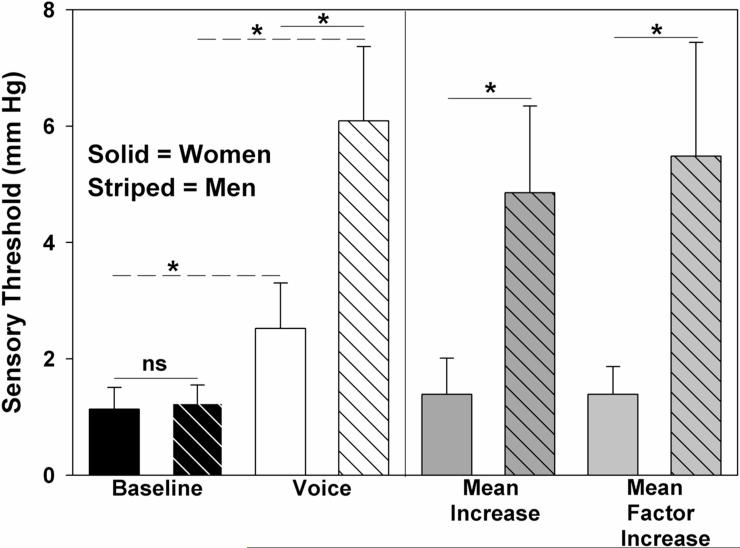
Laryngeal mechanosensory detection thresholds for each of the 10 participants are displayed in Figure 2. Summary statistics are displayed in Table 1 and Figure 3. We observed significant main effects for task [F(1,19)=14.89, p = 0.005, ηp2 = 0.65, Cohen's d =1.47] and sex [F(1, 19)=5.68, p = 0.044, ηp2 = 0.39, Cohen's d = 1.36]. Sensory thresholds were elevated for each participant during the voice task by an average of 3.44 mmHg, representing more than a 3-fold increase (p = 0.005). Men exhibited a greater 5-fold increase in threshold during the voice task; women exhibited a more modest 1.39-fold increase (p = 0.003).
Figure 2.
Laryngeal mechanosensory detection thresholds for baseline tidal breathing and voice conditions. (* p < 0.01) a. Circles represent individual threshold values for each participant and a line connects the circle (baseline and voice) for each participant. b. On the left, bar height represents mean (standard error) detection threshold for baseline and voice conditions. On the right, bar height represents mean increase and mean factor increase in detection threshold compared with baseline. (1 mm Hg = 133.32 Pa)
Table 1.
Mean (standard error) laryngeal mechanosensory detection threshold (mm Hg) for baseline and voice conditions. (1 mm Hg = 133.32 Pa) Mean increase and mean factor increase compared with baseline are included. Data are tabulated for all participants, then separately for women and men.
| Laryngeal Mechanosensory Detection Thresholds | |||
|---|---|---|---|
| All Participants | Women | Men | |
| Baseline Laryngeal Sensory Threshold | 1.18 (0.23) | 1.13 (0.38) | 1.23 (0.32) |
| Voice Laryngeal Sensory Threshold | 4.30 (0.92) | 2.52 (0.78) | 6.09 (1.28) |
| Mean Increase in Sensory Threshold | 3.12 (0.96) | 1.38 (0.62) | 4.85 (1.49) |
| Mean Factor Increase in Sensory Threshold | 3.44 (1.17) | 1.39 (0.48) | 5.48 (1.96) |
Figure 3.
Laryngeal mechanosensory detection thresholds for baseline tidal breathing and voice conditions for women (solid) and men (striped). (* p < 0.05) On the left, bar height represents mean (standard error) detection threshold for baseline and voice conditions. On the right, bar height represents mean increase and mean factor increase in detection threshold compared with baseline. (1 mm Hg = 133.32 Pa)
DISCUSSION
The influence of an active voice task on mechanosensory detection was previously unknown. We employed endoscopic assessment of laryngeal mechanosensory detection thresholds in healthy adults during baseline tidal breathing and during a voice task. With tidal breathing, participants exhibited an expected range of detection thresholds compared with healthy controls in previous work (Hammer 2009; Hammer and Barlow 2010; Hammer et al. 2013). However, with the voice task, detection thresholds were significantly higher. This primary finding suggests that the healthy human larynx exhibits voice-related elevation of mechanosensory detection thresholds. Such modulation has important implications for laryngeal control.
