Abstract
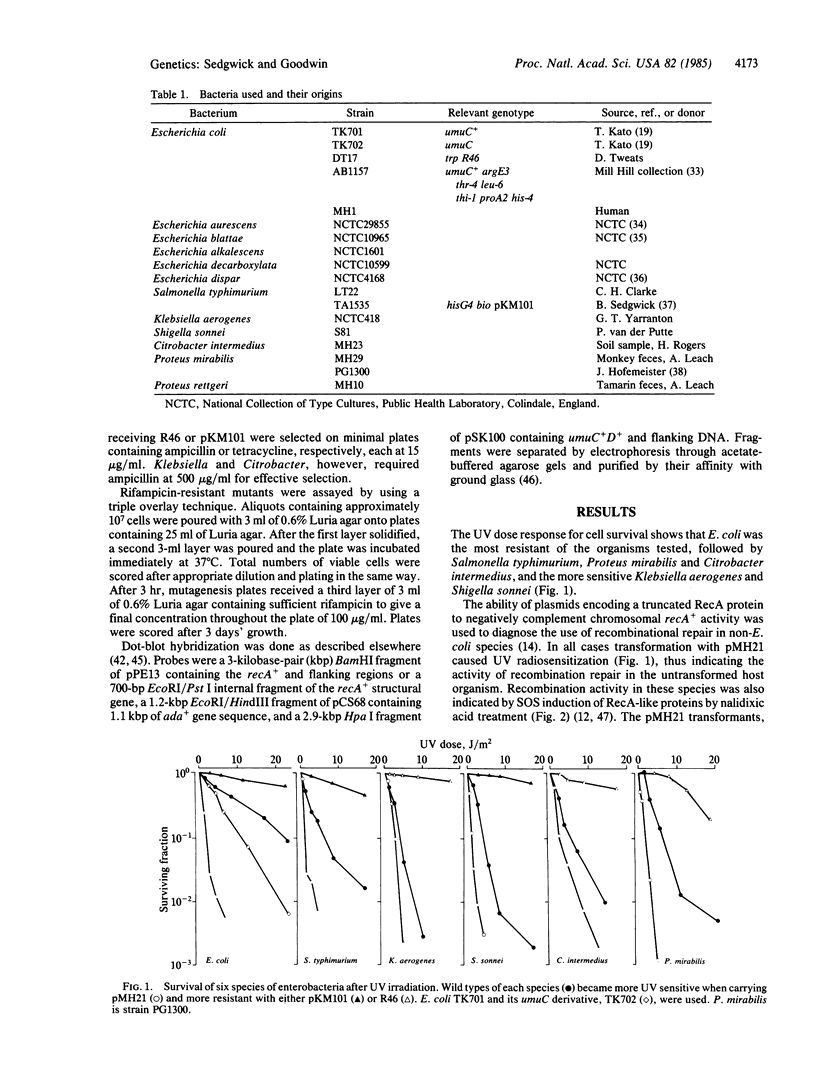
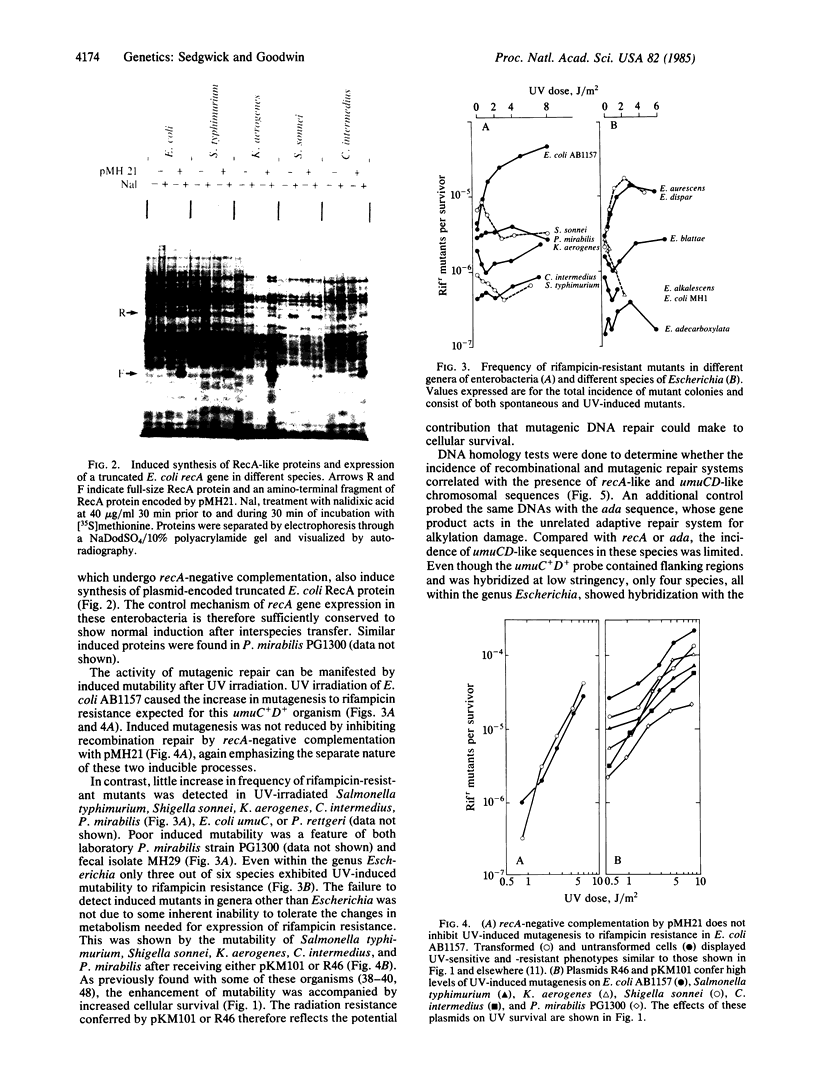
The incidence of recombinational DNA repair and inducible mutagenic DNA repair has been examined in Escherichia coli and 11 related species of enterobacteria. Recombinational repair was found to be a common feature of the DNA repair repertoire of at least 6 genera of enterobacteria. This conclusion is based on observations of (i) damage-induced synthesis of RecA-like proteins, (ii) nucleotide hybridization between E. coli recA sequences and some chromosomal DNAs, and (iii) recA-negative complementation by plasmids showing SOS-inducible expression of truncated E. coli recA genes. The mechanism of DNA damage-induced gene expression is therefore sufficiently conserved to allow non-E. coli regulatory elements to govern expression of these cloned truncated E. coli recA genes. In contrast, the process of mutagenic repair, which uses umuC+ umuD+ gene products in E. coli, appeared less widespread. Little ultraviolet light-induced mutagenesis to rifampicin resistance was detected outside the genus Escherichia, and even within the genus induced mutagenesis was detected in only 3 out of 6 species. Nucleotide hybridization showed that sequences like the E. coli umuCD+ gene are not found in these poorly mutable organisms. Evolutionary questions raised by the sporadic incidence of inducible mutagenic repair are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Kenyon C. J., Walker G. C. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5749–5753. doi: 10.1073/pnas.78.9.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4204–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. RecA + -dependent mutagenesis occurring before DNA replication in UV- and -irradiated Escherichia coli. Mutat Res. 1971 Sep;13(1):1–8. doi: 10.1016/0027-5107(71)90120-5. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Woodgate R. Mutagenic repair in Escherichia coli. X. The umuC gene product may be required for replication past pyrimidine dimers but not for the coding error in UV-mutagenesis. Mol Gen Genet. 1984;196(2):364–366. doi: 10.1007/BF00328073. [DOI] [PubMed] [Google Scholar]
- Burgess N. R., McDermott S. N., Whiting J. Aerobic bacteria occurring in the hind-gut of the cockroach, Blatta orientalis. J Hyg (Lond) 1973 Mar;71(1):1–7. doi: 10.1017/s0022172400046155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Dowden S. B., Glazebrook J. A., Strike P. UV inducible UV protection and mutation functions on the I group plasmid TP110. Mol Gen Genet. 1984;193(2):316–321. doi: 10.1007/BF00330687. [DOI] [PubMed] [Google Scholar]
- Drabble W. T., Stocker B. A. R (transmissible drug-resistance) factors in Salmonella typhimurium: pattern of transduction by phage P22 and ultraviolet-protection effect. J Gen Microbiol. 1968 Aug;53(1):109–123. doi: 10.1099/00221287-53-1-109. [DOI] [PubMed] [Google Scholar]
