Abstract
Molecular imaging is highly advantageous as various insidious inflammatory events can be imaged in a serial and quantitative fashion. Combined with the conventional imaging modalities like computed tomography (CT), magnetic resonance (MR) and nuclear imaging, it helps us resolve the extent of ongoing pathology, quantify inflammation and predict outcome. Macrophages are increasingly gaining importance as an imaging biomarker in inflammatory cardiovascular diseases. Macrophages, recruited to the site of injury, internalize necrotic or foreign material. Along with phagocytosis, activated macrophages release proteolytic enzymes like matrix metalloproteinases (MMPs) and cathepsins into the extracellular environment. Pro-inflammatory monocytes and macrophages also induce tissue oxidative damage through the inflammatory enzyme myeloperoxidase (MPO). In this review we will highlight recent advances in molecular macrophage imaging. Particular stress will be given to macrophage functional and enzymatic activity imaging which targets phagocytosis, proteolysis and myeloperoxidase activity imaging.
Keywords: Molecular imaging, macrophage, cardiovascular, inflammation, enzyme activity, protease, atherosclerosis, myocardial infarction, transplant rejection, phagocytes, myeloperoxidase
Introduction
Inflammation plays a pivotal role in the pathogenesis of cardiovascular diseases. Oxidative stress and inflammatory cytokines generated from inflammatory cells, especially monocytes and macrophages, contribute to cellular infiltration, atherosclerotic plaque expansion, thrombosis and adverse cardiovascular events leading to myocardial infarction (MI), ventricular remodeling and heart failure [1]. Therefore, there has been a pressing need to go beyond the capabilities of conventional imaging techniques; to image not only whole organs but also to probe at the cellular and molecular levels. This would allow us to visualize various in vivo biological events that govern tissue structural integrity and functional homeostasis. Equally important is to temporally decipher cellular and molecular events in vivo that, in case of any pathological insult, culminate in disease. Molecular imaging is highly advantageous to visualize these complex pathologies as various inflammatory events that underlie or precede tissue morphological changes can be imaged in a serial and quantitative fashion. Combined with the conventional imaging modalities like computed tomography (CT), magnetic resonance (MR) and nuclear imaging, it helps us resolve extent of ongoing pathology, quantify residing inflammation and predict future outcome [2]. Better understanding of the molecular and cellular events also paves way to identify therapeutic targets that would regulate the course the inflammation and improve outcome before the irreversible damage ensues. Another translational advantage is to monitor treatment effects in vivo due to the (semi)quantitative nature of molecular imaging [3, 4].
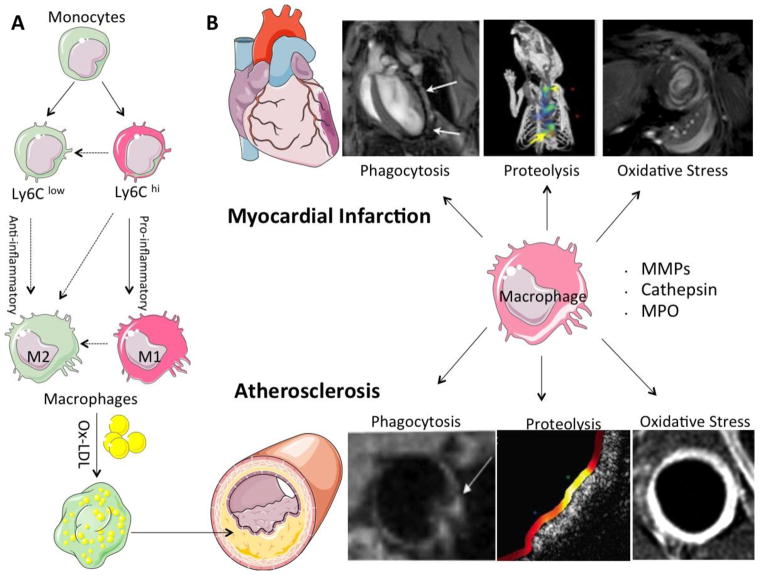
To grasp the full potential of molecular imaging advantages, it is important to identify imaging targets that play prominent roles in disease pathogenesis, and if subjected to therapeutic intervention, may lead to improved outcome. In preclinical studies, various molecular agents targeting inflammation, apoptosis, thrombosis, fibrosis, angiogenesis etc. have been synthesized and successfully imaged with various techniques [3]. Of these, macrophages are increasingly gaining importance due to the multitudes of roles they play in inflammatory cardiovascular diseases (Fig. 1A). In atherosclerosis, macrophages extravasate to the site of endothelial activation, phagocytose oxidized low density lipoproteins (LDL) and become foam cells. Later they release proteolytic enzymes which contribute to vulnerable plaque formation and acute ischemic events. After MI, there is a sequential monocytosis of the precursor pro-inflammatory Ly6Chi and then anti-inflammatory Ly6Clo monocytes [5]. These are recruited to the infarcted myocardium and after differentiating into macrophages, help in clearing infarct debris, tissue repair and in case of excessive proteolysis, facilitate adverse tissue remodeling. Furthermore, various cardiovascular disease pathologies are linked: post-MI monocytosis perpetuates atherosclerosis through increased cellular infiltration and consequent inflammation [6]. Intra-lesional macrophages, which are abundant in the inflamed plaque microenvironment, are also capable of self-renewal through local proliferation, independent of the systemic monocyte influx [7]. As a link to adaptive immunity, macrophages express non-self antigens to orchestrate T cell mediated rejection of the cardiac allograft transplant [8]. Therefore, imaging strategies that target macrophages, and its functions and products, would benefit in a wide variety of cardiovascular diseases.
Fig. 1.
(A) Macrophage phenotypic and functional plasticity. Circulating monocytes cross endothelial barrier at the site of injury. Depending upon tissue microenvironment, precursor Ly6Chi and Ly6Clow monocytes will give rise to M1 and M2 macrophages with pro- and anti-inflammatory functions respectively. (Dashed arrows: possible pathways yet to be proven in cardiovascular disease settings; derived from Nahrendorf et al. [87]). In atherosclerotic plaque, macrophages gobble up oxidized low density lipoproteins (ox-LDL), become foam cells and contribute to lesion burden (B) Schematic diagram for macrophage functional imaging with various molecular imaging techniques in myocardial infarction and atherosclerosis. Principal macrophage functions exploited for the purpose of cardiovascular molecular imaging are phagocytosis, proteolysis with matrix metalloproteinases (MMPs) and cathepsin, and induced oxidative stress with enzyme myeloperoxidase (MPO) (further details in respective sections).
Recent evidence suggests that there is considerable amount of phenotypic and functional plasticity in macrophage development in local tissue environment. Exposure to certain inflammatory cytokines such as interferon (IFN) and lipopolysaccharide (LPS) differentiates macrophages into classically activated, pro-inflammatory M1 macrophages, while exposure to IL-4 and IL-13 leads to a reparative and immunosuppressant M2 phenotype (Fig. 1A). Therapeutically, it may be important to identify M1 and M2 subsets with in vivo molecular imaging so that one with undesirable downstream effects can be targeted while others are spared. Functional and enzyme activity imaging may be particularly important in this respect as currently no single cell surface target can reliably identify these macrophage subsets [9].