Our findings are consistent with the central nervous system's ability to alter its afferent processing in a task dependent manner (Andreatta and Barlow 2003; Schmidt et al. 1990; Post et al. 1994; Feine et al. 1990) by filtering or gating expected movement-generated afferent inputs. Given the rich afference that accompanies the variety of centrally patterned laryngeal behaviors (Davis et al. 1996; Titze et al. 2008; Larson et al. 1994; Chiao et al. 1994; Larson 1991), the ability of the laryngeal sensorium to gate afferent inputs would be advantageous. One compelling observation is that mechanosensory information that yields a defensive response when an individual breathes may go largely unnoticed when the individual voices. Therefore, such modulation or gating may be important to maintain a fluent voice pattern in the presence of potentially distracting sensory input.
The observed modulation of mechanosensory detection thresholds in the present study is consistent with the role of efference copy in voice related laryngeal control (Liu et al. 2010; Larson et al. 2008) and with the growing evidence that motor driven predictions are used to detect and correct for errors in self-generated movements (Liu et al. 2011; Behroozmand and Larson 2011). In the present study, relatively small mechanosensory inputs were easily detected during the tidal breathing task. During the voice task, however, the finding that these small inputs were undetected reveals that small deviations in sensory input may be interpreted by the central nervous system as self-generated and thus remain undetected by the speaker. In contrast, the relatively large deviations in sensory input may be interpreted as other-generated stimuli and more easily detected by the speaker. Therefore, the modulation of mechanosensory detection thresholds observed in the present study may reflect the presence of sensory gating and efference copy within the cortical and subcortical regions subserving vocalization including corollary discharge mediated to regions including the superior temporal gyrus (Parkinson et al. 2012; Behroozmand et al. 2009). The fact that sensory detection thresholds change in a task dependent manner suggests that this may be a speech/vocalization specific phenomenon. Future work will be required to further elucidate these mechanosensory auditory interactions.
In the present study, women and men each exhibited elevated thresholds during the voice task. However, although women and men demonstrated similar thresholds during the baseline tidal breathing task, women had lower thresholds (greater sensitivity) than men during the voice condition, and thus less change in threshold. This finding parallels evidence of increased sensitivity to pain, touch, temperature, dyspnea, differences in functional connectivity and cortical/subcortical sensory representation, and increased sensitivity to citric acid induced cough in women than men (Dicpinigaitis and Rauf 1998; Ide and Aboitiz 2001; Fillingim and Ness 2000; Gui et al. 2010; Harju 2002; Kelsall et al. 2009; Linnman et al. 2012; Matos et al. 2011; Bajaj et al. 2001; Chesterton et al. 2003; Dicpinigaitis et al. 2001; Nishino 2011). Women also demonstrate less sensory gating in response to auditory stimuli, and less modulation of pain detection thresholds during dyspnea (Hetrick et al. 1996; Nishino et al. 2008; Patterson et al. 2008; Tomasi et al. 2008). Our finding of greater voice related sensitivity in women is particularly interesting given the higher prevalence of reported voice disturbances in women including those related to occupational voice use and laryngeal dystonia (Hunter et al. 2011; Smith et al. 1998; Titze 1989; Roy et al. 2005). Thus, women may be more aware of and sensitive to sensory phenomenon associated with potential voice disturbances. Of course, it may be possible that the greater sensitivity in women is simply a function of anatomical size. A smaller female larynx with smaller arytenoids may possess a higher density of mechanoreceptors. These differences require further investigation. Although this remains unexplored in the larynx, recent evidence suggests that this may be true for the cutaneous surface of the finger (Peters et al. 2009). However, in the present study, differences between women and men were only specific to the voice task. Therefore, women may rely more heavily on mechanosensory than auditory afference for voice control, and thus maintain more sensitivity to mechanosensory stimuli during vocalization (Chen et al. 2010; Larson et al. 2008). Women may be more aware of and sensitive to mechanosensory stimuli during vocalization and the sensory phenomenon associated with potential voice disturbances. Differences in laryngeal mechanosensation between women and men require further exploration.
