Abstract
Objective
Bipolar and cannabis use disorders commonly co-occur during adolescence, and neurochemical studies may help clarify the pathophysiology underlying this co-occurrence. This study compared metabolite concentrations in the left ventral lateral prefrontal cortex among: adolescents with bipolar disorder (bipolar group; n=14), adolescents with a cannabis use disorder (cannabis use group, n=13), adolescents with cannabis use and bipolar disorders (bipolar and cannabis group, n=25), and healthy adolescents (healthy controls, n=15). We hypothesized that adolescents with bipolar disorder (with or without cannabis use disorder) would have decreased N-acetyl aspartate levels in the ventral lateral prefrontal cortex compared to the other groups, and that the bipolar and cannabis group would have the lowest N-acetyl aspartate levels of all groups.
Methods
N-acetyl aspartate concentrations in the left ventral lateral prefrontal cortex were obtained using Proton Magnetic Resonance Spectroscopy.
Results
Adolescents with bipolar disorder showed significantly lower left ventral lateral prefrontal cortex N-acetyl aspartate levels, but post-hoc analyses indicated that this was primarily due to increased N-acetyl aspartate levels in the cannabis group. The cannabis use disorder group had significantly higher N-acetyl aspartate levels compared to the bipolar disorder and the bipolar and cannabis groups (p=0.0002 and p=0.0002, respectively). Pearson correlations revealed a significant positive correlation between amount of cannabis used and N-acetyl aspartate concentrations.
Conclusions
Adolescents with cannabis use disorder showed higher levels of N-acetyl aspartate concentrations that were significantly positively associated with the amount of cannabis used; however, this finding was not present in adolescents with comorbid bipolar disorder.
Keywords: magnetic resonance spectroscopy, 1H-MRS, N-acetyl aspartate, NAA, bipolar disorder, cannabis use, marijuana, adolescents
Individuals with bipolar disorder have a high rate of comorbid substance use disorders (Khan & Akella, 2009). Adolescents with co-occurring bipolar and substance use disorders have worse outcomes and poorer adherence than those with either disorder alone (Jarvis et al., 2008). Few studies have addressed the underlying neurophysiology of this common comorbidity. A more comprehensive understanding of the relationship between substance use and bipolar disorders might lead to more effective treatment options for patients.
Magnetic resonance spectroscopy (1H-MRS) studies indicate that abnormalities in neurochemical markers of neuronal integrity and metabolism are present in prefrontal brain regions of adolescents with bipolar disorder. Specifically, prior studies suggest that adolescents with bipolar disorder exhibit decreased levels of N-acetyl aspartate, as compared to healthy participants (Adler et al., 2007; DelBello et al., 2006). Youth with cannabis use disorders also exhibit decreased dorsolateral prefrontal N-acetyl aspartate/total creatine ratios (Hermann et al., 2007), and decreased anterior cingulate N-acetyl aspartate levels (Prescot et al., 2011) compared with healthy individuals. N-acetyl aspartate is a neuronal amino acid recognized as a putative marker of neuronal integrity and metabolic function (DelBello and Strakowski, 2004). To our knowledge, there is only one neuroimaging study examining abnormalities in adolescents with co-occurring bipolar and cannabis use disorders. This study demonstrated greater structural abnormalities in adolescents with bipolar and cannabis use disorders than in adolescents with bipolar disorder alone in frontal and temporal cortical regions, as well as in subcortical areas linked with emotion and motivational regulation (Jarvis et al., 2008).
With these considerations in mind, the aim of our study was to compare regional brain neurochemistry in the left ventral lateral prefrontal cortex among adolescents with co-occurring cannabis use and bipolar disorders, adolescents with bipolar disorder who do not use cannabis, adolescents with a cannabis use disorder who do not have a mood or psychotic disorder and healthy adolescents without any DSM-IV Axis I disorder. We hypothesized that 1) adolescents with bipolar disorder, independent of co-occurring cannabis use, would have decreased N-acetyl aspartate levels in the ventral lateral prefrontal cortex compared with adolescents without a mood disorder and 2) adolescents with co-occurring bipolar and cannabis use disorders would have the lowest N-acetyl aspartate levels compared to all other groups.
METHODS
Subjects
Adolescents were recruited from inpatient and outpatient clinics at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center into one of four groups: adolescents with bipolar disorder without cannabis use (bipolar disorder, n=14); adolescents with bipolar disorder and cannabis use disorder (bipolar and cannabis, n=25); adolescents with a cannabis use disorder without bipolar disorder (cannabis use disorder, n=13); and adolescents with no psychiatric or substance use disorders (healthy controls, n=15). Cananbis use was verified by a drug screen and all participants were unmedicated at the time of the scan. Subjects were group-matched for age, race, and sex (Table 1).