- Echols H. SOS functions, cancer and inducible evolution. Cell. 1981 Jul;25(1):1–2. doi: 10.1016/0092-8674(81)90223-3. [DOI] [PubMed] [Google Scholar]
- Eitner G., Adler B., Lanzov V. A., Hofemeister J. Interspecies recA protein substitution in Escherichia coli and Proteus mirabilis. Mol Gen Genet. 1982;185(3):481–486. doi: 10.1007/BF00334144. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. The muc genes of pKM101 are induced by DNA damage. J Bacteriol. 1983 Sep;155(3):1306–1315. doi: 10.1128/jb.155.3.1306-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J Mol Biol. 1974 Jul 25;87(1):103–119. doi: 10.1016/0022-2836(74)90563-4. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Seawell P. C., Mount D. W. Effect of tsl (thermosensitive suppressor of lex) mutation on postreplication repair in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):935–942. doi: 10.1128/jb.135.3.935-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofemeister J., Eitner G. Repair and plasmid R46 mediated mutation requires inducible functions in Proteus mirabilis. Mol Gen Genet. 1981;183(2):369–375. doi: 10.1007/BF00270642. [DOI] [PubMed] [Google Scholar]
- Hofemeister J., Köhler H., Filippov V. D. DNA repair in Proteus mirabilis. VI. Plasmid (R46-) mediated recovery and UV mutagenesis. Mol Gen Genet. 1979 Oct 3;176(2):265–273. [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., West S. C., Stasiak A. Role of RecA protein spiral filaments in genetic recombination. Nature. 1984 May 17;309(5965):215–219. doi: 10.1038/309215a0. [DOI] [PubMed] [Google Scholar]
- Kato T. Effects of chloramphenicol and caffeine on postreplication repair in uvr A- umuC- und uvrA- recF- strains of Escherichia coli K-12. Mol Gen Genet. 1977 Nov 14;156(2):115–120. doi: 10.1007/BF00283483. [DOI] [PubMed] [Google Scholar]
- Kato T., Ise T., Shinagawa H. Mutational specificity of the umuC mediated mutagenesis in Eschericha coli. Biochimie. 1982 Aug-Sep;64(8-9):731–733. doi: 10.1016/s0300-9084(82)80119-3. [DOI] [PubMed] [Google Scholar]
- Kato T., Nakano E. Effects of the umuC36 mutation on ultraviolet-radiation-induced base-change and frameshift mutations in Escherichia coli. Mutat Res. 1981 Oct;83(3):307–319. doi: 10.1016/0027-5107(81)90014-2. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Langer P. J., Shanabruch W. G., Walker G. C. Functional organization of plasmid pKM101. J Bacteriol. 1981 Mar;145(3):1310–1316. doi: 10.1128/jb.145.3.1310-1316.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L. Specificity of UV mutagenesis in the lac promoter of M13lac hybrid phage DNA. Nature. 1982 Jun 17;297(5867):596–598. doi: 10.1038/297596a0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Yarranton G. T. Inducible DNA repair in Ustilago maydis. Mol Gen Genet. 1982;185(2):245–250. doi: 10.1007/BF00330793. [DOI] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W., Yanisch-Perron C. R. Purified lexA protein is a repressor of the recA and lexA genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4199–4203. doi: 10.1073/pnas.78.