Although the present results are compelling, there are limitations in the study and important issues to address in future work. We demonstrated the ability of the laryngeal sensorium to modulate detection of mechanosensory input during a voice task with a relatively modest set of healthy participants. However, it is important to expand upon this work in future studies and to further elucidate the comparison between women with men with an analysis of self-perceived voice and airway function. It remains unknown whether mechanosensory modulation occurs with other non-voice laryngeal tasks and whether such modulation is altered in individuals who experience voice disturbances. Therefore, future work is required with individuals who experience voice disturbances to test for an association between laryngeal sensory and voice related motor function. The present study provides an important basis for such work.
In conclusion, our results are consistent with the presence of mechanosensory modulation in other motor systems and with observed sensory differences between women and men. Such modulation has important implications for understanding the underlying neural mechanisms of laryngeal control and how these mechanisms may operate in individuals with laryngeal disturbances. In the present work, we demonstrated that the healthy human larynx exhibits voice-related elevation of mechanosensory detection thresholds and that women may maintain a greater sensitivity (lower thresholds) than men. We would suggest that voice related modulation may be important to maintain fluent voice production, and that such modulation has important implications for laryngeal control.
Figure 1.
Stimulus delivery paradigm. The stimulus is directed to the laryngeal mucosa through the laryngoscope as visualized on the monitor. A +5V signal from a hand-held switch indicates when the participant feels the laryngeal somatosensory stimulus. A microphone connected to a sound level meter is used during the voice trials.
ACKNOWLEDGEMENTS
Dr. Hammer is supported through National Institutes of Health grants DC010900 and RR025012.
Footnotes
The authors have no conflicts of interest.
REFERENCES
- Andreatta RD, Barlow SM. Movement-related modulation of vibrotactile detection thresholds in the human orofacial system. Experimental brain research. 2003;149:75–82. doi: 10.1007/s00221-002-1336-x. [DOI] [PubMed] [Google Scholar]
- Andreatta RD, Mann EA, Poletto CJ, Ludlow CL. Mucosal afferents mediate laryngeal adductor responses in the cat. Journal of applied physiology. 2002;93:1622–1629. doi: 10.1152/japplphysiol.00417.2002. [DOI] [PubMed] [Google Scholar]
- Bajaj P, Arendt-Nielsen L, Bajaj P, Madsen H. Sensory changes during the ovulatory phase of the menstrual cycle in healthy women. European journal of pain. 2001;5:135–144. doi: 10.1053/eujp.2001.0236. [DOI] [PubMed] [Google Scholar]
- Behroozmand R, Karvelis L, Liu H, Larson CR. Vocalization-induced enhancement of the auditory cortex responsiveness during voice F0 feedback perturbation. Clinical neurophysiology. 2009;120:1303–1312. doi: 10.1016/j.clinph.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behroozmand R, Larson CR. Error-dependent modulation of speech induced auditory suppression for pitch shifted voice feedback. BMC neuroscience. 2011;12:54. doi: 10.1186/1471-2202-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan L, Cordo P, Carlton L, Carlton M. Proprioceptive coordination of movement sequences: discrimination of joint angle versus angular distance. Journal of neurophysiology. 1994;71:1862–1872. doi: 10.1152/jn.1994.71.5.1862. [DOI] [PubMed] [Google Scholar]
- Chen Z, Liu P, Jones JA, Huang D, Liu H. Sex-related differences in vocal responses to pitch feedback perturbations during sustained vocalization. The Journal of the Acoustical Society of America. 2010;128:EL355–360. doi: 10.1121/1.3509124. [DOI] [PubMed] [Google Scholar]
