Abstract
The left anterior descending (LAD) coronary artery is the most frequently involved vessel in coronary artery dissection, a cause of acute coronary syndrome or sudden cardiac death. The biomechanical mechanisms underlying arterial dissection are not well understood. This study investigated the dissection properties of LAD specimens harvested from explanted hearts at the time of cardiac transplantation, from patients with primary dilated cardiomyopathy (n=12). Using a previously validated approach uniquely modified for these human LAD specimens, we quantified the local energy release rate, G, within different arterial layers during experimental dissection events (tissue tearing). Results show that the mean values of G during arterial dissection within the intima and within the media in human LADs are 20.7±16.5 J/m2 and 10.3±5.0 J/m2, respectively. The difference in dissection resistance between tearing events occurring within the intima and within the media is statistically significant. Our data fall in the same order of magnitude as most previous measurements of adhesive strength in other human arteries, with the differences in measured values of G within the layers most likely due to histologically observed differences in the structure and composition of arterial layers.
Keywords: coronary artery, arterial dissection, media, intima, energy release rate, peeling experiments
1. Introduction
Spontaneous or traumatic arterial dissection results in separation of the different layers of the arterial wall, with the creation of a false lumen. A photomicrograph of an arterial dissection event is shown in Figure 1. Arterial dissection has been observed in many different vessels, including the coronary arteries, carotid artery and aorta [1-3]. Clinical evidence indicates that hemorrhage into the false lumen and resulting thrombus formation can compress the true lumen, leading to obstruction of blood flow and causing myocardial ischemia, ischemic stroke and other lethal outcomes [2-4]. The left anterior descending (LAD) coronary artery accounts for 60% of cases of coronary artery dissection [3].
Fig. 1. Histopathological image of dissection of human thoracic aorta. Stains are Victoria Blue and H&E.
This file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license (http://creativecommons.org/licenses/by-sa/3.0/deed.en).
Arterial dissection often begins with an intimal tear which extends into the vessel wall in response to hemodynamic forces. Separation of layers could occur (a) between the intima and the media, (b) between the media and the adventitia, or (c) within the intima or media [5]. Arterial dissection is associated with many predisposing factors, such as elastin fragmentation, loss of smooth muscle cells, atherosclerosis, and hypertension [1,5]. Coronary atherosclerosis is one of the most common pathologies associated with spontaneous coronary artery dissection [3].
Dissection properties of the media from human carotid arteries and aortas have been studied. In these studies, the dissection strength of different interfaces has been measured in peeling experiments [4,6,7]. However, data regarding the dissection properties of human LAD coronary arteries have not been reported in the literature.
The purpose of the present study is to quantify the dissection properties of human coronary arteries. The peeling experiments performed in this study are based on the recently reported methodology developed by the authors [8,9], and are designed to measure the dissection strength in different layers by measuring the local energy release rate, G, as separation occurs along interfaces between layers or within the arterial wall. By quantifying G, a measure of dissection strength, we can obtain insight into mechanisms of arterial dissection and potential interventions. Furthermore, measured values of energy release rate will serve as input to future computational modeling studies of arterial dissection by employing cohesive zone models. To complement our mechanical experiments, we also performed histological studies to examine the exact dissection locations and the microstructural characteristics along the separated surfaces.
2. Experimental Procedure
2.1 Specimen preparation
Twelve human LAD coronary arteries were tested in this study. Samples were harvested from hearts explanted during transplant surgeries.1 The LAD was dissected at approximately 1 cm below the origin from the left main coronary artery and continued to the 2nd diagonal branch. The epicardial fat surrounding the vessel was not dissected and the epicardium was also left intact in order not to disturb the native architecture of the vessel. The underlying cardiac disease was primary cardiomyopathy, of both non-ischemic and ischemic etiology (n=10 and n=2, respectively).
The specimens were transported and stored in University of Wisconsin organ preservation solution [10] at 4°C until use. Specimens were tested within 48 hours after dissection. Ultrasound scanning was used to examine the pathological condition of specimens and to identify the presence of atherosclerotic plaques, enabling us to open the artery longitudinally with surgical scissors to expose any plaques without introducing additional damage. The proximal end of the specimen was identified by the larger diameter of the artery. Segments of the opened vessel (2-5 cm) were mounted and prepared for peeling experiments as described in the next section.