In the past, several functional activities of macrophages have been exploited for in vivo macrophage imaging [10]. Phagocytosis and proteolysis are the main tissue effector functions of macrophages which help in clearing and digesting necrotic cellular and tissue materials after injury. Macrophages, recruited to the site of injury, internalize necrotic or foreign material. Along with phagocytosis, activated macrophages release their proteolytic enzymes like matrix metalloproteinases (MMPs) and cathepsins into the extracellular environment which break down extracellular matrix and attract more inflammatory cells by releasing chemokine and cytokines. Pro-inflammatory monocytes and macrophages also induce tissue oxidative damage through inflammatory enzyme myeloperoxidase (MPO) after ischemic or inflammatory insult. Cell surface targets have also been exploited for macrophage imaging and may provide useful information about macrophage quantification. Various cell surface receptors including vascular cell adhesion molecule-1 (VCAM) [11], inter-cellular adhesion molecule-1 (ICAM) [12] and integrins [13] have been tagged with different imaging reporters. However specificity of these agents is mixed as neutrophils, monocytes and endothelial cells may also share the same receptors. Furthermore, these mainly report macrophage content rather than pro-inflammatory activity directly.
Recent advances in molecular imaging techniques have made it possible to image these diverse macrophage activities in vivo. In addition to anatomical imaging, these molecular imaging strategies provide useful information about the ways macrophage interact with and modify the inflammatory milieu. Molecular imaging of macrophages has greatly expanded our understanding of the biological role of these important cells at the tissue, cellular, and molecular level in a wide variety of physiological and pathological conditions. We will highlight the principal functions of macrophages: (I) phagocytosis, (II) proteolysis, and (III) oxidative stress in inflammatory cardiovascular diseases (Fig. 1B).
Imaging Phagocytic activity
After reaching the site of injury, an important function of macrophages is the phagocytosis of the foreign pathogens or senescent cells [14]. This initiates a cascade of downstream events in which macrophages continue to link and orchestrate innate and adaptive immune responses depending upon the type of injury or pathogen involved. Being an attractive imaging target, macrophage phagocytosis function has been exploited with the help of specialized nanoscale constructs called magnetic nanoparticles (MNP) [15]. MNP are less than 100nm particles with an iron oxide [16] or gadolinium (Gd) core [17, 18] and a surface coat of dextran or related carbohydrates [19], lipids [20] or various polymers. There are several advantages in the structural properties of MNP making them highly suitable for molecular cardiovascular and monocyte/macrophage cellular imaging [10].
The outer surface coating allows identification and phagocytosis, while the inner magnetic core makes MRI imaging possible. It is possible to modify the surface to target certain cell-type or cell-state. For example, high-throughput surface-modified MNP screening revealed specific nanoparticles for both resting and activated macrophages, endothelial and cancer cells [21]. Target specificity can also be conferred by attaching certain antibodies or peptides which can be recognized by macrophages receptors [22]. Newer generation of MNP’s have considerably longer plasma half life and higher contrast on MRI imaging. Additionally, their smaller size allows better tissue penetration, and after phagocytosis, these tend to be concentrated within sub-cellular compartment (lysosomes) generating more MRI contrast [23]. All these properties make them suitable to sensitively image phagocytes such as monocyte and macrophages in atherosclerotic plaques [24] and inflamed myocardium [25].
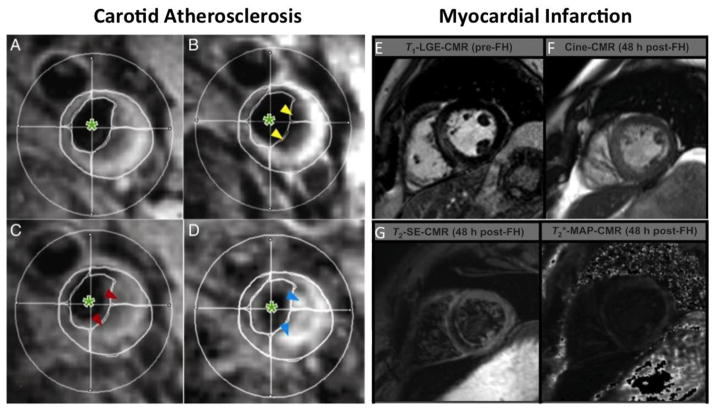
MNP have been extensively categorized to image macrophage phagocytic activity both in experimental preclinical and clinical studies. Owing to the superior sensitivity and favorable pharmacokinetics, ultrasmall superparamagnetic iron oxide nanoparticles (USPIO) have been utilized for rodents in vivo imaging of atherosclerosis [24] and myocardial infarction (MI) [25]. Similarly, in clinical studies, intralesional macrophages have been successfully imaged in inflamed carotid atherosclerotic plaques [26] and ST elevation MI (Fig. 2E–G) [27, 28], where macrophage MNP uptake was seen not only in infarct core and peri-infarct tissue but also in non-infarcted remote myocardium. Macrophage infiltration in these remote areas may orchestrate tissue remodeling and further contribute to chronic heart failure. In addition to diagnostic utility, MNP cardiac MR imaging has also proven useful for treatment follow up. In these settings, treatment responses to various anti-inflammatory treatments which either suppress macrophage phagocytic activity or decrease their recruitment to inflamed plaque, have been successfully imaged in human [29](Fig. 2A–D), mice [30, 31], and rabbits [32]. Another possible clinical utility is to non-invasively image cardiac transplant rejections with USPIO, where pre-clinical studies have shown encouraging results [33]. However, it has been shown in cell culture studies that MNP uptake may affect macrophage biology by increasing cell proliferation and reactive oxygen species formation [34]. This fact should be cautiously considered while performing in vivo imaging.
Fig. 2.
Clinical human Imaging with USPIO: Response to high dose statin therapy as imaged by T2*-weighted imaging of a human carotid artery atherosclerosis before and after ultrasmall superparamagnetic iron oxide (USPIO) infusion at week zero (A and B) and 12 weeks (C and D) after treatment. (B) USPIO uptake can be seen in the plaque before treatment (yellow arrowheads). (D) The plaque enhances at 12 weeks (blue arrowheads), indicating that the high-dose statin treatment has damped the USPIO-defined inflammation. Cross hairs: middle of the lumen. Green asterisk: blood pool (reproduced from Tang et al. with permission [29]). (E) Imaging myocardial infarction with USPIO. Late gadolinium enhancement (LGE) [pre-Feraheme™ (FH; a USPIO agent)] compared with (F) cine-cardiovascular magnetic resonance (CMR) images, (G)T2-weighted hypoenhancement andT2*-mapping signal voids; each 48 h after ferumoxytol administration (post-Feraheme™) (modified from Yilmaz et al. with permission [27]).