TABLE 1.
Demographic and Clinical Characteristics of Participants at Baseline
| Characteristic | HC (n = 15) |
CU (n = 13) |
BP (n = 14) |
BPCU (n = 25) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Sex (female) | 9 (60) | 5 (38) | 7 (50) | 11 (44) |
| Race (non-Caucasian) | 5 (33) | 3 (23) | 4 (29) | 5 (20) |
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Age | 18.4 (2.0) | 19.2 (1.1) | 17.2 (3.0) | 18.4 (2.1) |
| IQa | 115 (12) | 107 (7) | 100 (10) | 97 (9) |
| Manic symptomsa | 1 (2) | 2 (4) | 29 (9) | 24 (5) |
| Joints smoked per montha | 0 (1) | 36 (30) | 0 (1) | 80 (85) |
Note. HC = adolescents with no substance use or psychiatric disorder; CU adolescents with cannabis use disorder without bipolar disorder; BP = adolescents with bipolar disorder without cannabis use; BPCU = adolescents with bipolar and cannabis use disorder.
p < .0001 between groups.
DSM-IV-TR diagnoses were confirmed by trained raters (kappa > 0.9) who administered the Washington University in St. Louis Kiddie Schedule of Affective Disorders and Schizophrenia (WASH-U KSADS) to assess for DSM-IV-TR Axis I disorders other than substance use disorders and the Semi-Structured Assessment for the Genetics of Alcoholism, Adolescent version (SSAGA-A) to evaluate for substance use disorders (Black et al., 2012; Hesselbrock et al., 1999; Bucholz et al., 1994). Participants were not eligible if they had an IQ of 70 or lower on the Wechsler Abbreviated Scales of Intelligence, any unstable medical or neurological illness as determined by a study physician, a positive urine pregnancy test, any contraindication to undergoing an MRI scan (e.g., braces or claustrophobia), a Tanner Score of less than 3, current use of a psychotropic medication, or any history of significant head trauma resulting in a loss of consciousness for greater than five minutes. Only those who were not taking their prescribed psychotropic medications at the time of scan were included.
The Teen-Addiction Severity Index (T-ASI) was also administered to all subjects (McLellan et al., 1980; Kaminer et al., 1991). The T-ASI is a semi-structured interview developed as a standardized instrument for periodic evaluation of adolescent substance use. The T-ASI is an age-appropriate modification of the Addiction Severity Index. It yields 70 ratings in seven domains: substance use, school status, employment/support status, family relations, peer/social relationships, legal status, and psychiatric status. It has established validity and inter-rater reliability. Adolescents were interviewed separately from their legal guardians and the T-ASI was used to collect the amount of cannabis used.
The Young Mania Rating Scale (YMRS) and the Children’s Depression Rating Scale (CDRS) were administered by raters with established high inter-rater reliability (ICC>0.90) to evaluate manic and depressed symptoms, respectively (Fristad et al., 1992; Young et al., 1978; Poznanski et al., 1979).
The study was approved and monitored by the University of Cincinnati Institutional Review Board. After study procedures were fully explained, all study participants under the age of 18 provided written assent and their legal guardians provided written informed consent, while all study participants aged 18 and older provided written consent. The study was conducted in accordance with the Declaration of Helsinki.
Magnetic Resonance Spectroscopy (1H-MRS) Procedures
All scans were completed at the University of Cincinnati’s Center for Imaging Research using a 4-Tesla Varian Unity INOVA Whole Body MRI/MRS system (Varian, Inc.) equipped with a head RF coil (MR Instruments, Inc.). To minimize subject motion during scanning procedures, all subjects were instructed to remain still and foam packing was inserted around their head.
To obtain magnetic resonance spectroscopy metabolite measurements, an experienced spectroscopist positioned the voxel in the left ventral lateral prefrontal cortex using anatomical landmarks to ensure consistent placement and to avoid signal artifacts from the orbits as described elsewhere (DelBello et al., 2006). Spectra were acquired from these voxels using Point-RESolved Spectroscopy pulse sequence (echo time = 23 ms, repetition time = 2000ms with 128 averages). Data were processed using the LC Model program (Provencher 1993). Concentrations of N-acetyl-aspartate were determined in each spectrum (Kraguljac et al., 2012; Patel et al., 2006; DelBello et al., 2006). Statistical Parametric Mapping (SPM; University College London, 2005) was completed to segment each voxel of each participant to identify and adjust for the gray matter/white matter/cerebral spinal fluid percentages in each voxel (DelBello et al., 2006; Patel et al., 2006).