Delamination experiments
2.1.1 Experimental Device
All peeling experiments were performed on the computer-controlled biomechanical testing machine (Bose ElectroForce 3200 Test Instrument, Eden Prairie, MN, USA) shown in Figure 2.2 The accuracy of the displacement transducer and the load cell were estimated experimentally as +/-30 μm and +/-0.02 g, respectively. The mechanical test bed was interfaced with the computer vision system (see Fig. 2), comprised of a 28mm lens, a monochrome Q-Imaging QICAM 10-bit camera, and a 1934 PCMCIA card for image acquisition. The experiments were performed on a floating optical bench to isolate the experiment from laboratory vibrations.
Fig. 2. Photographs and diagram of overall experimental setup.

Top: Bose 3200 ELF system is used to apply displacement and to measure load. Camera on top used to visually estimate delamination area during each loading cycle. Middle inset: Close-up view of gripping tweezers with human artery specimen during delamination process. Bottom right: Schematic of the experimental loading process shown above.
2.1.2 Experimental Protocol
Figure 2 shows the loading apparatus, as well as a close-up photo and a schematic of the peeling experiment. While viewing the specimen at low magnification under a dissecting microscope, the authors used a pair of fine forceps to initiate a small flaw at the proximal end of the non-atherosclerotic vessel segment so that peeling proceeded from the proximal to distal end. This process creates an upper tongue for the peeling experiments. The procedure for initially creating the peel arm is “blind” in the sense that the boundary between the intima and the media is not clearly visible in the fresh, unstained tissue. Although efforts were made to initiate the flaw at a consistent depth, in some cases it started in the intima and in other cases it started in the media, due to variations in the thickness of the layers. As shown in Figure 2, the upper “tongue” was flipped over parallel to the luminal surface and attached to the micro-clamp grips, with tearing occurring in the axial direction. The lower tongue was clamped to the mounting plate at its proximal end to further restrain the outward motion of the artery during delamination, as shown schematically in Fig. 2. The bottom portion of the specimen is secured to a plastic mesh with sutures and the plastic mesh is bonded to a stiff lower plate. The plate-mesh combination is mounted within the Bose 3200 test machine via a serrated clamping fixture, providing a stable platform for the delamination experiments. Though not shown clearly in Fig. 2, the sutured lower specimen is also held down along the longitudinal edges by plastic tie-downs that added additional restraint to reduce the upward motion of the lower portion of the artery during the experiment. All results reported here refer to samples which were separated along the axial (flow) direction.
During the peeling process, the actuator applied sequential loading-unloading cycles to the upper “tongue” as separation proceeded axially towards the distal end of the artery in delamination increments of ~12mm. Sample loading was applied at an actuator velocity of 0.05mm/s for all artery peeling experiments. More details regarding the experimental setup and data processing can be found in [9].
Load-Displacement Measurements during Delamination
Figure 3a shows the typical load-displacement measurements acquired during a single tearing cycle. The area enclosed by the loading and unloading curves is an estimate of the fracture energy, ΔE, for the current peeling cycle. Images were taken before and after a peeling cycle to obtain the corresponding newly exposed area (ΔA). Newly exposed area and energy release rate G were calculated as described in [9]. Figure 3b shows typical results for G vs. ΔA obtained during the peeling experiments.
Fig. 3. Representative load-displacement curve during a single loading-unloading cycle of artery delamination (a) and Energy release rate (G) vs. Total area (b).

a. The jagged horizontal segments indicate the rupture in the arterial wall that occurs during delamination. The black arrows show the original loading path and unloading path respectively, during the delamination process. The area within the black curves corresponds to a first order estimate of the fracture energy for this delamination event. b. Three representative curves corresponding to all loading and unloading cycles for each of three LADs. Each point represents an individual delamination event (step). The legend indicates individual LAD identification numbers.