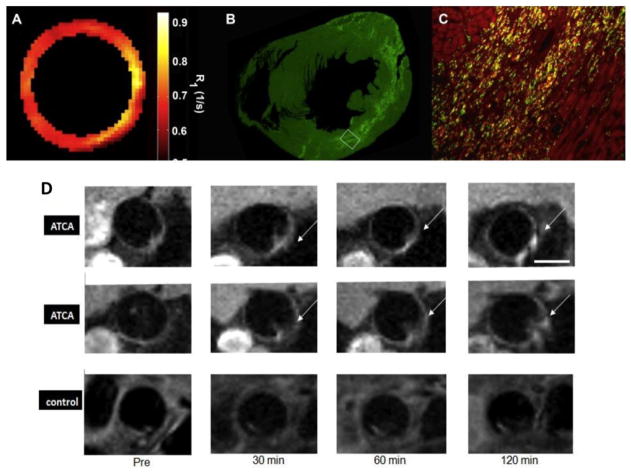
Iron oxide MNPs are superparamagnetic and after phagocytosis, generate negative contrast on T2* weighted images [15] with superior sensitivity as compared to traditional gadolinium based contrast agents which continue to stay in the extracellular fluid. Some imaging artifacts such as blood flow or cardiac motion related abnormalities, air and calcification may also produce negative contrast and need to be differentiated from MNP specific signal. For this purpose, certain off-resonance techniques have been developed which detect MNPs as a bright signal. Another approach is to use Gd-based nanoparticles with a surface coat of liposomes with or without foam cells specific antibody (CD36). This liposome coat is identified and internalized by macrophages with resultant contrast concentration, increased signal-to-noise ratio and enhanced sensitivity. Imaging is then performed by T1-weighted sequences generating positive contrasts. This technique has been utilized to label and track serial monocytes/macrophages responses after MI (Fig. 3A–C) [35] and also in a recent study, to specifically identify intra-lesional macrophages with atherosclerotic-targeting contrast agent (ATCA) (Fig. 3D) [36]. Imaging at clinical MR strength may also be possible with Gd-based nanoparticles which demonstrated increased lesional uptake in human aorta harvested from cadavers and incubated with CD36 targeted nanoparticles ex vivo [37]. Recently, monocyte/macrophages have been imaged with 19Fluorine-MRI based positive contrast. For this purpose, perfluorocarbon (PFC) nanoemulsions containing naturally occurring fluorine isotope (19F) were synthesized. 19F is not present in the body and any signal generated is due to exogenously injected 19F which can be superimposed on conventional MRI imaging. This technique has been utilized to image myocardial infarction, stroke [38] and cardiac transplant rejection [39].
Fig. 3.
(A–C) Correspondence between R1 map, CD68-positive monocytes/macrophages and liposomes containing Gd and Dil dye (DLL) in mouse model of myocardial infarction. (A) R1 map of infarcted heart at day 4 post-MI. (B) Fluorescence immunohistochemistry low magnification. C, High-power (×20) magnification of region bounded by white box in B. CD68-positive monocytes and/or macrophages are in green, DLL in bright red and double positive in yellow (reproduced from Naresh, et al. with permission [35]). (D) Atherosclerotic-specific contrast agent (ATCA) is composed of Gd metallofullerences and liposome coat carrying CD36 antibody for macrophage targeting. Representative aortic plaque images from two mice injected with (ATCA) and one control mouse with non-targeted agent (without CD36). Post-contrast images show enhanced plaque areas (arrows) only in the (ATCA) injected mice as compared to control (modified from Dellinger et al. [36] with permission).
Perhaps one the most significant advantages of MNP is that various fluorescent probes and radioligands for PET or SPECT can be attached to the MNP surface such that they still can be phagocytized by macrophages. At the same time, the fluorescent or nuclear probes could report additional information or utilized for validation. These particles can be imaged not only with MRI but also with other complementary imaging modalities both in vivo and ex vivo. MRI provides excellent anatomical details while fluorescent or nuclear imaging provides superior sensitivity. Ex vivo fluorescence detection techniques are also capable to image at cellular resolutions. These complementary functions have greatly expanded the role of MNP in modern era multi-modality molecular imaging where various aspects of macrophage biology and their interactions with surrounding cells and tissues can be serially imaged in vivo and validated further in vitro.
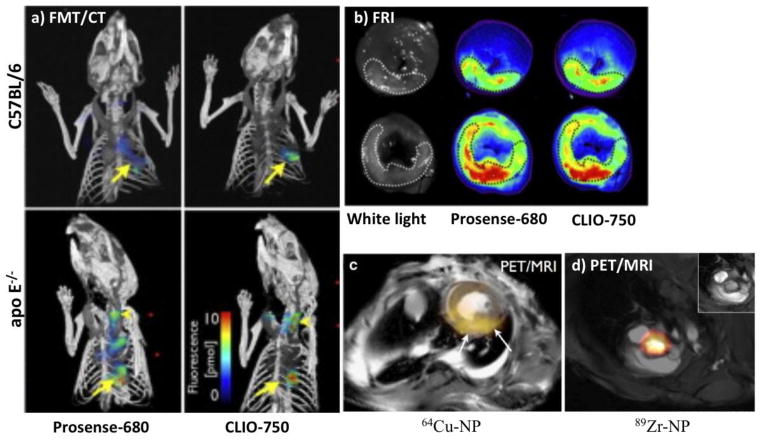
In experimental studies, fluorescent tagged MNPs facilitate their visualization during particle synthesis, early validation and specificity studies. Furthermore, after in vivo MRI imaging, tissues can be harvested and imaged ex vivo with fluorescent reflectance imaging (FRI), microscopy, or analyzed with flow cytometry in order to ascertain target cell uptake. Near infrared fluorescent (NIRF) probes are generally used for this purpose because of decreased auto-fluorescence and less tissue light scatter leading to greater photon penetration and depth of imaging. Recent advances in the 3D reconstruction algorithm have also made possible to perform in vivo small animal fluorescence molecular tomography (FMT). Images are also co-registered with CT or MRI at the same time to better resolve spatial resolution and anatomical details. This technique has been utilized to non-invasively image infarcted hearts [25, 40] (Fig. 4a-b) and inflamed aorta [24] in mice. Another noteworthy NIRF probe is indocyanine green, which is an FDA approved agent for various diagnostics application since 1959. Recently it has been shown that indocyanine green is taken up by foam cells and lipid-rich inflamed plaques as imaged by in vivo intravascular NIRF imaging catheters and in vitro validation studies [41]. Being widely available and FDA approved, it has a considerable promise for catheter-based human coronary imaging.
Fig. 4.
(a) 3-dimentional combined fluorescence molecular tomography and computed tomography (FMT-CT). C57BL/6 mice and genetically modified mice prone to atherosclerosis (apo E−/−) were imaged 5 days after myocardial infarction with both protease probe (Prosense) and phagocytic probe (CLIO). Arrows denote apical infarct signal, arrow heads denote fluorescence signal in the carotid artery; both are increased in apo E−/− mice. (b) Ex vivo fluorescence reflectance imaging (FRI) corroborates in vivo FMT findings (modified from Panizzi et al. [40] with permission). c) Combined positron emission tomography and magnetic resonance imaging (PET-MRI) of myocardial infarction and aortic root with 64Cu and 89Zr [50] labeled iron oxide nanoparticles respectively (NP: nanoparticles).