Statistical analyses were performed using the Statistical Analysis System (SAS Institute, 2002–2008). Analyses of variance (ANOVA) and chi-square tests were used to examine group differences in demographic characteristics. Analyses of covariance (ANCOVA), using IQ as the covariate, were used to examine differences in N-acetyl aspartate metabolite measurements between groups. Pearson correlations between N-acetyl aspartate metabolite levels and both YMRS scores and the number of joints smoked during the past month were performed in the bipolar and cannabis and cannabis use disorder groups.
RESULTS
Clinical Characteristics
There were no statistically significant group differences in age, sex, or race among the groups (Table 1). Statistically significant differences in IQ were observed between groups (p < 0.0001) and therefore, IQ was used as a covariate in all analyses.
Magnetic Resonance Spectroscopy
Adolescents with bipolar disorder (i.e., bipolar disorder and bipolar and cannabis groups) had significantly lower left ventral lateral prefrontal cortex N-acetyl aspartate levels when compared to the non-bipolar disorder groups (cannabis use disorder and healthy control groups; F(1, 65) = 7.72, p=0.007 (see Figure 1). Post-hoc comparisons revealed that this difference was largely due to the greater left ventral lateral prefrontal cortex N-acetyl aspartate levels in the cannabis use disorder group compared to the bipolar disorder and bipolar and cannabis groups (p= 0.0002 and p= 0.0002, respectively; Figure 1). Adolescents with bipolar disorder did not have significantly lower left ventral lateral prefrontal cortex N-acetyl aspartate levels when compared to healthy controls. No other significant differences in N-acetyl aspartate levels were observed among other groups. No significant correlations between YMRS scores and N-acetyl aspartate levels for the bipolar disorder or bipolar and cannabis groups were observed.
Figure 1.
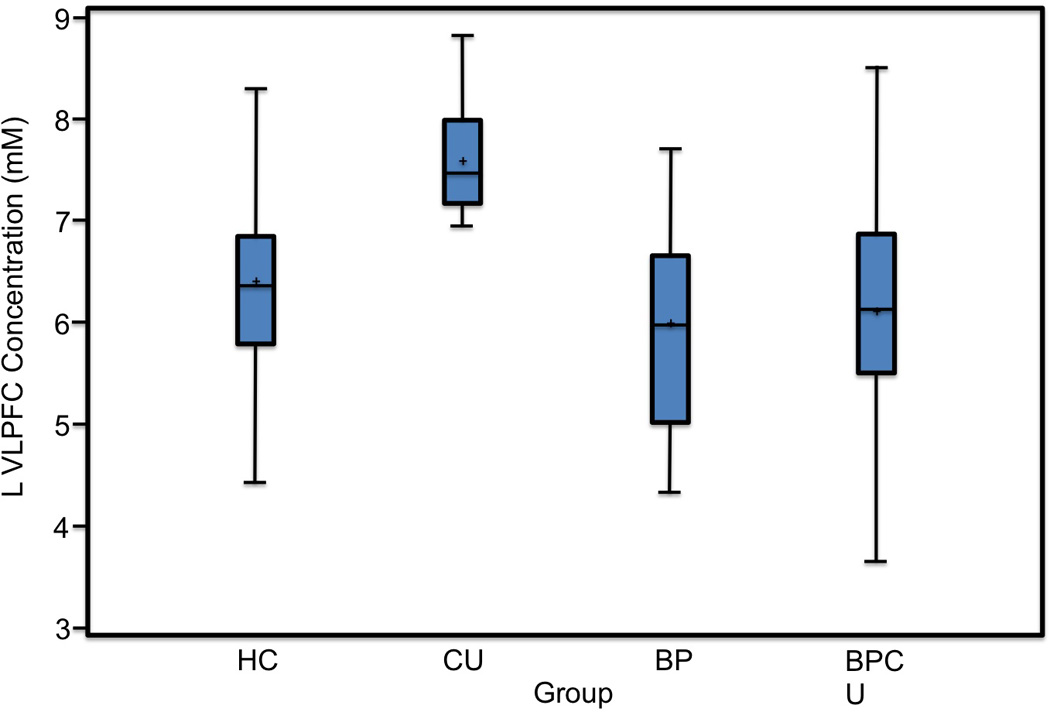
Group differences in left ventral lateral prefrontal cortex (VLPFC) N-acetyl aspartate concentrations. Note. HC = adolescents with no substance use or psychiatric disorder; CU adolescents with cannabis use disorder without bipolar disorder; BP = adolescents with bipolar disorder without cannabis use; BPCU = adolescents with bipolar and cannabis use disorder. The cannabis use disorder group had significantly higher left ventral lateral prefrontal cortex N-acetyl aspartate levels when compared to the bipolar disorder and the bipolar and cannabis groups (p=0.0002 and p=0.0002, respectively).