2.2 Histology
To identify the rupture surface, we examined longitudinal paraffin sections of partially-delaminated coronary arteries using Masson’s Trichrome staining. Following the peeling experiments, artery segments were fixed for at least 24 hours in 10% neutral buffered formalin, dehydrated in a tissue processor (Leica ASP300), and embedded in paraffin. Paraffin blocks were sectioned at 5 μm on a microtome, and sections were collected individually onto glass microscope slides for staining. Figure 4 presents representative photographs of the stained specimens.
Fig. 4. Histological demonstration of delamination surface locations.

Masson’s Trichrome staining of longitudinal sections of delaminated arteries, showing dissection (tearing) within the intima (a) and within the media (b), respectively (100X, scale bar = 100μm). The arrows indicate rupture locations. Collagen is stained blue, nuclei purple-black, and smooth muscle cells red.
3. Discussion of Results
3.1 Histology
As shown in Figure 4, histological studies at 100X magnification indicate that separation propagated within different layers of individual specimens, either within the intima (WI) or within the media (WM). In one or two cases, separation was not confined to one layer, but crossed different layers as the delamination propagated. For every specimen, the measured values of G obtained were identified with the specific layer in which the separation occurred. If there was any ambiguity regarding the layer in which dissection occurred, the G values were not included in the subsequent analysis.
3.2 Energy release rate measurements during dissection in human coronary arteries
Values of G obtained experimentally were expressed as mean ± standard deviation (SD). For comparison of group means of G values, a two-tailed Mann-Whitney test was carried out. In all cases, statistical significance was concluded where the two-tailed probability was <0.05.
Figure 3a shows a representative curve of load vs. displacement during a single tearing event for an LAD specimen. Using data obtained from three LAD specimens, Figure 3b demonstrates that G varies somewhat randomly along the length of each individual artery. Figure 5 presents three histograms showing the distribution of G values for all specimens. It is important to state that the G-ΔA data were segregated according to experimentally observed dissection plane. In total, we obtained 98 dissection/delamination energy measurements from 19 experiments performed using LAD specimens from 12 patients.
Fig. 5. Distributions of local energy release rate, G.
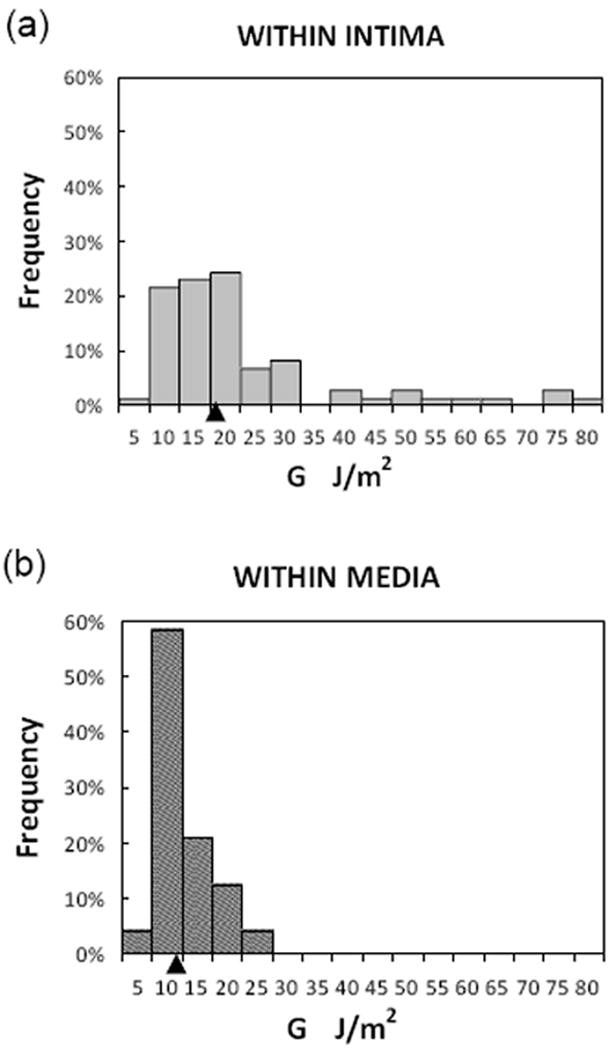
a. Rupture propagated within the intima layer; b. Rupture propagated within the media layer. Statistical parameters are given in Table. Arrowhead on x-axis indicates position of median value.