However, without invasive techniques, FMT cannot image tissue beyond a certain depth and therefore not currently suitable for human studies. For clinical studies, superior sensitivity and deep tissue imaging can be achieved with PET and SPECT nuclear imaging. Potential draw backs are radiation exposure and suboptimal spatial resolution which requires combined imaging with either CT or MRI. However, nuclear imaging is rapidly expanding around the globe and lack of integrated PET/CT or PET/MR scanners is less of a problem these days. Therefore, translational potential for a radioligand, which may specifically report lesion macrophage burden, may be promising for clinical trials. 18F-Fluorodeoxyglucose (18F-FDG), a glucose analogue, is thought to be taken up by early foam cells due to high metabolic activity [42]. It has already been used clinically to measure plaque inflammation [43, 44] as well as treatment response to delcetrapid [18, 45] (a cholesterol modulating agent which works by increasing beneficial HDL) and statin where it showed significant dose dependent reduction in 18F-FDG uptake in patients undergoing statin therapy [46]. However, it is not macrophage specific and may be taken up by other metabolically active and/or hypoxic cells [47, 48] in the vicinity of infarcted tissue. In order to improve macrophage specificity, 18F and newer radioligands with long plasma half life (64Cu and 89Zr) were derivatizated and linked to MNPs. After injection, these particles were phagocytized by macrophages and then successfully imaged with combined PET/CT or PET/MRI in mice models of atherosclerosis [49, 50] (Fig. 4d), aortic aneurysm [51], cardiac transplant rejection[52] and cancer [53]. In future, these multimodality radioligands may prove to be attractive candidates for quantifying lesion macrophage burden and therapeutic response follow up in clinical studies.
It is worth noting that while macrophages are the dominant phagocytes, other cells can also have phagocytic activity. As such, MNPs are not specific to macrophages, and small amounts can be taken up by smooth muscle cells, endothelial cells [24] or other inflammatory cells, including anti-inflammatory cells such as Ly6Clo monocytes [54].
Imaging Proteolytic Activity
Macrophage secretes proteases that can play prominent roles in the pathogenesis of cardiovascular diseases. In atherosclerotic plaques, macrophages phagocytose oxidized low density lipoproteins to become foam cells and release their battery of extracellular matrix degrading and pro-inflammatory proteases including MMP’s [55] and cathepsins [56]. These proteases can lead to fibrous cap thinning, plaque rupture and acute coronary events. In the infarcted myocardium, unchecked proteolytic activity also contributes to adverse ventricular remodeling, dilatation, aneurysm formation or in worse cases, ventricular rupture. Due to the diverse pathophysiological roles they play in cardiovascular diseases, both MMP’s and cathepsins have been explored as imaging biomarkers for intra-lesional macrophage load and increased proteolytic activity. Clinically it may have potential to identify high-risk patients with vulnerable plaques [57] who might benefit from coronary stent placement before an acute event occurs.
Due to limited stoichiometry, targeted imaging of MMP’s and cathepsins requires high sensitivity for detection in ranges that are detectable by nuclear imaging but difficult to achieve by conventional MRI techniques. For this purpose, tissue inhibitors of metalloproteinases (TIMP) attached with radioligands for PET/SPECT have been synthesized with different target specificities and have been successfully used to image atherosclerosis [58], vascular aneurysms [59] and MI [60] in both small and large animal studies. Co-registration with MRI or CT adds on anatomical information while in vitro studies confirm signal co-localization with macrophages.
It is also possible to not only image ex vivo but also in vivo (using FMT) protease enzyme activity rather than enzyme presence with the use of activatable smart fluorescent probes. For this purpose, NIRF probes have been synthesized such that in the native form, fluorescent molecules lie in close proximity to each other with resultant signal quenching. However, these molecules can be linked with synthetic linker peptides which are substrates for protease. Upon exposure, the linker peptide bond is broken, resulting in an increase in the physical distance between fluorochromes and consequent signal dequenching. Depending on how active the protease is, several fluorochromes may be activated by a single molecule which amplifies the signal. It is possible to tailor the linker peptide with amino acid sequences specific for different MMP’s or cathepsins [61]. With the help of these probes, macrophage protease activity has been successfully reported in atheroma [62], aortic aneurysm [63] and infarcted myocardium [40, 64] in small animals (Fig. 4a).
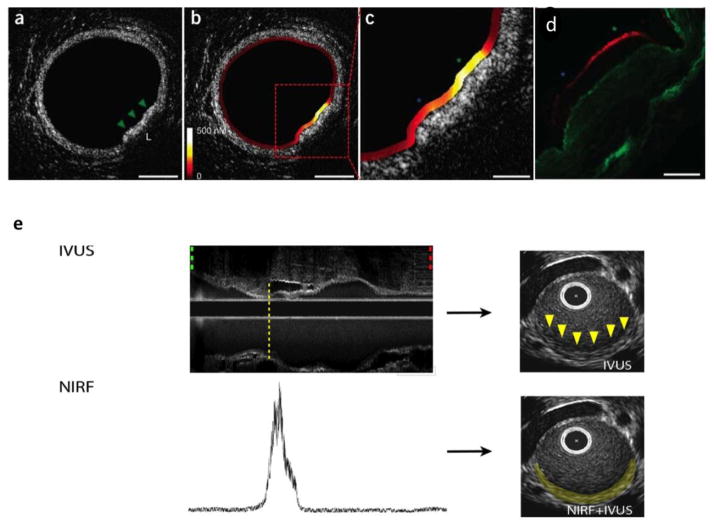
However, as FMT is limited only to small animal studies, there has been an increasing trend towards intravascular catheter based NIRF imaging which has translational potential of imaging intracoronary pathology in clinical studies. This technique employs custom made catheters to image inflammatory plaques in rabbit aorta which has a comparable vessel diameter to human coronary arteries (diameter 3–4mm). In a proof-of-concept study, a custom based catheter loaded with NIRF detection system was placed in rabbit iliac vessels (slightly smaller than the human coronary) 24 hours after injection of a cathepsin specific activatable probe (Prosense/VM110), and fluorescence signal was recorded [65]. In a later study, two-dimentional NIRF imaging was performed with the help of automated rotational catheter and later co-registered with intravascular ultrasound (IVUS) to better delineate plaque anatomy (fig. 5e). This study provided highly detailed in vivo imaging of the inflamed plaque as well as stent induced vessel injury [66], where increased cathepsin activity was visible along the edges of the stent placement. Ex vivo fluorescence reflectance imaging and microscopy corroborated in vivo imaging findings with NIR signal co-localized with cathepsin and macrophage immunostaining.
Fig. 5.