- Chesterton LS, Barlas P, Foster NE, Baxter GD, Wright CC. Gender differences in pressure pain threshold in healthy humans. Pain. 2003;101:259–266. doi: 10.1016/S0304-3959(02)00330-5. [DOI] [PubMed] [Google Scholar]
- Chiao GZ, Larson CR, Yajima Y, Ko P, Kahrilas PJ. Neuronal activity in nucleus ambiguous during deglutition and vocalization in conscious monkeys. Experimental brain research. 1994;100:29–38. doi: 10.1007/BF00227276. [DOI] [PubMed] [Google Scholar]
- Cordo P, Carlton L, Bevan L, Carlton M, Kerr GK. Proprioceptive coordination of movement sequences: role of velocity and position information. Journal of neurophysiology. 1994;71:1848–1861. doi: 10.1152/jn.1994.71.5.1848. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Zhang SP, Bandler R. Midbrain and medullary regulation of respiration and vocalization. Progress in brain research. 1996;107:315–325. doi: 10.1016/s0079-6123(08)61873-7. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Allusson VR, Baldanti A, Nalamati JR. Ethnic and gender differences in cough reflex sensitivity. Respiration; international review of thoracic diseases. 2001;68:480–482. doi: 10.1159/000050554. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Rauf K. The influence of gender on cough reflex sensitivity. Chest. 1998;113:1319–1321. doi: 10.1378/chest.113.5.1319. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. Journal of neurophysiology. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analyses of dynamic strain sensitivity in human skin mechanoreceptors. Journal of neurophysiology. 2004;92:3233–3243. doi: 10.1152/jn.00628.2004. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. The Journal of physiology. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feine JS, Chapman CE, Lund JP, Duncan GH, Bushnell MC. The perception of painful and nonpainful stimuli during voluntary motor activity in man. Somatosensory & motor research. 1990;7:113–124. doi: 10.3109/08990229009144702. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neuroscience and biobehavioral reviews. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Gui P, Ebihara S, Kanezaki M, Suda C, Nikkuni E, Ebihara T, Yamasaki M, Kohzuki M. Gender differences in perceptions of urge to cough and dyspnea induced by citric acid in healthy never smokers. Chest. 2010;138:1166–1172. doi: 10.1378/chest.10-0588. [DOI] [PubMed] [Google Scholar]
- Hammer MJ. Design of a new somatosensory stimulus delivery device for measuring laryngeal mechanosensory detection thresholds in humans. IEEE transactions on bio medical engineering. 2009;56:1154–1159. doi: 10.1109/TBME.2008.2007968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer MJ, Barlow SM. Laryngeal somatosensory deficits in Parkinson's disease: implications for speech respiratory and phonatory control. Experimental brain research. 2010;201:401–409. doi: 10.1007/s00221-009-2048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer MJ, Murphy CA, Abrams TM. Airway Somatosensory Deficits and Dysphagia in Parkinson's Disease. Journal of Parkinson's Disease. 2013;3:39–44. doi: 10.3233/JPD-120161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harju EL. Cold and warmth perception mapped for age, gender, and body area. Somatosensory & motor research. 2002;19:61–75. doi: 10.1080/08990220120113057. [DOI] [PubMed] [Google Scholar]
- Hetrick WP, Sandman CA, Bunney WE, Jr., Jin Y, Potkin SG, White MH. Gender differences in gating of the auditory evoked potential in normal subjects. Biological psychiatry. 1996;39:51–58. doi: 10.1016/0006-3223(95)00067-4. [DOI] [PubMed] [Google Scholar]
- Hunter EJ, Tanner K, Smith ME. Gender differences affecting vocal health of women in vocally demanding careers. Logopedics, phoniatrics, vocology. 2011;36:128–136. doi: 10.3109/14015439.2011.587447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide A, Aboitiz F. A sex difference in the postcentral sulcus of the human brain. Brain research. 2001;890:330–332. doi: 10.1016/s0006-8993(00)03215-7. [DOI] [PubMed] [Google Scholar]