Separated by layer in which tearing occurred, (a) 74 data points were obtained for intimal dissection (WI) from 12 LAD specimens that were removed from 9 patients, and (b) 24 data points were obtained for medial dissection (WM) from 5 LAD specimens that were removed from 4 patients. When the specimens were segregated by disease etiology (ischemic cardiomyopathy (ICM) vs. non-ischemic cardiomyopathy (NICM)), all measured separation events in ICM specimens (8 data points from 2 individual LAD coronaries) were found to be confined to the intimal layer. In arteries from patients with NICM, tearing events in both the intima and the media were observed. The frequent observation of dissection within the intima reflects the fact that human coronary arteries have a relatively thick intimal layer.
As shown in Table 1, the positive skewnesses of 2.01 and 1.40 for G value distributions in the intimal (WI) and medial (WM) dissection groups differ significantly from 0 (P<0.05), indicating that these are non-normal distributions. The measured kurtosis values for both intimal and medial dissection are also significantly greater than 0 (P<0.05), indicating that the distributions are leptokurtic. The reported standard deviation (SD) serves as an indicator of variability.
Table 1. Statistical Parameters for Distributions of Experimental G Measurements.
| G (J/m2) | Within Intima (WI) | Within media (WM) |
|---|---|---|
| Mean | 20.7 | 10.3 |
| Median | 16.0 | 9.2 |
| SD | 16.5 | 5.0 |
| Q1 | 11.1 | 7.0 |
| Q3 | 21.3 | 12.3 |
| IQR | 10.2 | 5.3 |
| Kurtosis | 3.77 | 1.81 |
| Skewness | 2.01 | 1.40 |
| Minimum | 2.5 | 4.6 |
| Maximum | 77.9 | 24.5 |
| Observations | 74 | 24 |
Q1: first quartile; Q3: third quartile; IQR: interquartile range
The mean values of G for dissection events WI and WM are 20.7±16.5 J/m2 and 10.3±5.0 J/m2, respectively. Statistical analysis indicates that the mean values of G from the WI and WM groups are significantly different (p<0.05). When samples were stratified in terms of etiology of the cardiomyopathic disease process (ICM vs. NICM), we found no significant difference in G values for intimal tearing between ICM and NICM specimens (24.5 ± 18.3 J/m2 vs. 20.2 ± 16.3 J/m2; NS); therefore, ICM and NICM data were combined for subsequent analysis.
In one specimen from a patient with ICM, we observed G values much higher than those typical for most of our delamination experiments. Analysis of the specimen confirmed that it had extensive arterial calcification. The calcified artery was rigid and difficult to tear experimentally. Due to the major difference in material properties for this case, the data has been excluded from the results presented here.
4. Additional Remarks and Discussion
To the best of our knowledge, quantifying the dissection resistance of human LAD coronary arteries using peeling experiments and the corresponding local energy release rate (G) has not been reported previously. The G measurements provide a quantifiable metric of arterial interfacial adhesive strength. Furthermore, histological studies indicate that our peeling experiments reflect the adhesive strengths of different layers in the vascular wall during tearing (within the media or within the intima), as measured by the local energy release rate, G.
In our study, the mean measured values of G for dissection within the intima are 20.7 J/m2, while the mean value of G within the media is 10.3 J/m2. Our results fall within the same order of magnitude for all layers, but are two to seven times lower than those of published studies that have reported mean energy release rates for dissection/delamination resistance of human abdominal aortic media, human carotid artery and human ascending aorta, as listed in Table 2.