Pearson correlations in the cannabis use disorder group revealed a positive correlation between the number of joints smoked in the past month and N-acetyl aspartate concentration levels of the left ventral lateral prefrontal cortex (r=0.7, p=0.03). No significant correlations between N-acetyl aspartate concentration levels and amount of cannabis used were observed in the bipolar and cannabis group.
DISCUSSION
This study found that adolescents with a cannabis use disorder, and no mood disorder, showed higher levels of N-acetyl aspartate concentrations in the ventral lateral prefrontal cortex that were significantly positively associated with the amount of cannabis used, when compared to all other groups; however, this finding was not seen in adolescents with comorbid bipolar disorder. N-acetyl aspartate is a neuronal amino acid recognized as a putative marker of neuronal integrity and metabolic function (DelBello and Strakowski, 2004). An increase in N-acetyl aspartate concentration, as reported in the cannabis use disorder group, may reflect increased neurons or glia in the left ventral lateral prefrontal area. Alternatively, N-acetyl aspartate is produced in the mitochondria and N-acetyl aspartate synthesis is reduced by mitochondrial respiratory chain inhibitors (DelBello and Strakowski, 2004). Therefore, our finding of increased N-acetyl aspartate concentrations in adolescents with a cannabis use disorder, but no mood disorder, may reflect enhanced mitochondrial energy production in this group (Weber et al., 2013; Stork & Renshaw, 2005). This finding, however, is in contrast to other studies that reported decreased N-acetyl aspartate levels in the dorsolateral prefrontal cortex (Hermann et al., 2007) and the anterior cingulate (Prescot et al., 2011) of youth with a cannabis use disorder.
Although there was a significant difference between adolescents with and without bipolar disorder, this was largely driven by the increase in the cannabis group. The large variance in N-acetyl aspartate levels in the healthy control group might have contributed to the lack of difference between the bipolar disorder and healthy control groups (Figure 1).
A positive correlation was found between the amount of cannabis used by the cannabis use disorder group and their N-acetyl aspartate metabolite concentrations in the left ventral lateral prefrontal cortex. This finding is consistent with a study that reported significantly positive correlations between N-acetyl aspartate levels and nonpsychotropic cannabinoid in the putamen/globus pallidum of cannabis users (Hermann, et al., 2007). Nonpsychotropic cannabinoid has neuroprotective properties that have been shown in animal studies of brain injury and could be potentially beneficial in treating diseases such as Parkinson’s disease (Hermann, et al., 2007; Marsicano, et al., 2003; Panikashvili, et al., 2001). The positive correlation between cannabis use and N-acetyl aspartate levels was not seen in the bipolar and cannabis group, suggesting that cannabis use does not have similar effects on prefrontal N-acetyl aspartate as some anti-manic medications (DelBello et al., 2006).
There are several limitations that should be considered when interpreting the results of our study. First, the number of subjects in each group was relatively small, limiting the power to detect smaller findings. Second, this study is cross-sectional so that conclusions cannot be drawn regarding the causation of the reported results; that is, increased N-acetyl aspartate may be a risk factor associated with developing a cannabis use disorder in non-bipolar adolescents. Third, differences in clinical characteristics between groups (e.g., depressive symptoms) may have impacted our findings. Finally, we did not adjust for time since last cannabis use or total amount of cannabis used, which might have impacted the findings. Nonetheless, despite these limitations, to our knowledge this study is the first to examine the neurochemical effects of cannabis use in adolescents with bipolar disorder. Future studies to determine the effects of cannabis use on the neurodevelopment of adolescents with bipolar disorder are needed.
ACKNOWLEDGMENT
The authors would like to thank Jennifer Beavers, MS for her assistance with patient recruitment.
FUNDING
This study was supported by a grant from the National Institute on Drug Abuse (NIDA), Grant # R01 DA022221 (DelBello).
Footnotes
DISCLOSURES
Ms. Bitter, Mr. Weber, Dr. Chu, and Dr. Eliassen report no disclosures. Dr. Adler has received research support from AstraZeneca, Amylin, Eli Lilly, GlaxoSmithKline, Lundbeck, Martek, Merck, Novartis, Otsuka, Pfizer, Takeda, and Shire. He has been on the lecture bureau for Merck and Sunovion, for which he received honoraria. Dr. Strakowski chairs Data and Saftey Monitoring Boards for Sunovion and is a consultant to Procter & Gamble. Dr. DelBello has received research support from AstraZeneca, Amylin, Eli Lilly, Pfizer, Otsuka, GlaxoSmithKline, Merck, Martek, Novartis, Lundlbeck, and Shire. Dr. DelBello is also on the lecture bureau for Otsuka, Merck, and Bristol-Meyers Squibb. She has received Consulting/Advisory Board/Honoraria/Travel for Merck, Pfizer, Dey, Lundlbeck, Sunovian, and Otsuka.