(a–c) Combined dual catheter NIRF-OFDI imaging in rabbit aorta dilineating plaque outline and superimposed cathepsin activity with high spatial resolution. (d) Fluorescence imaging and immunohistochemistry (not shown) revealed increased cathepsin B activity along the plaque surface (red) which co-localized with intravascular NIRF imaging signal. Green asterisk: higher protease signal; blue asterisk: lower signal. (Modified from Yoo et al. [67] with permission) (e) Combined IVUS-NIRF imaging with 2D rendering showing increased cathepsin activity in rabbit aortic plaque (Modified from Jaffer et al. [66] with permission).
In search of even superior plaque imaging at resolutions better than IVUS, newer catheters have been developed which combine intravascular NIRF imaging with optical frequency domain imaging (OFDI) [67]. OFDI mainly is based upon the principles of optical coherence tomography (OCT) with faster intravascular imaging acquisition and capability to delineate plaques structural details at microscopic resolutions (~10 μm) [68]. When paired with catheter NIRF imaging, it provides a powerful tool to image in vivo plaque inflammation superimposed on detailed vessel anatomy (Fig. 5a-d). In the future this technique may be utilized for therapeutic monitoring for drugs designed to reduce plaque inflammation as well as plaque thickness. Vulnerable plaque can also be identified at the same time and may help in deciding which patients may benefit from percutaneous coronary intervention and stenting.
MPO activity imaging
In addition to the MMPs and cathepsins, primary granules of myeloid cells and intra-plaque macrophages also contain abundant amount of the inflammatory enzyme MPO which is released into the surrounding tissue environment to generate oxidative stress, and attract and activate additional inflammatory cells [69]. There has been mounting evidence implicating MPO’s role in various CVD’s including atherosclerosis, myocardial infarction and cardiac transplant rejection. Unlike MMP and cathepsins, MPO is involved very early on in the pathogenesis of atherosclerosis [70]. It affects endothelial function by disrupting physiological balance of nitric oxide [71]. During plaque initiation, it impairs cholesterol transport by oxidizing LDL which are then taken up by macrophages leading to foam cell formation [70]. MPO and its products can also modify high density lipoproteins (HDL) leading to decreased cholesterol transport out of the plaque [72, 73]. In mature plaque, MPO is present in lesional macrophages where it interacts with and activates proteases. In the infarcted tissue, myeloid cells infiltrate ischemic myocardium and release MPO to contribute to adverse cardiac remodeling and chronic heart failure [74].
Therefore, MPO has emerged as a prominent CVD biomarker and has also been suggested for prognostic risk stratification [75]. Indeed, plasma MPO levels strongly predict prevalence of coronary artery disease [76] as well as adverse future outcome including need for revascularization [77] in patients with suspected coronary event [78] as well as in healthy individuals [79]. This makes MPO an ideal target for macrophage molecular imaging in inflammatory cardiovascular diseases [80]. Successful in vivo MPO molecular imaging may provide diagnostic as well as prognostic information for making informed management decisions. Furthermore, it may also help to follow treatment response to emerging anti-MPO therapies.
An activatable MRI-based smart imaging agent called MPO-Gd has been synthesized, which is specific for extracellular MPO activity [81, 82]. MPO-Gd molecule carries two serotonin moieties attached to Gd, which, upon exposure to active enzyme, is oxidized and forms oligomers. This increases physical size of the agent which in turn increases longitudinal relaxivity (r1) that can be visualized on T1-weighted MRI images as increased enhancing signal. These oligomers can crosslink to proteins to further amplify the signal [83]. The larger molecular size and protein binding further result in retention of the activated agents and further enhance the signal at sites of elevated MPO activity. Consequent increase in sensitivity as well as positive contrast advantages make it suitable for in vivo molecular imaging at clinically available MR field strengths. MRI also provides high structural detail and soft tissue contrast. In addition, systolic and diastolic cardiac functions can be quantified and correlated with molecular information at the same time.
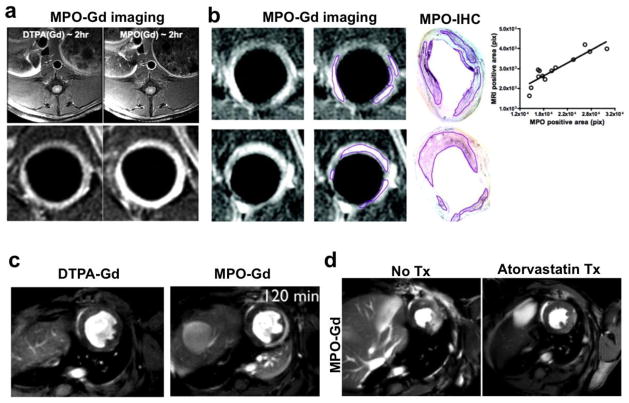
MPO-Gd has been successfully used for imaging inflamed atherosclerotic plaques [84], myocardial infarction [85], stroke [86] and heart transplant rejection [87]. In a rabbit atherosclerotic disease model, delayed images obtained two hours after contrast injection showed two-fold signal increase in inflamed aortic lesions as compared to normal wall (Fig. 6a). Because of the superior spatial resolution, focal areas of MPO induced signal enhancement were also identified which corresponded extremely well with MPO immunohistochemistry (fig. 6b)[84]. Similarly, MPO-Gd successfully imaged MPO activity from neutrophil and monocyte/macrophage influx after ischemic insult to the infarcted myocardium in vivo (Fig. 6c), later confirmed by histology and MPO activity assays [85]. Specificity of the agent was confirmed by lack of signal enhancement in infarcted MPO-knockout mice. Another noteworthy aspect of this study was the in vivo treatment response monitoring with anti-inflammatory therapy atorvastatin. Serial MPO-Gd imaging has not been found to affect the course of the disease process when comparing clinical outcome, flow cytometry, and histological markers with and without agent injection in animals models of neurological and cardiovascular diseases [82, 86–89], further underpinning the translational potential of this imaging technique (Fig. 6d).
Fig. 6.
(a) Delayed contrast MRI at two hour shows brighter signal in MPO-Gd imaging as compared to DTPA-Gd in rabbit model of atherosclerosis (reproduced from Ronald et al. [84] with permission). (b) MPO-Gd also precisely localizes focal areas of inflamed plaque as outlined and confirmed with MPO immunohistochemistry visually as well as correlation analysis. (c) At two hours post contrast injection, much of the DTPA-Gd is washed away however MPO-Gd still lights up the infarcted myocardium in mouse model. (d) Follow up of atorvastatin treatment response with MPO-Gd imaging reveals decreased inflammation and in vivo MPO activity (modified from Nahrendorf et al. [85] with permission).