Table 2. Published Experimental Measurements of Arterial Fracture Energy.
| Tissue | Fracture energy (J/m2) | Reference |
|---|---|---|
| Human ascending thoracic aorta, medial layer | 100.0±4.1~149.0±7.6 (axial) 88.4±4.1~126.0±6.6 (circumferential) |
[6] |
| Human carotid artery | 65±27 (adventitia-media interface) 52±31 (intima-media interface) 60±16~75±24 (within media) |
[7] |
| Human abdominal aorta, medial layer | 76±27 (axial) 51+6 (circumferential) |
[4] |
| Mouse atherosclerotic plaque-internal elastic lamina interface | 24.5±18.6 | [9] |
| Human LAD coronary artery | 20.7±16.5 (within the intima) 10.3±5.0 (within the media) |
Present study |
The variations in energy release rate which we measured are nominally consistent with reported standard deviation values for previously published measurements. However, our results suggest that G values are not normally distributed, while results from other studies appear to assume a normal distribution. The effect of a non-normal distribution is particularly significant when using the variability to estimate probability of occurrence, especially when considering the medical implications of such decisions.
Even though our results fall in the same order of magnitude as those of previous studies, there are several possible explanations for the differences in range. First of all, the published studies investigated dissection properties of different vessels, including ascending thoracic aorta, carotid artery and abdominal aorta, rather than coronary artery as in our study. The human aorta has a larger diameter (~1 cm vs. 0.4 cm) than the LAD coronary artery and thicker walls with more elastic lamellae. In addition, the coronary arteries have developmental origins distinct from that of the aorta and its branches [11], which may account for differences in structure and even strength of the tissues in the layers.
Secondly, most of the previous results refer to dissection only within the arterial media. One group reported their results with respect to multiple, specific separation planes [7]. However, their histological study indicated that dissections did not always occur precisely at the desired location, though their data was not segregated by layer. As noted earlier, the authors identified the layer where the separation was occurring for every specimen post-hoc using histology and categorized the results accordingly. Since the different layers of the arterial wall are structurally distinct from each other, by identifying the precise dissection plane, our results provide more detailed information about dissection resistance of arteries.
Another issue regarding comparison of data sets is that other groups performed peeling as a single complete separation event, averaging the effects of layer change and path change to generate a single value for each specimen. Since the composition of the arterial wall will vary along the axial direction, particularly when pathological intimal thickening is present, the average values of G calculated from a single peeling event will not have information regarding the heterogeneity expected within a single layer or within a single specimen. By conducting cyclic peeling tests with separation of data according to tissue layer, the values of G calculated reflect the variation of composition and microstructure in arterial walls along the axial direction. In this regard, as shown in Fig. 3b, there is considerable variation in the measured local energy release rate, G. These variations are believed to be a function of the diverse pathological conditions of the LADs.
Our results indicate that the mean value of local energy release rate decreases in the radial direction from the inner layer to the middle layer (media) of the arterial wall. Based on our data, the intima appears to be the layer most resistant to dissection or tearing, which can be explained by its well-organized structure composed of smooth muscle cells, elastin and proteoglycans [12] and high collagen content (see Figure 4a). Intimal thickening is common in adult coronary arteries and increases with age [12]. This may explain why arterial dissection rarely happens spontaneously. However, once an initial tear is made in the intima, either by trauma or accidentally during intravascular surgeries, blood under pressure can force its way into the arterial wall and cause the separation to propagate. As shown in our results, dissection resistance within the media is lower than that measured within the intima. Therefore, a dissection initiating in the intima would tend to propagate into the weakest layer, the media, where a false lumen forms as observed and defined in previous studies [5,3]. We also observed that delamination could alternate between layers during propagation, which is consistent with the clinical observation that an arterial dissection can create a false lumen by propagating through the media but may eventually re-enter the true lumen at a more distal location [6]. Such events are readily understandable when considering the variability that exists in the measured values of G in the layers.
In related work by the authors, a series of loading-unloading studies on atherosclerotic mouse aortas have shown that energy dissipation effects make a relatively small (<10%) contribution to the measured values of G [9]. The delamination steps used in the present study are about 15-40 times larger than those used in the mouse model. Therefore, a smaller relative contribution to the measured energy release from specimen deformations is expected in the experiments reported here.
Finally, the measured values of energy release rate will prove useful as input to computational studies of arterial dissection, leading to a better understanding of forces required to initiate and propagate this acute emergent condition.