Contributor Information
Samantha M. Bitter, Email: bittersa@mail.uc.edu.
Wade A. Weber, Email: weberw@ucmail.uc.edu.
Caleb M. Adler, Email: adlercb@ucmail.uc.edu.
James C. Eliassen, Email: eliassjc@ucmail.uc.edu.
Stephen M. Strakowski, Email: strakosm@ucmail.uc.edu.
Melissa P. DelBello, Email: melissa.delbello@uc.edu.
REFERENCES
- 4-Tesla Varian Unity INOVA Whole Body MRI/MRS system. Palo Alto, CA: Varian, Inc.; 2005. [Google Scholar]
- Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biological Psychiatry. 2007;61(6):776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Black JJ, Heffner JL, Anthenelli RM, Beavers JN, Albertz A, Blom T, DelBello MP. Diagnosing Alcohol and Cannabis Use Disorders in Adolescents with Bipolar Disorder: A Preliminary Investigation. Journal of Dual Diagnosis. 2012;8:13–18. doi: 10.1080/15504263.2012.647349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR. Semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability for the SSAGA. Journal of Studies on Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Strakowski SM. Neurochemical predictors of response to pharmacologic treatments for bipolar disorder. Current Psychiatry Reports. 2004;6:466–472. doi: 10.1007/s11920-004-0012-1. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Cecil KM, Adler CM, Daniels JP, Strakowski SM. Neurochemical effects of olanzapine in first-hospitalization manic adolescents: A proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2006;31:1264–1273. doi: 10.1038/sj.npp.1300950. [DOI] [PubMed] [Google Scholar]
- Fristad MA, Weller EB, Weller RA. The Mania Rating Scale: Can it be used in children? A preliminary report. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(2):252–257. doi: 10.1097/00004583-199203000-00011. [DOI] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skoop G, Ende G, Mann K. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biological Psychiatry. 2007;61(11):1281–1289. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--A comparison with the SCAN. Addiction. 1999;94(9):1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Jarvis K, DelBello MP, Mills N, Elman I, Strakowski S, Adler CM. Neuroanatomic comparison of bipolar adolescents with and without cannabis use disorders. Journal of Child and Adolescent Psychopharmacology. 2008;18(6):557–563. doi: 10.1089/cap.2008.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer Y, Burkstein OG, Tarter RE. The Teen Addiction Severity Index: Rationale and reliability. The International Journal of the Addictions. 1991;26:219–226. doi: 10.3109/10826089109053184. [DOI] [PubMed] [Google Scholar]
- Khan MA, Akella S. Cannabis-induced bipolar disorder with psychotic features. Psychiatry. 2009;6(12):44–48. [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti A. Neurometabolites in schizophrenia and bipolar disorder - A systemic review and meta-analysis. Psychiatry Research. 2012:111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough K, Monory H, Hermann H, Eder M, Cannich A, Lutz B. CB1 cannabinoid receptors and on demand defense against excitotoxicity. Science. 2003;302(5642):84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instument for substance abuse patients. The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. Endogenous cannabinoid (2-AG): Is neuroprotective after brain injury. Nature. 2001;413(6855):527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Patel NC, DelBello MP, Cecil KM, Adler CM, Bryan HS, Stanford KE, Strakowski S. Lithium treatment effects on myoinositol in adolescents with bipolar depression. Biological Psychiatry. 2006;60:998–1004. doi: 10.1016/j.biopsych.2006.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescents chronic marijuana smokers: A proton MRS study. NeuroImage. 2011;57:69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- RF Coil. Minneapolis, MN: MR Instruments Inc.; [Google Scholar]
- SPM Software System. London: The FIL Methods Group, University College London; 2005. [Google Scholar]
- Statistical Analysis System. Cary, NC: SAS Institute; 2002–2008. [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Weber WA, Dudley J, Lee JH, Strakowski SM, Adler CM, Delbello MP. A pilot study of alterations in high energy phosphoryl compounds and intracellular pH in unmedicated adolescents with bipolar disorder. Journal of Affective Disorders. 2013;150(3):1109–1113. doi: 10.1016/j.jad.2013.04.047. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity, and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]