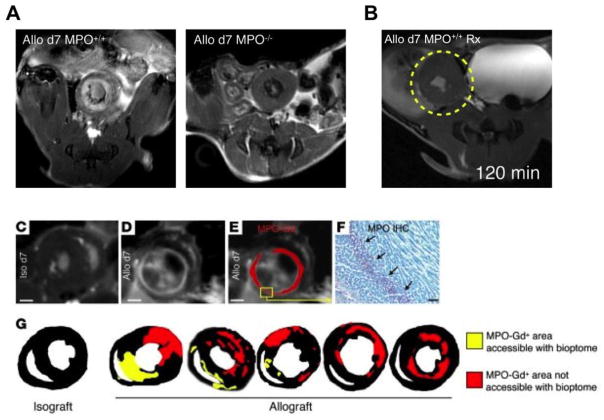
Serial cardiac biopsy is the current clinical standard for temporally resolving events that govern cardiac transplant rejection. However, it is invasive and subject to sampling error as not all areas of myocardium are accessible through biopsy. In a study investigating allograft transplant rejection, MPO-Gd was successfully used to identify and characterize cellular influx of MPO rich inflammatory monocytes to detect transplant rejection in sites that are not accessible by biopsy (Fig. 7C–G). Furthermore, treatment response with immunosuppressive therapy can be tracked, demonstrating decreased signal enhancement in the treated allograft (Fig. 7A–B). This study suggested an additional role of MPO-Gd imaging as a useful non-invasive alternative for serial cardiac biopsy [87].
Fig. 7.
(A) Cadiac allografts showed MPO dependent signal enhancement in wild type mice but no signal in the MPO-KO mice confirming specificity of imaging agent. (B) Immunosuppressive therapy resulted in attenuation of inflammation and signal enhancement in cardiac allograft. (C) Magnified MR image of isograft 120 minutes after injection of MPO-Gd. Some focal bright signal reflects spin refreshment effects due to blood flow. Scale bars: 1 mm. (D) Allograft 120 minutes after injection of MPO-Gd. (E) Same data as in D, but the MPO-Gd signal was thresholded and pseudocolored in red. (F) Immunoreactive staining for MPO in the area that enhances in D and E. Scale bar: 20 μm. (G) Thresholded data of signal enhancement 120 minutes after injection of MPO-Gd show typical enhancement patterns. To highlight the more comprehensive sampling of imaging over the clinical standard, areas that would be accessible to heart biopsies, which are routinely taken from the right ventricular cavity, are color-coded in yellow. Red encodes foci of rejection that could not be reached with routine transvenous right ventricular biopsies (modified with permission from Swirski et al. [87])
Conclusion
One of the driving questions in the field of molecular imaging is how to identify and quantify disease process before it has advanced to the point that available therapeutic intervention do not work affectively. In cardiovascular medicine, this translates into identifying patients with inflamed atherosclerotic plaques about to rupture or patients with pathological inflammatory stresses during early phase of post-MI wound repair as well as initiation of events culminating in cardiac transplants rejection. Furthermore, to keep pace with the drug development research, new technologies are needed which shed light on relevant biological process and monitor in vivo anti-inflammatory therapeutic effects. Indeed, current macrophage functional and enzymatic activity imaging has the potential to help answer these important questions with a promise of future clinical translation. 18F-PET and USPIO have already been used in the clinical trials for successful macrophage imaging, plaque inflammation quantification and treatment effects monitoring [29, 44–46].
Although not used in patients so far, pre-clinical agents targeting protease and MPO activity have a potential to open new ways to understand cellular and tissue microenvironment interactions. Individual patients or populations with certain genetic polymorphism, which may predispose them to excessive MPO [90, 91] or protease activity [92], can be identified for making timely and individually tailored management decisions. Additionally, there is a rapidly expanding field for identifying and characterizing distinct macrophage cell phenotypes which orchestrate divergent pro- and anti-inflammatory functions. Surface or enzymatic biomarkers, which may more specifically define these cells, should be validated in unperturbed tissue microenvironment. Macrophage imaging, as described here, will help to expand this effort to study cellular and molecular events and downstream inflammatory effects for improving our understanding of biological events in cardiovascular diseases. More importantly, macrophage imaging can identify distinct phenotypes, track disease progression, monitor treatment response, and predict prognosis noninvasively in a variety of inflammatory cardiovascular diseases to improve future patient care.
Footnotes
Conflict of Interest
Muhammad Ali, Benjamin Pulli, and John W. Chen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83(2):456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 2.Majmudar MD, Nahrendorf M. Cardiovascular molecular imaging: the road ahead. J Nucl Med. 2012;53(5):673–6. doi: 10.2967/jnumed.111.099838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahrendorf M, et al. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging. 2009;2(1):56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinusas AJ, et al. Multimodality cardiovascular molecular imaging, part I. Circ Cardiovasc Imaging. 2008;1(3):244–56. doi: 10.1161/CIRCIMAGING.108.824359. [DOI] [PubMed] [Google Scholar]
- 5.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta P, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–9. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Robbins CS, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19(9):1166–72. doi: 10.1038/nm.3258. (In this well elaborated study, authors explained that macrophages within atherosclerotic lesion are capable of local proliferation through scavenger receptor A. Furthermore, macrophage self-renewal capacity is independent of systemic monocyte infiltration and identifies macrophages as important therapeutic target in cardiovascular diseases.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyburn KR, et al. The role of macrophages in allograft rejection. Transplantation. 2005;80(12):1641–7. doi: 10.1097/01.tp.0000173903.26886.20. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann F, et al. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10(6):453–60. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012;111(2):231–44. doi: 10.1161/CIRCRESAHA.112.268144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahrendorf M, et al. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2(10):1213–22. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi KS, et al. Inflammation-specific T1 imaging using anti-intercellular adhesion molecule 1 antibody-conjugated gadolinium diethylenetriaminepentaacetic acid. Mol Imaging. 2007;6(2):75–84. [PubMed] [Google Scholar]
- 13.Kitagawa T, et al. Integrin-Targeted Molecular Imaging of Experimental Abdominal Aortic Aneurysms by 18F-FPPRGD2 Positron Emission Tomography. Circ Cardiovasc Imaging. 2013 doi: 10.1161/CIRCIMAGING.113.000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 15.Sosnovik DE, Nahrendorf M, Weissleder R. Magnetic nanoparticles for MR imaging: agents, techniques and cardiovascular applications. Basic Res Cardiol. 2008;103(2):122–30. doi: 10.1007/s00395-008-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wunderbaldinger P, Josephson L, Weissleder R. Crosslinked iron oxides (CLIO): a new platform for the development of targeted MR contrast agents. Acad Radiol. 2002;9(Suppl 2):S304–6. doi: 10.1016/s1076-6332(03)80210-6. [DOI] [PubMed] [Google Scholar]
- 17.Frias JC, et al. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc. 2004;126(50):16316–7. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 18.Lipinski MJ, et al. MRI to detect atherosclerosis with gadolinium-containing immunomicelles targeting the macrophage scavenger receptor. Magn Reson Med. 2006;56(3):601–10. doi: 10.1002/mrm.20995. [DOI] [PubMed] [Google Scholar]
- 19.Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc Chem Res. 2011;44(10):842–52. doi: 10.1021/ar200084x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morawski AM, et al. Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn Reson Med. 2004;51(3):480–6. doi: 10.1002/mrm.20010. [DOI] [PubMed] [Google Scholar]