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLIGO J. E., PARR L. W., ROBBINS M. L. Escherichia aurescens (Parr) comb. nov., a pigmented species. J Bacteriol. 1955 Nov;70(5):498–500. doi: 10.1128/jb.70.5.498-500.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortelmans K. E., Stocker B. A. Segregation of the mutator property of plasmid R46 from its ultraviolet-protecting property. Mol Gen Genet. 1979 Jan 2;167(3):317–327. doi: 10.1007/BF00267425. [DOI] [PubMed] [Google Scholar]
- Mortelmans K. E., Stocker B. A. Ultraviolet light protection, enhancement of ultraviolet light mutagenesis, and mutator effect of plasmid R46 in Salmonella typhimurium. J Bacteriol. 1976 Oct;128(1):271–282. doi: 10.1128/jb.128.1.271-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Kosel C. K., Walker A. Inducible, error-free DNA Repair in tsl recA mutants of E. coli. Mol Gen Genet. 1976 Jul 5;146(1):37–41. doi: 10.1007/BF00267980. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Doudney C. O. Different modes of loss of photoreversibility of mutation and lethal damage in ultraviolet-light resistant and sensitive bacteria. Mutat Res. 1969 Sep-Oct;8(2):215–228. doi: 10.1016/0027-5107(69)90001-3. [DOI] [PubMed] [Google Scholar]
- Pierré A., Paoletti C. Purification and characterization of recA protein from salmonella typhimurium. J Biol Chem. 1983 Mar 10;258(5):2870–2874. [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G. Inducible error-prone repair in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2753–2757. doi: 10.1073/pnas.72.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Yarranton G. T. Cloned truncated recA genes in E. coli. I. Effect on radiosensitivity and recA+ dependent processes. Mol Gen Genet. 1982;185(1):93–98. doi: 10.1007/BF00333796. [DOI] [PubMed] [Google Scholar]
- Shinagawa H., Kato T., Ise T., Makino K., Nakata A. Cloning and characterization of the umu operon responsible for inducible mutagenesis in Escherichia coli. Gene. 1983 Aug;23(2):167–174. doi: 10.1016/0378-1119(83)90048-3. [DOI] [PubMed] [Google Scholar]
- Skavronskaya A. G., Stepanova N. F., Andreeva I. V. UV-mutable hybrids of Salmonella incorporating Escherichia coli region adjacent to tryptophan operon. Mol Gen Genet. 1982;185(2):315–318. doi: 10.1007/BF00330804. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- Steinborn G. Uvm mutants of Escherichia coli K12 deficient in UV mutagenesis. I. Isolation of uvm mutants and their phenotypical characterization in DNA repair and mutagenesis. Mol Gen Genet. 1978 Sep 20;165(1):87–93. doi: 10.1007/BF00270380. [DOI] [PubMed] [Google Scholar]