- Jurgens U. Neural pathways underlying vocal control. Neuroscience and biobehavioral reviews. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Jurgens U, Kirzinger A. The laryngeal sensory pathway and its role in phonation. A brain lesioning study in the squirrel monkey. Experimental brain research. 1985;59:118–124. doi: 10.1007/BF00237672. [DOI] [PubMed] [Google Scholar]
- Kelsall A, Decalmer S, McGuinness K, Woodcock A, Smith JA. Sex differences and predictors of objective cough frequency in chronic cough. Thorax. 2009;64:393–398. doi: 10.1136/thx.2008.106237. [DOI] [PubMed] [Google Scholar]
- Kirchner JA, Wyke BD. Articular reflex mechanisms in the larynx. The Annals of otology, rhinology, and laryngology. 1965;74:749–768. doi: 10.1177/000348946507400315. [DOI] [PubMed] [Google Scholar]
- Kogo M, Iida S, Senoo H, Ishii S, Hamaguchi M, Enomoto A, Matsuya T. Effects of subglottal air pressure on velopharyngeal muscle activity in dogs. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2003;40:351–355. doi: 10.1597/1545-1569_2003_040_0351_eosapo_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Larson CR. On the relation of PAG neurons to laryngeal and respiratory muscles during vocalization in the monkey. Brain research. 1991;552(1):77–86. doi: 10.1016/0006-8993(91)90662-f. [DOI] [PubMed] [Google Scholar]
- Larson CR, Altman KW, Liu H, Hain TC. Interactions between auditory and somatosensory feedback for voice F0 control. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2008;187:613–621. doi: 10.1007/s00221-008-1330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CR, Yajima Y, Ko P. Modification in activity of medullary respiratory related neurons for vocalization and swallowing. Journal of neurophysiology. 1994;71:2294–2304. doi: 10.1152/jn.1994.71.6.2294. [DOI] [PubMed] [Google Scholar]
- Linnman C, Beucke JC, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2012;153:444–454. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Behroozmand R, Larson CR. Audio – vocal interactions in the mammalian brain. In: Brudzynski SM, editor. Handbook of Mammalian Vocalization: An Integrative Neuroscience Approach. Academic Press; San Diego: 2010. pp. 393–401. [Google Scholar]
- Liu H, Meshman M, Behroozmand R, Larson CR. Differential effects of perturbation direction and magnitude on the neural processing of voice pitch feedback. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2011;122:951–957. doi: 10.1016/j.clinph.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks TM, Poletto CJ, Saxon KG, Ludlow CL. Laryngeal muscle responses to mechanical displacement of the thyroid cartilage in humans. Journal of applied physiology. 2005;99:922–930. doi: 10.1152/japplphysiol.00402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malannino N. Proceedings: Laryngeal neuromuscular spindles and their possible function. Folia phoniatrica. 1974;26:291–292. doi: 10.1159/000263789. [DOI] [PubMed] [Google Scholar]
- Matos R, Wang K, Jensen JD, Jensen T, Neuman B, Svensson P, Arendt-Nielsen L. Quantitative sensory testing in the trigeminal region: site and gender differences. Journal of orofacial pain. 2011;25:161–169. [PubMed] [Google Scholar]
- Matsuo H, Shin T. Distribution of intraepithelial nerve fibers in the feline glottis. Otolaryngology--head and neck surgery. 1994;111:91–99. doi: 10.1177/019459989411100117. [DOI] [PubMed] [Google Scholar]
- Nishino T. Dyspnoea: underlying mechanisms and treatment. British journal of anaesthesia. 2011;106:463–474. doi: 10.1093/bja/aer040. [DOI] [PubMed] [Google Scholar]
- Nishino T, Isono S, Ishikawa T, Shinozuka N. Sex differences in the effect of dyspnea on thermal pain threshold in young healthy subjects. Anesthesiology. 2008;109:1100–1106. doi: 10.1097/ALN.0b013e31818d8f43. [DOI] [PubMed] [Google Scholar]