5. Limitations of Current Study
In this study, we tested de-identified coronary artery specimens discarded after heart transplantation surgeries. Thus, our dissection experiments were limited by a specific issue, the variation in pathological condition of the specimens obtained from patients with NICM or ICM. In most cases, corresponding clinical and demographic data were not available. Thus, the authors were unable to quantify the effect of age, gender or pathological condition on the dissection resistance of human LAD coronary arteries.
In addition, the authors have not been able to examine any physiologically normal coronary arteries for control purposes. One of the LAD specimens from a patient with ICM contained highly calcified plaques, rendering it unsuitable for delamination testing, while the others had only thickened intima, which is common in human arteries [12]. In future we will need to segregate specimens more precisely by type and severity of cardiomyopathy in order to draw more complete conclusions regarding the effect of ischemia on arterial wall dissection resistance.
Our present histological study did not include a detailed investigation of the microstructural origins of the difference in dissection resistance for arterial intima and media. More specific histological studies, focusing on the microstructure at the delamination interface, will be required to draw such conclusions.
Acknowledgments
The authors would like to thank the staff of the Cardiothoracic Surgery Research unit at the Medical University of South Carolina for their assistance in specimen collection and transport. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103444, and by the National Science Foundation under grant numbers NSF CMMI-1200358 and EPS-0903795. Dr. Francis Spinale is supported by the Research Service of the Department of Veterans Affairs. The study sponsors have played no role in the study design, analysis, or manuscript preparation.
Footnotes
Use of materials from human subjects was approved by the IRB at the Medical University of South Carolina, where the tissue harvest was carried out.
Estimation of G in this work assumes the artery has minimal deformation during delamination. This assumption can be readily modified to include the effect of the elasticity of the artery, e.g., use of an elastic foundation where the elastic properties of the aorta are determined separately.
References
- 1.Celik SK, Sagcan A, Altintig A, Yuksel M, Akin M, Kultursay H. Primary spontaneous coronary artery dissections in atherosclerotic patients. Report of nine cases with review of the pertinent literature. Eur J Cardiothorac Surg. 2001;20(3):573–576. doi: 10.1016/s1010-7940(01)00864-8. [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 3.Vrints CJ. Spontaneous coronary artery dissection. Heart. 2010;96(10):801–808. doi: 10.1136/hrt.2008.162073. [DOI] [PubMed] [Google Scholar]
- 4.Sommer G, Gasser TC, Regitnig P, Auer M, Holzapfel GA. Dissection properties of the human aortic media: an experimental study. J Biomech Eng. 2008;130(2):021007. doi: 10.1115/1.2898733. [DOI] [PubMed] [Google Scholar]
- 5.Pratt B, Curci J. Arterial elastic fiber structure. Function and potential roles in acute aortic dissection. J Cardiovasc Surg (Torino) 2010;51(5):647–656. [PubMed] [Google Scholar]
- 6.Pasta S, Phillippi JA, Gleason TG, Vorp DA. Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. J Thorac Cardiovasc Surg. 2012;143(2):460–467. doi: 10.1016/j.jtcvs.2011.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong J, Sommer G, Regitnig P, Holzapfel GA. Dissection properties and mechanical strength of tissue components in human carotid bifurcations. Ann Biomed Eng. 2011;39(6):1703–1719. doi: 10.1007/s10439-011-0264-y. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Johnson JA, Fulp A, Sutton MA, Lessner SM. Adhesive strength of atherosclerotic plaque in a mouse model depends on local collagen content and elastin fragmentation. J Biomech. 2013;46:716–722. doi: 10.1016/j.jbiomech.2012.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Ning J, Johnson JA, Sutton MA, Lessner SM. Development of a quantitative mechanical test of atherosclerotic plaque stability. J Biomech. 2011;44(13):2439–2445. doi: 10.1016/j.jbiomech.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–247. doi: 10.1146/annurev.med.46.1.235. [DOI] [PubMed] [Google Scholar]
- 11.Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O’Rourke BP, Sharp DJ, Zheng D, Lenz J, Baldwin HS, Chang CP, Zhou B. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151(5):1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res. 2008;79(1):14–23. doi: 10.1093/cvr/cvn099. [DOI] [PubMed] [Google Scholar]