- 21.Weissleder R, et al. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23(11):1418–23. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 22.Segers FM, et al. Scavenger receptor-AI-targeted iron oxide nanoparticles for in vivo MRI detection of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2013;33(8):1812–9. doi: 10.1161/ATVBAHA.112.300707. [DOI] [PubMed] [Google Scholar]
- 23.Simon G, et al. T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5T and 3T clinical MR scanning. European Radiology. 2006;16(3):738–745. doi: 10.1007/s00330-005-0031-2. [DOI] [PubMed] [Google Scholar]
- 24.Jaffer FA, et al. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging. 2006;5(2):85–92. [PubMed] [Google Scholar]
- 25.Sosnovik DE, et al. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation. 2007;115(11):1384–91. doi: 10.1161/CIRCULATIONAHA.106.663351. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi RA, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26(7):1601–6. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 27**.Yilmaz A, et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. European Heart Journal. 2013;34(6):462–475. doi: 10.1093/eurheartj/ehs366. (In this clinical trial, authors compared ferumoxytol (Feraheme™, FH), an ultrasmall superparamagnetic iron oxide nanoparticle (USPIO), to conventional gadolinium based agents in patients with acute myocardial infarction. T2* contrast was recognized not only in the infarct core and peri-infarct tissue but also in the remote myocardium suggesting macrophage infiltration and possible remote tissue remodeling.) [DOI] [PubMed] [Google Scholar]
- 28.Alam SR, et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ Cardiovasc Imaging. 2012;5(5):559–65. doi: 10.1161/CIRCIMAGING.112.974907. [DOI] [PubMed] [Google Scholar]
- 29.Tang TY, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53(22):2039–50. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Olzinski AR, et al. Pharmacological inhibition of C-C chemokine receptor 2 decreases macrophage infiltration in the aortic root of the human C-C chemokine receptor 2/apolipoprotein E−/− mouse: magnetic resonance imaging assessment. Arterioscler Thromb Vasc Biol. 2010;30(2):253–9. doi: 10.1161/ATVBAHA.109.198812. [DOI] [PubMed] [Google Scholar]
- 31.Sigovan M, et al. Anti-Inflammatory Drug Evaluation in ApoE / Mice by Ultrasmall Superparamagnetic Iron Oxide–Enhanced Magnetic Resonance Imaging. Investigative Radiology. 2012;47(9):546–552. doi: 10.1097/RLI.0b013e3182631e68. [DOI] [PubMed] [Google Scholar]
- 32*.Millon A, et al. Monitoring plaque inflammation in atherosclerotic rabbits with an iron oxide (P904) and (18)F-FDG using a combined PET/MR scanner. Atherosclerosis. 2013;228(2):339–45. doi: 10.1016/j.atherosclerosis.2013.03.019. (By using a combined PET/MRI scanner, authors compared (18)F-FDG PET and USPIO based MRI to assess plaque inflammation changes induced by atorvastatin and dietary change in a rabbit model of atherosclerosis. There was a decrease in the standard uptake value of (18)F-FDG after six months of treatment. Similar response was also measured in R2* with the help of USPIO imaging.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu YL, et al. Noninvasive evaluation of cardiac allograft rejection by cellular and functional cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2(6):731–41. doi: 10.1016/j.jcmg.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang CY, et al. Mechanism of cellular uptake and impact of ferucarbotran on macrophage physiology. PLoS One. 2011;6(9):e25524. doi: 10.1371/journal.pone.0025524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naresh NK, et al. Monocyte and/or macrophage infiltration of heart after myocardial infarction: MR imaging by using T1-shortening liposomes. Radiology. 2012;264(2):428–35. doi: 10.1148/radiol.12111863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dellinger A, et al. Functionalization of gadolinium metallofullerenes for detecting atherosclerotic plaque lesions by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:7. doi: 10.1186/1532-429X-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipinski MJ, et al. Macrophage-Specific Lipid-Based Nanoparticles Improve Cardiac Magnetic Resonance Detection and Characterization of Human Atherosclerosis. JACC: Cardiovascular Imaging. 2009;2(5):637–647. doi: 10.1016/j.jcmg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flögel U, et al. In Vivo Monitoring of Inflammation After Cardiac and Cerebral Ischemia by Fluorine Magnetic Resonance Imaging. Circulation. 2008;118(2):140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flögel U, et al. Noninvasive Detection of Graft Rejection by In Vivo 19F MRI in the Early Stage. American Journal of Transplantation. 2011;11(2):235–244. doi: 10.1111/j.1600-6143.2010.03372.x. [DOI] [PubMed] [Google Scholar]
- 40.Panizzi P, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55(15):1629–38. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Vinegoni C, et al. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci Transl Med. 2011;3(84):84ra45. doi: 10.1126/scitranslmed.3001577. (This study explains that the indocyanin green is taken up by lipid rich foam cells and can be imaged with intravenous near infrared detection catheter in rabbit vessels. Being widely available and FDA approved, indocyanin has a promise for clinical macrophage imaging in human coronary disease as well. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa M, et al. What can be seen by 18F-FDG PET in atherosclerosis imaging? The effect of foam cell formation on 18F-FDG uptake to macrophages in vitro. J Nucl Med. 2012;53(1):55–8. doi: 10.2967/jnumed.111.092866. [DOI] [PubMed] [Google Scholar]
- 43.Truijman MT, et al. Combined 18F-FDG PET-CT and DCE-MRI to Assess Inflammation and Microvascularization in Atherosclerotic Plaques. Stroke. 2013 doi: 10.1161/STROKEAHA.113.003140. [DOI] [PubMed] [Google Scholar]
- 44.Rudd JH, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50(9):892–6. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 45.Fayad ZA, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378(9802):1547–59. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Tawakol A, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909–17. doi: 10.1016/j.jacc.2013.04.066. (In this similar clinical trial, PET/CT imaging with (18)F-FDG was used to assess high and low dose atorvastatin treatment response in patients with carotid atherosclerosis. Again, high dose treatment produced significant reductions in FDG uptake that may represent changes in atherosclerotic plaque inflammation. This study signifies (18)F-FDG based macrophage imaging as an important tool for therapeutic response follow up.) [DOI] [PubMed] [Google Scholar]
- 47.Peterson LR, Gropler RJ. Radionuclide imaging of myocardial metabolism. Circ Cardiovasc Imaging. 2010;3(2):211–22. doi: 10.1161/CIRCIMAGING.109.860593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folco EJ, et al. Hypoxia But Not Inflammation Augments Glucose Uptake in Human MacrophagesImplications for Imaging Atherosclerosis With 18Fluorine-Labeled 2-Deoxy-D-Glucose Positron Emission Tomography. Journal of the American College of Cardiology. 2011;58(6):603–614. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 49.Nahrendorf M, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117(3):379–87. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majmudar MD, et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res. 2013;112(5):755–61. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nahrendorf M, et al. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011;31(4):750–7. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Ueno T, et al. Nanoparticle PET-CT detects rejection and immunomodulation in cardiac allografts. Circ Cardiovasc Imaging. 2013;6(4):568–73. doi: 10.1161/CIRCIMAGING.113.000481. (Trimodality contrats nanoagents have been used in the past as well. In this recent study, Ueno et al. reported use of a macrophage avid trimodality PET and magnetofluorescent nanoparticle in mouse model of cardiac transplant rejection. Additionally, immunosuppressive response with angiotensin converting enzyme inhibitor was also quantified with this novel imaging agent which resulted in better allograft survival.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keliher EJ, et al. 89Zr-labeled dextran nanoparticles allow in vivo macrophage imaging. Bioconjug Chem. 2011;22(12):2383–9. doi: 10.1021/bc200405d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Settles M, et al. Different capacity of monocyte subsets to phagocytose iron-oxide nanoparticles. PLoS One. 2011;6(10):e25197. doi: 10.1371/journal.pone.0025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995;77(5):863–8. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- 56.Lutgens SP, et al. Cathepsin cysteine proteases in cardiovascular disease. FASEB J. 2007;21(12):3029–41. doi: 10.1096/fj.06-7924com. [DOI] [PubMed] [Google Scholar]