- Parkinson AL, Flagmeier SG, Manes JL, Larson CR, Rogers B, Robin DA. Understanding the neural mechanisms involved in sensory control of voice production. NeuroImage. 2012;61:314–322. doi: 10.1016/j.neuroimage.2012.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, Potkin S, Bunney WE., Jr. P50 sensory gating ratios in schizophrenics and controls: a review and data analysis. Psychiatry research. 2008;158:226–247. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Peters RM, Hackeman E, Goldreich D. Diminutive digits discern delicate details: fingertip size and the sex difference in tactile spatial acuity. The Journal of neuroscience. 2009;29:15756–15761. doi: 10.1523/JNEUROSCI.3684-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post LJ, Zompa IC, Chapman CE. Perception of vibrotactile stimuli during motor activity in human subjects. Experimental brain research. 1994;100:107–120. doi: 10.1007/BF00227283. [DOI] [PubMed] [Google Scholar]
- Roy N, Merrill RM, Gray SD, Smith EM. Voice disorders in the general population: prevalence, risk factors, and occupational impact. The Laryngoscope. 2005;115:1988–1995. doi: 10.1097/01.mlg.0000179174.32345.41. [DOI] [PubMed] [Google Scholar]
- Sampson S, Eyzaguirre C. Some Functional Characteristics of Mechanoreceptors in the Larynx of the Cat. Journal of neurophysiology. 1964;27:464–480. doi: 10.1152/jn.1964.27.3.464. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Mathew OP. Laryngeal receptors and their reflex responses. Clinics in chest medicine. 1986;7:211–222. [PubMed] [Google Scholar]
- Sapir S, Baker KK, Larson CR, Ramig LO. Short-latency changes in voice F0 and neck surface EMG induced by mechanical perturbations of the larynx during sustained vowel phonation. Journal of speech, language, and hearing research : JSLHR. 2000;43(1):268–276. doi: 10.1044/jslhr.4301.268. [DOI] [PubMed] [Google Scholar]
- Schmidt RF, Schady WJ, Torebjork HE. Gating of tactile input from the hand. I. Effects of finger movement. Experimental brain research. 1990;79:97–102. doi: 10.1007/BF00228877. [DOI] [PubMed] [Google Scholar]
- Shiba K, Miura T, Yuza J, Sakamoto T, Nakajima Y. Laryngeal afferent inputs during vocalization in the cat. Neuroreport. 1999;10:987–991. doi: 10.1097/00001756-199904060-00017. [DOI] [PubMed] [Google Scholar]
- Shiba K, Yoshida K, Nakajima Y, Konno A. Influences of laryngeal afferent inputs on intralaryngeal muscle activity during vocalization in the cat. Neuroscience research. 1997;27:85–92. doi: 10.1016/s0168-0102(96)01136-4. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Afferent cortical connections of the motor cortical larynx area in the rhesus monkey. Neuroscience. 2005a;130:133–149. doi: 10.1016/j.neuroscience.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jurgens U. Afferent subcortical connections into the motor cortical larynx area in the rhesus monkey. Neuroscience. 2005b;130:119–131. doi: 10.1016/j.neuroscience.2004.06.071. [DOI] [PubMed] [Google Scholar]
- Smith E, Kirchner HL, Taylor M, Hoffman H, Lemke JH. Voice problems among teachers: differences by gender and teaching characteristics. Journal of voice : official journal of the Voice Foundation. 1998;12:328–334. doi: 10.1016/s0892-1997(98)80022-2. [DOI] [PubMed] [Google Scholar]
- Titze IR. Physiologic and acoustic differences between male and female voices. The Journal of the Acoustical Society of America. 1989;85:1699–1707. doi: 10.1121/1.397959. [DOI] [PubMed] [Google Scholar]
- Titze IR. Principles of voice production. Allyn & Bacon, Inc.; 1994. [Google Scholar]
- Titze IR, Finnegan EM, Laukkanen AM, Fuja M, Hoffman H. Laryngeal muscle activity in giggle: a damped oscillation model. Journal of voice : official journal of the Voice Foundation. 2008;22:644–648. doi: 10.1016/j.jvoice.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, Ernst T. Sex differences in sensory gating of the thalamus during auditory interference of visual attention tasks. Neuroscience. 2008;151:1006–1015. doi: 10.1016/j.neuroscience.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Tanaka Y, Hirano M, Nakashima T. Sensory innervation of the pharynx and larynx. The American journal of medicine. 2000;108(Suppl 4a):51S–61S. doi: 10.1016/s0002-9343(99)00342-3. [DOI] [PubMed] [Google Scholar]