- Teo I., Sedgwick B., Demple B., Li B., Lindahl T. Induction of resistance to alkylating agents in E. coli: the ada+ gene product serves both as a regulatory protein and as an enzyme for repair of mutagenic damage. EMBO J. 1984 Sep;3(9):2151–2157. doi: 10.1002/j.1460-2075.1984.tb02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C., Pinney R. J. Expression of eight unrelated Muc+ plasmids in eleven DNA repair-deficient E. coli strains. Mutat Res. 1983 Oct;112(5):261–273. doi: 10.1016/0167-8817(83)90002-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R., Margossian L. J., Clark A. J. Evidence that rnmB is the operator of the Escherichia coli recA gene. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1786–1790. doi: 10.1073/pnas.78.3.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R., Spencer D. F., Clark A. J. Indirect and intragenic suppression of the lexA102 mutation in E. coli B/r. Mol Gen Genet. 1979;177(1):129–137. doi: 10.1007/BF00267262. [DOI] [PubMed] [Google Scholar]
- Walker G. C., Dobson P. P. Mutagenesis and repair deficiencies of Escherichia coli umuC mutants are suppressed by the plasmid pKM101. Mol Gen Genet. 1979 Apr 17;172(1):17–24. doi: 10.1007/BF00276210. [DOI] [PubMed] [Google Scholar]
- Walker G. C., Dobson P. P. Mutagenesis and repair deficiencies of Escherichia coli umuC mutants are suppressed by the plasmid pKM101. Mol Gen Genet. 1979 Apr 17;172(1):17–24. doi: 10.1007/BF00276210. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Plasmid (pKM101)-mediated enhancement of repair and mutagenesis: dependence on chromosomal genes in Escherichia coli K-12. Mol Gen Genet. 1977 Mar 28;152(1):93–103. doi: 10.1007/BF00264945. [DOI] [PubMed] [Google Scholar]
- West S. C., Countryman J. K., Howard-Flanders P. Purification and properties of the recA protein of Proteus mirabilis. Comparison with Escherichia coli recA protein; specificity of interaction with single strand binding protein. J Biol Chem. 1983 Apr 10;258(7):4648–4654. [PubMed] [Google Scholar]
- West S. C., Little J. W. P. mirabilis RecA protein catalyses cleavage of E. coli LexA protein and the lambda repressor in vitro. Mol Gen Genet. 1984;194(1-2):111–113. doi: 10.1007/BF00383505. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Persistence and decay of thermoinducible error-prone repair activity in nonfilamentous derivatives of tif-1, Escherichia coli B/r: the timing of some critical events in ultraviolet mutagenesis. Mol Gen Genet. 1975 Dec 29;142(2):87–103. doi: 10.1007/BF00266092. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., Wermundsen I. E. Targeted and untargeted mutagenesis by various inducers of SOS functions in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):881–886. doi: 10.1101/sqb.1979.043.01.095. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Skopek T. R., Hutchinson F. Changes in DNA base sequence induced by targeted mutagenesis of lambda phage by ultraviolet light. J Mol Biol. 1984 Mar 5;173(3):273–291. doi: 10.1016/0022-2836(84)90121-9. [DOI] [PubMed] [Google Scholar]
- Yarranton G. T., Sedgwick S. G. Cloned truncated recA genes in E. coli II. Effects of truncated gene products on in vivo recA+ protein activity. Mol Gen Genet. 1982;185(1):99–104. doi: 10.1007/BF00333797. [DOI] [PubMed] [Google Scholar]