- 57.Hermann S, et al. Non-FDG imaging of atherosclerosis: will imaging of MMPs assess plaque vulnerability? J Nucl Cardiol. 2012;19(3):609–17. doi: 10.1007/s12350-012-9553-6. [DOI] [PubMed] [Google Scholar]
- 58.Razavian M, et al. Atherosclerosis Plaque Heterogeneity and Response to Therapy Detected by In Vivo Molecular Imaging of Matrix Metalloproteinase Activation. Journal of Nuclear Medicine. 2011;52(11):1795–1802. doi: 10.2967/jnumed.111.092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Razavian M, et al. Molecular imaging of matrix metalloproteinase activation to predict murine aneurysm expansion in vivo. J Nucl Med. 2010;51(7):1107–15. doi: 10.2967/jnumed.110.075259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sahul ZH, et al. Targeted imaging of the spatial and temporal variation of matrix metalloproteinase activity in a porcine model of postinfarct remodeling: relationship to myocardial dysfunction. Circ Cardiovasc Imaging. 2011;4(4):381–91. doi: 10.1161/CIRCIMAGING.110.961854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaffer FA, et al. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115(17):2292–8. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 62.Nahrendorf M, et al. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler Thromb Vasc Biol. 2009;29(10):1444–51. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheth RA, Maricevich M, Mahmood U. In vivo optical molecular imaging of matrix metalloproteinase activity in abdominal aortic aneurysms correlates with treatment effects on growth rate. Atherosclerosis. 2010;212(1):181–7. doi: 10.1016/j.atherosclerosis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nahrendorf M, et al. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res. 2007;100(8):1218–25. doi: 10.1161/01.RES.0000265064.46075.31. [DOI] [PubMed] [Google Scholar]
- 65.Jaffer FA, et al. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118(18):1802–9. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaffer FA, et al. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011;57(25):2516–26. doi: 10.1016/j.jacc.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.Yoo H, et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat Med. 2011;17(12):1680–4. doi: 10.1038/nm.2555. (This study combines macrophage protease activity with high resolution structural imaging (optical frequency domain imaging) as imaged in rabbit vessels comparable in size to human coronary arteries. Both technologies were mounted on a same catheter and provided a powerful tool to image in vivo plaque inflammation superimposed on detailed vessel anatomy.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bezerra HG, et al. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv. 2009;2(11):1035–46. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11(11):2899–937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 70.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25(6):1102–11. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 71.Vita JA, et al. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110(9):1134–9. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng L, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114(4):529–41. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergt C, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101(35):13032–7. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Askari AT, et al. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197(5):615–24. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schindhelm RK, et al. Myeloperoxidase: A Useful Biomarker for Cardiovascular Disease Risk Stratification? Clinical Chemistry. 2009;55(8):1462–1470. doi: 10.1373/clinchem.2009.126029. [DOI] [PubMed] [Google Scholar]
- 76.Zhang R, et al. ASsociation between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286(17):2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 77.Baldus S, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108(12):1440–5. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 78.Brennan ML, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349(17):1595–604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 79.Meuwese MC, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;50(2):159–65. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 80.Chen JW, et al. Human myeloperoxidase: a potential target for molecular MR imaging in atherosclerosis. Magn Reson Med. 2004;52(5):1021–8. doi: 10.1002/mrm.20270. [DOI] [PubMed] [Google Scholar]
- 81.Luo Y, et al. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97(2):435–48. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- 82.Pulli B, et al. Measuring myeloperoxidase activity in biological samples. PLoS One. 2013;8(7):e67976. doi: 10.1371/journal.pone.0067976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriguez E, et al. Activatable magnetic resonance imaging agents for myeloperoxidase sensing: mechanism of activation, stability, and toxicity. J Am Chem Soc. 2010;132(1):168–77. doi: 10.1021/ja905274f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ronald JA, et al. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation. 2009;120(7):592–9. doi: 10.1161/CIRCULATIONAHA.108.813998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nahrendorf M, et al. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation. 2008;117(9):1153–60. doi: 10.1161/CIRCULATIONAHA.107.756510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Breckwoldt MO, et al. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci U S A. 2008;105(47):18584–9. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87**.Swirski FK, et al. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120(7):2627–34. doi: 10.1172/JCI42304. (For cardiac transplant rejection, serial biopsy is the current standard, which is invasive and prone to sampling error. In this study, Swirski et al. reported use of a gadolinium based agent specific for enzyme myeloperoxidase (MPO-Gd), which can be used as an imaging biomarker for cardiac rejection. Additionally, MPO-Gd was also able to follow immunosuppressive response non-invasively) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen JW, et al. Myeloperoxidase-targeted imaging of active inflammatory lesions in murine experimental autoimmune encephalomyelitis. Brain. 2008;131(Pt 4):1123–33. doi: 10.1093/brain/awn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Forghani R, et al. Demyelinating diseases: myeloperoxidase as an imaging biomarker and therapeutic target. Radiology. 2012;263(2):451–60. doi: 10.1148/radiol.12111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pecoits-Filho R, et al. A functional variant of the myeloperoxidase gene is associated with cardiovascular disease in end-stage renal disease patients. Kidney Int Suppl. 2003;(84):S172–6. doi: 10.1046/j.1523-1755.63.s84.32.x. [DOI] [PubMed] [Google Scholar]
- 91.Nikpoor B, et al. A functional myeloperoxidase polymorphic variant is associated with coronary artery disease in French-Canadians. American heart journal. 2001;142(2):336–339. doi: 10.1067/mhj.2001.116769. [DOI] [PubMed] [Google Scholar]
- 92.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis the good, the bad, and the ugly. Circulation research. 2002;90(3):251–262. [PubMed] [Google Scholar]